Control of the Action Potential Duration
The duration of the cardiac action potential is 100 times longer than the action potential duration (APD) in neurons. Unlike neurons, whose repolarization rate is less than one order of magnitude slower than the depolarization rate, the repolarization rate in the heart is between 2 and 3 orders of magnitude slower than that of depolarization. Thus, in the heart the upstroke velocity is more than 200 V/s during depolarization, but the change in membrane potential during phase 2 (the plateau) is only about 0.1 V/s. The rate of change of membrane potential is roughly proportional to the net ion current flowing across the membrane particularly during the plateau when the membrane conductance is low (1), so a 0.1 V/s change of voltage means that a net current of 0.1 pA/pF is flowing. It is this very small net current that predisposes to substantial changes in the cardiac APD. Many currents are flowing simultaneously during the plateau, and an increase in a single inward current or a decrease in an outward current will increase the APD. In this review we will focus on four currents that flow during the human ventricular action potential plateau: the rapid delayed rectifier current IKr, the slow delayed rectifier current IKs, the L-type calcium current ICaL and the persistent sodium current INaP. The amplitude of each current 200 ms into the human action potential during steady state stimulation at a cycle length of 1 s is shown in Figure 1 (adapted from (2)). Given the small net current flowing (0.1 pA/pF), a significant change in any of these four currents will have an effect on the APD. The degree to which this heterogeneity of current flow can give rise to action potential prolongation is indicated by the many causes of congenital long QT syndrome (cLQTS) (3). Both decreased outward currents and increased inward currents can be the underlying cause.
Figure 1. Four major currents that flow during the plateau phase of the human action potential.
Current density of two outward currents [IKr (rapid delayed rectifier) and IKs (slow delayed rectifier)] and two inward currents [ICaL (L-type calcium current) and INaP (persistent sodium current)] at 200 ms in a simulation of the human action potential stimulated at 1 Hz and at steady state. The net current INet is also shown. Estimates made from (2).
Brief Historical Perspective on Congenital and Acquired Long QT Syndromes
With the genetic information and improved sequencing technology developed during the human genome project, it became possible to identify mutated genes in families associated with a prolonged QT interval on the electrocardiogram. Not surprisingly, mutations in multiple ion channels were identified as causes of cLQTS (3), with the vast majority of patients possessing congenital forms of LQT1 (changes in KCNQ1 that reduce IKs), LQT2 (changes in hERG that reduce IKr) and LQT3 (changes in the long-lasting or “persistent” component of the tetrodotoxin-sensitive sodium current that increase INaP). LQT8 is due to rare mutations that cause an increase in ICaL. For individuals afflicted with these mutations, the APD is prolonged, predisposing to both early afterdepolarizations (EADs) and the potentially lethal arrhythmia torsades de pointes.
Besides the congenital basis for LQTS, it has become increasingly apparent that drugs can also produce this abnormality. In this circumstance the disease is called acquired long QT syndrome (aLQTS) (4). Clinical interest in drug-induced aLQTS reemerged in the late 1980s (5) when the non-soporific antihistamine Seldane (terfenadine) caused multiple sudden deaths. The lengthening of the APD (and consequently the QT interval) was attributed to block of the IKr channel (6). Subsequently, a large number of additional drugs with multiple therapeutic modalities were demonstrated to cause aLQTS by reducing IKr (7). It was initially proposed that these drugs act by direct blockade of a large internal vestibule in the channel which communicates with the narrower pore through which potassium ions flow (Figure 2; adapted from (8)). Reduced trafficking of the hERG protein to the cell surface was also described as a mechanism by which some other medications including fluoxetine (Prozac) cause aLQTS (9, 10).
Figure 2. The original hypothesis for the genesis of drug-induced aLQTS.
The product of the human ether-a-go-go related gene (HERG) is the alpha subunit of the IKr channel. It has a large internal vestibule that can be blocked by drugs, thereby reducing IKr and prolonging action potential duration (APD). When APD is sufficiently prolonged to allow for recovery of the L-type calcium channel from inactivation, the outcome is an early afterdepolarization (EAD). APD prolongation results in a prolonged QT interval, while the EADs can give rise to triggered action potentials, leading to torsades de pointes that can degenerate into ventricular fibrillation (VF). The figure was adapted from (8).
Evidence for Phosphoinositide 3-kinase Inhibition as a Mechanism of aLQTS
Phosphoinositide 3-kinase signaling
Class I phosphoinositide 3-kinases (PI3Ks) phosphorylate phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) to form the lipid second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP3) and are activated in response to growth factors, hormones or other environmental cues. These PI3Ks are complexes consisting of a catalytic subunit (p110α, p110β, p110δ, or p110γ) bound to one of several regulatory subunits (11, 12). The PI3Kα, PI3Kβ, and PI3Kδ complexes are activated by intracellular tyrosine kinases or receptor tyrosine kinases. PI3Kγ and PI3Kβ are activated by G protein-coupled receptors. PIP3 produced by Class I PI3Ks leads to the activation of effectors including the protein kinases Akt, PDK1, mTORC2 and atypical PKCs (13). PI3K signaling is turned off by the lipid phosphatase PTEN, which converts PIP3 to PI(4,5)P2. The mammalian heart expresses the p110α, p110β, and p110γ PI3K catalytic isoforms along with several different regulatory subunits. Figure 3 (modified from (14) illustrates the pathway from activation of PI3Kα by a receptor tyrosine kinase to its multiple effectors and finally to the ion currents that are affected.
Figure 3. Activation of phosphoinositide 3-kinase α (PI3Kα) signaling and regulation of ion channels.
Ligand binding to a receptor tyrosine kinase (RTK) such as the insulin receptor causes the activation of PI3Kα. Activated PI3Kα converts phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to phosphatidylinositol 3,4,5-trisphosphate (PIP3), which leads to the activation of downstream effectors such as mechanistic target of rapamycin complex 2 (mTORC2), phosphoinositide-dependent protein kinase 1 (PDK1), Akt, and atypical PKC (aPKC) isoforms. Other effectors of PIP3 that are not shown might also be involved in ion channel regulation. PI3Kα signaling suppresses the persistent sodium current (INaP) and enhances the rapid and slow delayed rectifiers (IKr and IKs, respectively), the L-type calcium current (ICaL) and the peak sodium current (INa). The currents are regulated in the opposite manner by PI3K inhibition or diabetes. Dephosphorylation of PIP3 by the lipid phosphatase PTEN turns off PI3Kα signaling. Adapted from (14).
Effects of PI3K inhibition on the cardiac action potential and cardiac ion currents
Over the past two decades, several new tyrosine kinase inhibitors designed to treat chronic myeloid leukemia have entered clinical use (15). The FDA’s approval of one of these, nilotinib, includes a black box warning for possible aLQTS. In 2012 we reported that nilotinib and some other drugs act through a novel mechanism to cause aLQTS (16). We investigated the effects of nilotinib and several other tyrosine kinase inhibitors and found that those that reduced PI3K activity in cardiac myocytes also lengthened the cardiac APD (Figure 4) and led to a significant increase in the incidence of pro-arrhythmic EADs. This effect of the tyrosine kinase inhibitors was not immediate, requiring several hours to be significant. A broad-spectrum PI3K inhibitor (PI-103) caused similar effects (Figure 5), so we concluded that the changes in APD induced by nilotinib are mediated by inhibition of PI3K. This conclusion was further investigated in mice with cardiac-specific knockout of the PI3K catalytic subunits p110α, p110β, or both isoforms. The results of these experiments supported our conclusion based on the pharmacologic studies and further demonstrated that the p110α, regulates the APD.
Figure 4. Prolonged APD caused by tyrosine kinase inhibitors in canine myocytes and reversal by PIP3 infusion.
(A) Myocytes were treated with vehicle (Veh) or dasatinib (Das), nilotinib (Nilo), or sunitinib (Su) at 1 μM for 1 h and then stimulated with 7.5% fetal bovine serum for 5 min. PI3K activity was assayed in anti-phosphotyrosine immunoprecipitates of cell lysates. The upper panel is an autoradiograph from a representative assay showing [32P]phosphatidylinositol 3-phosphate (PIP), and the lower graph summarizes data from three independent experiments. Data are means ± SE. (B) Sample traces of action potentials in myocytes treated with vehicle or drugs (1 μM for 2 h) with or without intracellular infusion of 1 μM PIP3. (C) Summary data of APD90 (APD at 90% repolarization) in myocytes treated with tyrosine kinase inhibitors with or without infusion of 1 μM phospholipids. Data are means ± SE. The number of cells studied is above each bar. Adapted from (16).
Figure 5. PI3K inhibition lengthens APD.
Sample traces of action potentials in myocytes treated with the PI3K inhibitor PI-103 (500 nM for 2 h) or left untreated as control, with or without intracellular infusion of 1 μM PIP3. Adapted from (16).
We then proceeded to investigate which ion currents are altered when PI3K is inhibited. Four currents were reduced: the peak sodium current INa (which generates the action potential upstroke) and the three plateau currents IKr, IKs and ICaL. A fifth current that also flows during the plateau, INaP, was increased. No change in any one current contributed more than 40% to the APD lengthening, but the combined changes in IKr and INaP accounted for more than 70% of the overall lengthening (Figure 6).
Figure 6. Computer simulation of the effects of PI-103 on the canine ventricular action potential.
PI-103 induced changes in IKr, IKs, ICaL, INaP and INa were determined experimentally and then incorporated into a computer model to simulate effects on the action potential (solid red line) as compared to the control. A simulation using the effects of PI-103 on IKr and INaP only is also shown (dashed red line). For these simulations IKr was reduced by 60% and INaP was increased by 180%. Modified from (16).
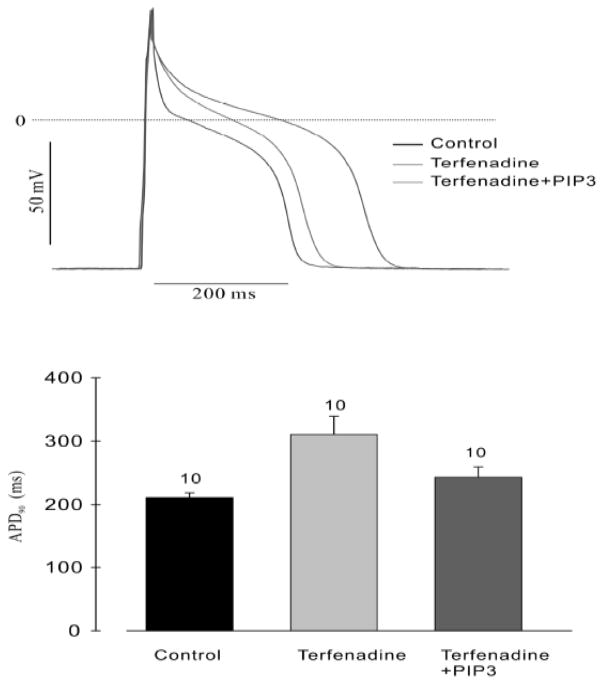
We next asked whether terfenadine, the compound on which the original IKr hypothesis of drug-induced aLQTS was based, might also act by inhibiting PI3K. The results are shown in Figure 7 and Figure 8. Perfusion of cardiac myocytes with terfenadine increased the APD, but the majority of this prolongation was absent when PIP3 was included in the pipette solution. The minor degree of APD prolongation remaining is probably due to direct blockade of IKr (Figure 7). In addition, terfenadine caused an increase in INaP that was prevented when PIP3 was added to the pipette solution (Figure 8).
Figure 7. Prolongation of the APD by terfenadine and partial reversal by PIP3.
(Top) Representative action potentials recorded in isolated canine ventricular myocytes treated with or without 1 μM terfenadine for 2 h and in the presence or absence of PIP3 in the patch pipette. (Bottom) Summary graph of APD90 for the three conditions. The number of cells studied is above each bar. From (16).
Figure 8. Increase in INaP by terfenadine and reversal by PIP3.
INaP was measured in canine ventricular myocytes using 10 mM external [Na+]. (A) Representative traces of INaP in untreated control cells, in the presence of terfenadine and with PIP3 in the pipette solution. (B) Current-voltage relationships for the 3 conditions in (A). From (16).
The question then was whether this action of drugs on PI3K is a common mechanism of aLQTS or a rare one. In 2014, Yang et al. investigated this question by testing a number of structurally diverse drugs already identified as IKr blockers for the ability to inhibit PI3K and increase INaP (17). Their study demonstrated that some of these drugs produced increases in INaP (dofetilide, E-4031, d-sotalol, thioridazine, haloperidol and erythromycin), while others had no effect (moxifloxacin and verapamil). Among the drugs studied, dofetilide increased INaP the most, and this effect was reversed by adding PIP3 to the pipette solution (Figure 9). The drugs that increased INaP were known to be more likely to induce the arrhythmia torsades de pointes. In their experiments, like ours with the tyrosine kinase inhibitors, the increase in INaP took many hours to days to be well evidenced. By contrast, the block of IKr is rapid and occurs in minutes.
Figure 9. Chronic dofetilide exposure increases INaP in mouse ventricular myocytes.

Individual and summary data for the effects of dofetilide treatment (1 μM for 5 h) on INaP. PIP3 (1 μM) in the pipette solution prevented the augmentation produced by dofetilide (data after 200 ms at 30 mV; n=6–10 each). From (17).
Since sodium channel blockers are available, it should be possible to determine how IKr block and INaP activation induced by a specific drug contribute to the development of APD prolongation over time. Qiu et al. performed such experiments on canine ventricular strips treated with dofetilide in vitro as well as in anesthetized dogs infused with dofetilide in vivo (18). For both in vitro (Figure 10) and in vivo (Figure 11) conditions the results were the same. Low dose lidocaine to inhibit INaP had little effect on the APD in vitro or the rate-corrected QT interval (QTc) in vivo when applied 30–60 minutes after dofetilide, while it dramatically shortened the APD and QTc when applied after 6 hours of dofetilide exposure. These results confirmed the two actions of dofetilide and the different time scales over which they act and indicated that INaP activation plays an important role in dofetilide-induced QTc prolongation in vivo.
Figure 10. Effects of lidocaine on APD after acute and chronic dofetilide superfusion in vitro.
After 3 h of adaptation in control solution, endocardial strips were superfused starting at 0 min with control solution containing 0.2 μM dofetilide, 100 μM moxifloxacin (Mox) or vehicle. Lidocaine (Lido; 20 μM for 20 min) was added to the superfusate at 30 min for dofetilide and 60 min for moxifloxacin, and again at 390 min. APD90 was measured periodically throughout the experiment. The largest effect of lidocaine is seen at 390 min in the dofetilide-treated sample. From (18).
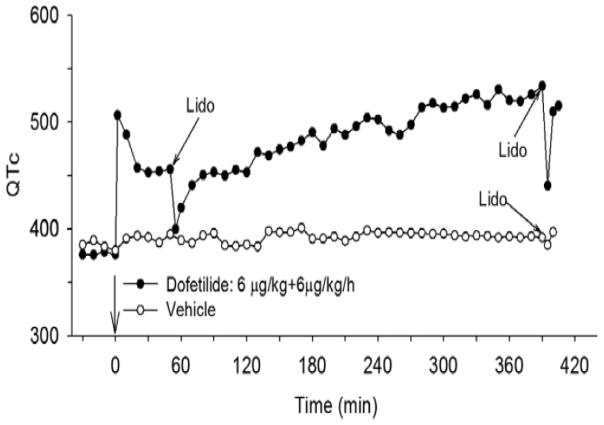
Figure 11. Effects of lidocaine on QTc after acute and chronic dofetilide infusion in vivo.
At 0 min, one dog received an intravenous bolus of dofetilide (6 μg/kg) followed by constant infusion of 6 μg/kg/h dofetilide for 7 h. The control animal was infused with vehicle for 7 h. An intravenous bolus of lidocaine (Lido; 2 mg/kg) was administered at the times indicated. QT interval and heart rate were recorded every 10 min and QTc was calculated. From (18).
Increased INaP in diabetes
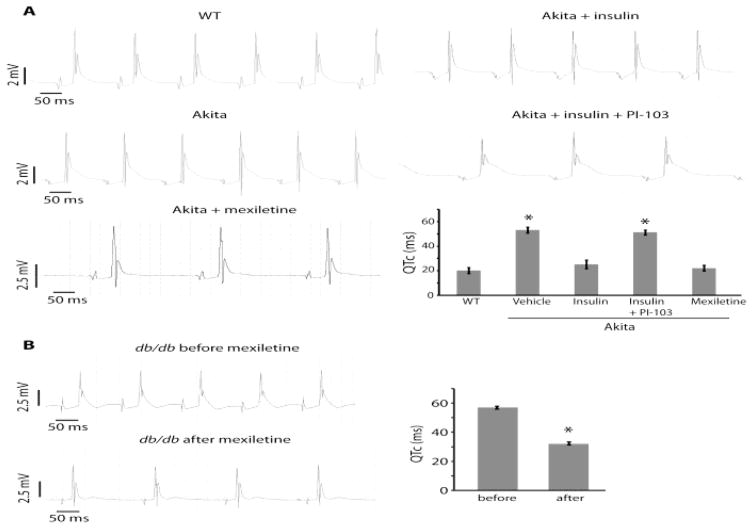
Insulin stimulates PI3K activity by binding to and activating the insulin receptor tyrosine kinase (see Figure 3). Diabetes is a disease in which there is reduced or absent insulin secretion (type 1) or reduced sensitivity to insulin in target tissues including the heart (type 2). Thus, it is not surprising that diabetes is associated with reduced cardiac PI3K activity, prolonged QT interval and an increase in the incidence of sudden death (19). Given the evidence that PI3K regulates INaP, Lu et al. (20) investigated whether an increase in this current plays an important role in cardiac repolarization in mouse models of type 1 (Ins2Akita) and type 2 (db/db) diabetes. In both models the APD was prolonged as compared to that in control mice (Figure 12). This difference was eliminated when mexiletine (a blocker of INaP) was added to the perfusate. It was also eliminated when PIP3 was added to the pipette solution. INaP was increased as compared to the control in both diabetic models, and adding PIP3 to the pipette solution also eliminated this difference (Figure 13). Finally, QTc measured in isolated perfused hearts was longer in the diabetic models than in the control. Again, this difference was dramatically reduced by the INaP blocker mexiletine (Figure 14).
Figure 12. APD prolongation in diabetic cardiac myocytes and reversal by PIP3 infusion or mexiletine treatment.
Ventricular myocytes were prepared from diabetic Ins2Akita (Akita) and db/db mice. (A) Sample traces of action potentials with or without intracellular infusion of 1 μM PIP3. (B) Sample traces of action potentials in myocytes preincubated with or without 4 μg/mL mexiletine for 2 h. (C) Sample trace of an action potential in a nondiabetic wild-type (WT) myocyte. (D) Summary data of APD90. Phospholipids were infused intracellularly at 1 μM. Data are means ± SE. The number of cells studied is above each bar. All studies were performed in the presence of 4-aminopyridine. From (20).
Figure 13. Increased INaP in diabetic cardiac myocytes and reversal by PIP3 infusion.
(A) Sample traces of tetrodotoxin (TTX)-sensitive INaP in wild-type (WT), Ins2Akita (Akita), and db/db myocytes with or without intracellular infusion of 1 μM PIP3. TTX-sensitive currents were obtained by subtracting traces obtained in the presence of 10 μM TTX from the traces obtained in its absence (inset). (B) Summary graph of INaP current-voltage relationships showing means ± SE. INaP was elicited by depolarizing pulses ranging from −80 to 0 mV in 10-mV increments from a holding potential of 80 mV. n = 7 cells per group. From (20).
Figure 14. QT prolongation of Ins2Akita (Akita) and db/db hearts and reversal by mexiletine.
Cardiac electrical activity was recorded from spontaneously beating hearts mounted on a Langendorff apparatus. QTc was automatically calculated from the tracings using the Mitchell formula (23). (A) Representative tracings from Akita and wild-type (WT) hearts. Hearts were treated with insulin (1 unit/L), PI-103 (500 nM), or mexiletine (4 μg/mL) added to the perfusate. Summary QTc graph shows means ± SE (bottom right). n ≥ 4 hearts per group. *, significantly different from WT values (P < 0.05, ANOVA with post hoc Fisher least significant differences test). (B) Representative tracings from a db/db heart before and after mexiletine treatment. Summary QTc graph shows means ± SE (right). n = 5 hearts. *, significantly different from before values (P < 0.05, Student’s t-test). From (20).
Taken as a whole, this is strong evidence that the aLQTS associated with mouse models of diabetes is due in large part to increased INaP, thus suggesting that abnormalities in PI3K activity may play a significant role in the increased incidence of QT prolongation and sudden death in diabetic people.
Implications of the Regulation of Cardiac Ion Channels by PI3K
Drug testing requirements to identify aLQTS risk must change. Pharmaceutical companies are required to test for the effects of novel drug candidates on the hERG channel. This now seems insufficient to guarantee drug safety. Specific studies of PI3K-dependent effects that develop over time should be included in drug safety testing. Such longer term testing of tyrosine kinase inhibitors on human induced pluripotent stem cell-derived cardiac myocytes has already begun (21). Electrophysiological measurements of the sensitivity of the APD to INaP blockade as well as direct assays examining drug effects on the PI3K signaling pathway are starting points. Further in vivo studies should carefully examine the time necessary for QTc to reach a new steady state.
Drugs that inhibit PI3K must have their dose levels monitored over longer time periods to assure that a steady state is reached. It has long been standard practice to monitor patients put on dofetilide for several days in a hospital setting to maximize safety. This should also be done for patients taking drugs that inhibit PI3K and that are used for non-cardiac purposes (e.g., nilotinib) to avoid an increased incidence of potentially lethal arrhythmias.
INaP represents a novel target for therapeutic intervention. The development of new pharmaceuticals to treat or prevent aLQTS is already in high gear. Ranolazine, the first of this new class of pharmacologic agents, blocks INaP but also reduces IKr (22).
Conclusions
It has long been known that alterations in a number of single cardiac ion currents can give rise to LQTS. This is a lesson continually being taught to us by the ever-increasing causes of cLQTS. Investigation of PI3K pathway inhibitors has shown that a single drug can affect multiple ion channels simultaneously to lengthen the APD. In addition, agents that block IKr while also inhibiting the PI3K pathway induce APD prolongation on multiple time scales. Happily, new origins of the same disease produce new therapeutic targets. The importance of INaP in aLQTS of different etiologies has already stimulated the industrialized development of new pharmacologic agents that can be used for treatment of both congenital and acquired LQTS.
Acknowledgments
Sources of Funding
National Heart Lung Blood Institute subcontract from Washington Univ, WU1640 (Cohen)
National Institute of Diabetes and Digestive Kidney Disease, 1R01DK10898901 (Lin)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noble D. The initiation of the heartbeat. 2. Oxford New York: Clarendon Press; Oxford University Press; 1979. p. xiii.p. 186. [Google Scholar]
- 2.O’Hara T, Virag L, Varro A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS computational biology. 2011 May;7(5):e1002061. doi: 10.1371/journal.pcbi.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crotti L, Celano G, Dagradi F, Schwartz PJ. Congenital long QT syndrome. Orphanet journal of rare diseases. 2008 Jul 07;3:18. doi: 10.1186/1750-1172-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003 Nov;89(11):1363–72. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies AJ, Harindra V, McEwan A, Ghose RR. Cardiotoxic effect with convulsions in terfenadine overdose. BMJ. 1989 Feb 04;298(6669):325. doi: 10.1136/bmj.298.6669.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roy M, Dumaine R, Brown AM. HERG, a primary human ventricular target of the nonsedating antihistamine terfenadine. Circulation. 1996 Aug 15;94(4):817–23. doi: 10.1161/01.cir.94.4.817. [DOI] [PubMed] [Google Scholar]
- 7.Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003 Apr 01;58(1):32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 8.Kannankeril P, Roden DM, Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010 Dec;62(4):760–81. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckhardt LL, Rajamani S, January CT. Protein trafficking abnormalities: a new mechanism in drug-induced long QT syndrome. Br J Pharmacol. 2005 May;145(1):3–4. doi: 10.1038/sj.bjp.0706143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajamani S, Eckhardt LL, Valdivia CR, Klemens CA, Gillman BM, Anderson CL, et al. Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br J Pharmacol. 2006 Nov;149(5):481–9. doi: 10.1038/sj.bjp.0706892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nature reviews Molecular cell biology. 2010 May;11(5):329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 12.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nature reviews Cancer. 2015 Jan;15(1):7–24. doi: 10.1038/nrc3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nature reviews Molecular cell biology. 2010 Jan;11(1):9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 14.Ballou LM, Lin RZ, Cohen IS. Control of cardiac repolarization by phosphoinositide 3-kinase signaling to ion channels. Circ Res. 2015 Jan 02;116(1):127–37. doi: 10.1161/CIRCRESAHA.116.303975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CC, Shah NP. Tyrosine kinase inhibitor therapy for chronic myeloid leukemia: approach to patients with treatment-naive or refractory chronic-phase disease. Hematology American Society of Hematology Education Program. 2011;2011:121–7. doi: 10.1182/asheducation-2011.1.121. [DOI] [PubMed] [Google Scholar]
- 16.Lu Z, Wu CY, Jiang YP, Ballou LM, Clausen C, Cohen IS, et al. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci Transl Med. 2012 Apr 25;4(131):131ra50. doi: 10.1126/scitranslmed.3003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang T, Chun YW, Stroud DM, Mosley JD, Knollmann BC, Hong C, et al. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation. 2014 Jul 15;130(3):224–34. doi: 10.1161/CIRCULATIONAHA.113.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu XS, Chauveau S, Anyukhovsky EP, Rahim T, Jiang YP, Harleton E, et al. Increased Late Sodium Current Contributes to the Electrophysiological Effects of Chronic, but Not Acute, Dofetilide Administration. Circ Arrhythm Electrophysiol. 2016 Apr;9(4):e003655. doi: 10.1161/CIRCEP.115.003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker AM, Cubbon RM. Sudden cardiac death in patients with diabetes mellitus and chronic heart failure. Diabetes & vascular disease research. 2015 Jul;12(4):228–33. doi: 10.1177/1479164115573225. [DOI] [PubMed] [Google Scholar]
- 20.Lu Z, Jiang YP, Wu CY, Ballou LM, Liu S, Carpenter ES, et al. Increased persistent sodium current due to decreased PI3K signaling contributes to QT prolongation in the diabetic heart. Diabetes. 2013 Dec;62(12):4257–65. doi: 10.2337/db13-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med. 2017 Feb 15;9(377) doi: 10.1126/scitranslmed.aaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006 Jul;92(Suppl 4):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998 Mar;274(3 Pt 2):H747–51. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]