Abstract
Background
The Standardized Treatment of Pulmonary Exacerbations (STOP) program has the intent of defining best practices in the treatment of pulmonary exacerbations (PEx) in patients with cystic fibrosis (CF). The objective of this analysis was to describe the clinical presentations of patients admitted for intravenous (IV) antibiotics and enrolled in a prospective observational PEx study as well as to understand physician treatment goals at the start of the intervention.
Methods
We enrolled adolescents and adults admitted to the hospital for a PEx treated with IV antibiotics. We recorded patient and PEx characteristics at the time of enrollment. We surveyed treating physicians on treatment goals as well as their willingness to enroll patients in various study designs. Additional demographic and clinical data were obtained from the CF Foundation Patient Registry.
Results
Of 220 patients enrolled, 56% were female, 19% were adolescents, and 71% were infected with P. aeruginosa. The mean (SD) FEV1 at enrollment was 51.1 (21.6) % predicted. Most patients (85%) experienced symptoms for ≥7 days before admission, 43% had received IV antibiotics within the previous 6 months, and 48% received oral and/or inhaled antibiotics prior to IV antibiotic initiation. Forty percent had ≥10% FEV1 decrease from their best value recorded in the previous 6 months, but for 20% of patients, their enrollment FEV1 was their best FEV1 recorded within the previous 6 months. Physicians reported that their primary treatment objectives were lung function recovery (53%) and improvement of symptoms (47%) of PEx. Most physicians stated they would enroll patients in studies involving 10-day (72%) or 14-day (87%), but not 7-day (29%), treatment regimens.
Conclusions
Based on the results of this study, prospective studies are feasible and physician willingness for interventional studies of PEx exists. Results of this observational study will help design future PEx trials.
Keywords: FEV1, symptoms, Pseudomonas aeruginosa
1. INTRODUCTION
Patients with cystic fibrosis (CF) develop chronic lung infections and suffer from recurrent acute pulmonary exacerbations (PEx), generally described as a worsening of respiratory signs and symptoms that are typically treated with antibiotics (1). PEx are associated with considerable morbidity and increased healthcare costs (2–5). There is often loss of lung function that is not fully recovered following treatment (6, 7). It is possible that some PEx treatment decisions may account for poorer outcomes (8, 9); for example, in the US, treatment with IV antibiotics for less than 9 days and treatment entirely outside of the hospital have both been associated with an increased risk of retreatment with IV antibiotics within 30 days of PEx treatment completion, despite similar patient characteristics at IV antibiotic initiation (9).
There were more than 17,000 events treated with IV antibiotics recorded in the US CF Foundation Patient Registry (CFFPR) in 2014 (10). Despite this being such a common event, there is a paucity of evidence upon which to develop PEx treatment guidelines (11) and substantial variation in therapeutic decisions surrounding PEx (8, 10, 12–15). Identifying best practices and evidence to guide treatment decisions offers the potential to improve the treatment of, and outcomes after, PEx.
To design a study to begin to define optimal treatment strategies, several questions need to be addressed. The PEx treatment guidelines highlighted several questions that might warrant investigation (11), but it is not known whether clinicians and patients would be willing to participate in such trials. Additionally, there are several endpoints that might be relevant for a PEx intervention study, including FEV1, symptom recovery, and time to next exacerbation. Understanding physician goals at the time they initiate IV antibiotics is necessary to select a clinical efficacy endpoint that will be accepted in practice. To formally power a study in CF PEx, a better understanding is needed regarding the magnitude of treatment effect and variance for these measures, in addition to the optimal timing of the endpoint assessment. A better understanding is also needed regarding which factors might confound a clinical trial in PEx (e.g., inpatient vs outpatient setting, airway clearance techniques, antibiotic selection and dosing); delineating the impact of these potential confounders is essential to designing any future clinical trial in PEx.
A careful review of the literature found the answers to these questions lacking. Thus, the Standardized Treatment of Pulmonary Exacerbations (STOP) study (clinicaltrials.gov NCT02109822) was performed to gather additional information to define key clinical endpoints, their magnitude of response, and their variance in order to guide future interventional trials to optimize PEx therapy and outcomes. In addition, we sought input from treating clinicians on treatment goals and willingness to enroll patients in various potential PEx study designs. We describe herein the methods for the STOP study, the clinical presentations of these patients, and the results of a physician survey that will inform future study design.
2. METHODS
STOP was an observational study conducted at eleven US CF centers between January 2014 and January 2015. Centers were recruited based on their willingness to participate, and their ability to enroll study subjects efficiently. This study was approved by each of the participating center’s Institutional Review Board and all participants or guardians provided written informed consent and assent where required.
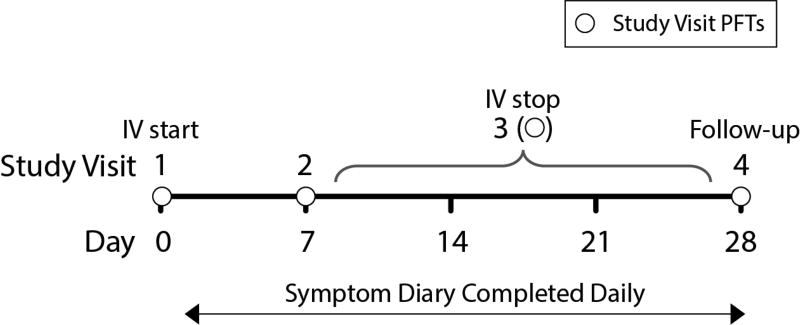
To be eligible for STOP, patients had to have a confirmed diagnosis of CF and be admitted to the hospital for treatment of a PEx with IV antibiotics. Because the characteristics of patients treated with IV antibiotics are generally similar whether they are admitted or not (9), we excluded patients whose IV antibiotics were initiated outside of the hospital in order to collect early response data during the most aggressive interventions. The diagnosis of PEx was determined by the treating physician. Patients were recruited within 24 hours of the start of IV therapy. Inclusion and exclusion criteria are included in the online supplement. Demographic and clinical data were collected at the time of enrollment and extracted from the CFFPR, including: age, sex, race/ethnicity, genotype, spirometry, respiratory microbiology, CF-related complications, pancreatic status, and history of previous PEx treatment. Additional data were collected specifically for STOP within the CFFPR at Days 1, 7, completion of IV antibiotics, and Day 28 (Figure 1). A survey (included in the online supplement) was performed on Day 1 that captured demographic and clinical data not available in the CFFPR, e.g., presence and duration of symptoms, auscultatory findings on chest exam, presence of non-massive hemoptysis, and prior treatment with oral and/or inhaled antibiotics. The physician survey also captured whether the treating physician’s primary goal was to recover lung function or to improve symptoms. If the primary treatment goal was to improve lung function, the clinician recorded a target FEV1 that would constitute treatment success. Finally, the physician survey asked for the clinician’s willingness to enroll the patient in hypothetical interventional trials including fixed treatment durations (7, 10 or 14 days), comparisons of different antibiotic treatments, and other treatments such as corticosteroids.
Figure 1.
Study design for STOP.
Spirometry performed at the time of enrollment (≤3 days before admission) was used for the admission FEV1. FEV1 % predicted was calculated using the Global Lung Initiative equations (16). Patients completed the validated CF Respiratory Symptom Diary - Chronic Respiratory Infection Symptom Score (CFRSD-CRISS) Questionnaire (17, 18) daily while enrolled in STOP. The CFRSD-CRISS is a CF-specific patient reported outcome measure designed to assess the severity of the most burdensome and frequent CF symptoms, and symptomatic response to treatment. It has been validated for adults and children 12 years and older; values range from 0 to 100, with lower scores indicating lower respiratory symptom burden.
Descriptive statistics were used to summarize demographics, symptom duration and distribution, prior PEx therapies, and spirometry at the time of enrollment, for the overall cohort, as well as by age group (<18 years or ≥18 years) and FEV1 % predicted (<50% predicted or ≥50% predicted). Two sample t-tests were used for comparisons of continuous variables or Fisher’s exact tests for categorical data. Analyses were performed using SAS (version 9.4, SAS Institute Inc., Cary, NC, 2013), and R (version 3.2.1, The R Foundation for Statistical Computing, Vienna, Austria, 2015).
3. RESULTS
3.1. Cohort characteristics
A total of 220 patients with CF were enrolled (Table 1). The mean (SD) age at admission was 26.3 (9.5) years. The mean (SD) body mass index (BMI) at admission for the 167 adult patients with available data was 21.0 (3.8) kg/m2. For 37 adolescent patients, the mean (SD) BMI percentile at admission was 32.7 (26.4) according to Center for Disease Control (CDC) standards. Among 216 patients with available microbiologic data collected within 6 months prior to enrollment, 71% had Pseudomonas aeruginosa isolated from respiratory secretions at least once. As might be expected, the prevalence of mucoid P. aeruginosa isolation was higher in adult patients (62% versus 31% in adolescent patients; difference = 31%, [95% CI = 14%, 45%]), while the prevalence of Staphylococcus aureus isolation was higher in adolescent patients (55% versus 32% in adult patients; difference = 23% [95% CI = 7%, 39%]). There were 16 patients with a history of NTM and 28 patients with a history of ABPA, but none were being actively treated at enrollment. Overall, our study cohort is similar to two recent PEx study cohorts in the US (see Table E1 in the online supplement).
Table 1.
Demographic and baseline characteristics at admission
| Characteristic | Study participants (N=220) |
||
|---|---|---|---|
| n | % | ||
| Gender | Female | 124 | 56 |
| Age distribution (years) | 12 to <18 | 42 | 19 |
| 18 to <30 | 116 | 53 | |
| ≥30 | 62 | 28 | |
| Race | White | 198 | 90 |
| Hispanic | 12 | 5 | |
| Unknown/Other | 10 | 5 | |
| Genotype | Homozygous F508del | 121 | 55 |
| Heterozygous F508del | 82 | 37 | |
| Other | 16 | 7 | |
| Not available | 1 | 1 | |
| Insurance status* | Enrolled in Medicaid | 76 | 37 |
| Pancreatic status* | Prescribed pancreatic enzymes | 196 | 89 |
| FEV1 % predicted* | <40 | 74 | 36 |
| 40–<70 | 84 | 41 | |
| 70–<100 | 42 | 21 | |
| ≥100 | 3 | 1 | |
| Respiratory microbiology*†‡ | Pseudomonas aeruginosa | 154 | 71 |
| Mucoid P. aeruginosa | 121 | 56 | |
| Staphylococcus aureus (methicillin susceptible) | 78 | 36 | |
| Methicillin-resistant S. aureus | 84 | 39 | |
| Stenotrophomonas maltophilia | 31 | 14 | |
| Achromobacter xylosoxidans | 20 | 9 | |
| Burkholderia cepacia complex | 6 | 3 | |
| Aspergillus spp. | 47 | 22 | |
| Non-tuberculous Mycobacteria (NTM)* | Yes | 16 | 7 |
| Allergic bronchopulmonary aspergillosis (ABPA)* | Yes | 28 | 13 |
| CF-related diabetes mellitus* | Yes | 86 | 39 |
| Chronic CF medications*†‡ | Inhaled tobramycin | 155 | 71 |
| Inhaled aztreonam | 111 | 51 | |
| Inhaled colistimethate | 30 | 14 | |
| Dornase alfa | 207 | 95 | |
| Hypertonic saline | 170 | 78 | |
| Azithromycin | 164 | 75 | |
| Ivacaftor | 8 | 4 | |
Excludes missing data for insurance status (n = 14), pancreatic status (n = 1), FEV1 % predicted (n = 17), respiratory microbiology (n = 4), non-tuberculous Mycobacteria (n = 2), ABPA (n = 1), CF-related diabetes mellitus (n = 1), chronic CF medications (n = 1)
Includes respiratory cultures recorded up to 6 months prior to admission and chronic medications recorded up to 12 months prior
Categories are not mutually exclusive and may add up to >100%
3.2. Symptoms and treatment prior to admission
The majority of patients had experienced symptoms for >7 days prior to admission, including hemoptysis (13%), wheezing (17%), or chest pain (24%) (Table 2). The mean (SD) CFRSD-CRISS score at admission was 47.5 (11.2), with a range from 0 (one patient reported no symptoms) to 73. The mean (SD) CFRSD-CRISS at admission was similar for adolescent patients, 43.0 (12.0), and adult patients, 48.6 (10.8). The mean [95% CI] BMI at enrollment had decreased from the best in the previous 6 months by 0.7 [0.5, 0.9] kg/m2 in adult patients and 7.6 [2.4, 12.8] percentile points in adolescent patients.
Table 2.
Characteristics of symptoms and treatments prior to enrollment.
| Adolescent patients (N=42) |
Adult patients (N=178) |
Total patients (N=220) | |||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Duration of symptoms | |||||||
| <7 days | 8 | 19 | 25 | 14 | 33 | 15 | |
| 7–21 days | 18 | 43 | 98 | 55 | 116 | 53 | |
| >21 days | 16 | 38 | 54 | 31 | 70 | 32 | |
| Non-massive hemoptysis* | 4 | 10 | 25 | 14 | 29 | 13 | |
| Wheezing* | 2 | 5 | 35 | 20 | 37 | 17 | |
| Chest pain/pleurisy* | 7 | 17 | 45 | 26 | 52 | 24 | |
| PEx treated with IV antibiotics in the 6 months before enrollment | 13 | 31 | 82 | 46 | 95 | 43 | |
| Initial outpatient antibiotic therapy prior to enrollment* | 31 | 76 | 72 | 41 | 103 | 48 | |
Excluding missing data for failed outpatient treatment (n = 5), symptom duration (n = 1), hemoptysis (n = 2), wheezing (n = 4), and chest pain (n = 4)
Many patients had recently been treated for a PEx: 43% received IV antibiotics within the 6 months prior to admission, with events more common in adults (46%) than adolescents (31%) (Table 2, difference = 15% [−2%, 29%]). Nearly half (48%) of patients had been treated for a PEx with oral and/or inhaled antibiotics prior to admission. More adolescent (76%) than adult patients (41%) were treated with oral and/or inhaled antibiotics prior to admission (difference = 34% [18%, 47%]).
3.3. Spirometry
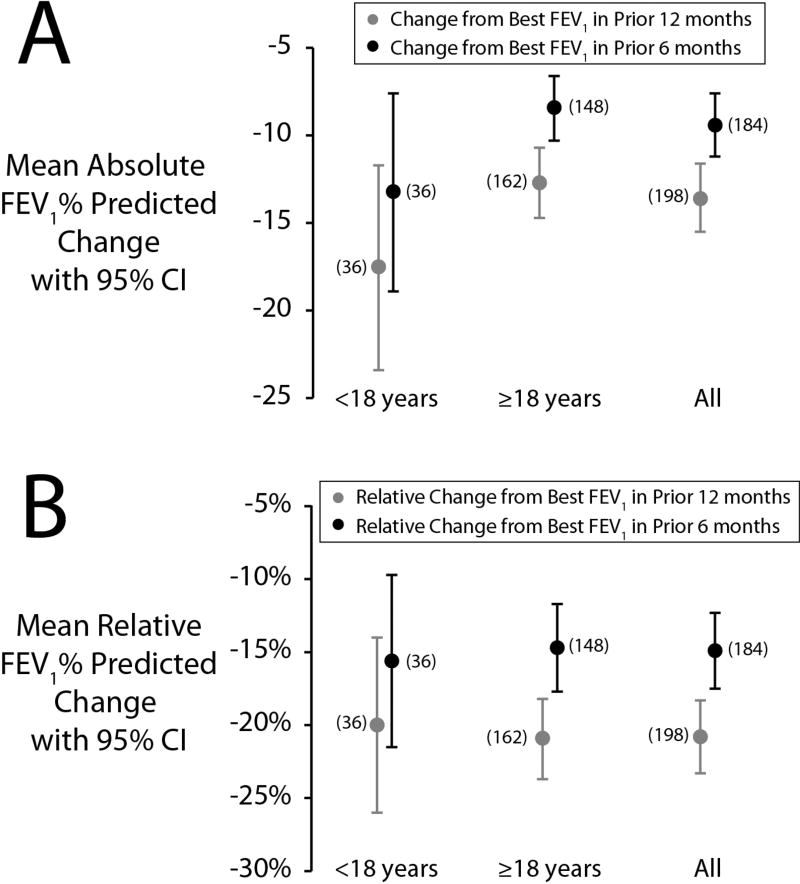
Within 3 days of admission, 203 (92%) patients performed spirometry. Mean (SD) FEV1 at enrollment was 51.1 (21.6)% predicted. At least one FEV1 measurement was recorded in the CFFPR for 200 (91%) patients within the preceding 6 months. The mean (SD) relative decrease from the best FEV1 in the previous 6 months was 14.9 (18.1)% predicted and the mean (SD) absolute decrease from the best FEV1 in the previous 6 months was 9.4 (12.6)% predicted (Figure 2). Among 216 (97%) patients with at least one FEV1 measured within the previous 12 months, the mean relative and absolute decreases were 20.8 (17.9) and 13.6 (13.7)% predicted, respectively (Figure 2). Among 184 patients with at least one measurement in the preceding 6 months and at enrollment, an absolute decline in FEV1 >10% from the best FEV1 in the 6 months prior to enrollment occurred in 74 patients (40%). However, for 20% of patients, the FEV1 measured at enrollment was the best FEV1 recorded within the previous 6 months.
Figure 2.
Mean absolute (A) and relative (B) change from 12-month and 6-month best FEV1 % predicted at the time of enrollment. Sample sizes are shown adjacent to point estimates. Vertical bars represent 95% confidence intervals.
Adolescent patients had higher FEV1 % predicted at enrollment and in the 6 months prior to enrollment than adult patients, but the drop from the best FEV1 in the 6 prior to enrollment was not statistically different between age groups (Figure 2). Patients whose 6-month best FEV1 was ≤50% predicted had smaller mean absolute decreases from their best FEV1 in the 6 months (5% vs 12%, difference = −7%, 95% CI = −10%, −4%) prior to enrollment than patients with FEV1 >50% predicted; however, the relative decreases in FEV1 % predicted were not significantly different.
3.4. Physician survey
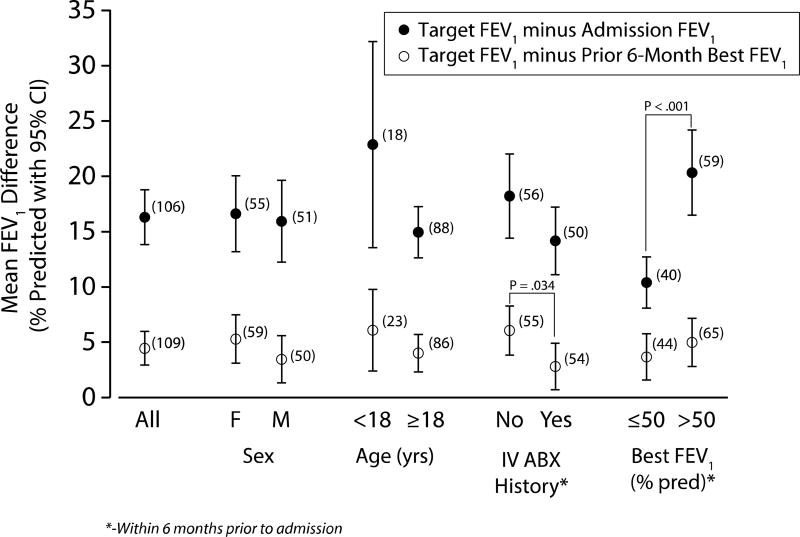
Physicians reported that their primary objective of treatment was recovery of lung function and improvement of symptoms in 53% and 47% of PEx, respectively. Forty-seven percent of physicians reported having a protocolized treatment duration with a mean planned duration of antibiotic therapy of 13.8 (1.6) days. Of the 116 physicians who chose lung function recovery as their primary objective, the absolute mean (SD) FEV1 recovery improvement goal was 16 (13)% predicted (Figure 3 and Table 3). This was larger in the adolescent patients, 23 (19)% predicted, than in the adult patients, 15 (11) % predicted, difference = 8% [−2%, 18%]. The absolute difference between admission and target FEV1 % predicted was smaller for patients with the best FEV1 in 6 months prior ≤50% predicted, as compared to those with best FEV1 in 6 months prior >50% predicted (10% vs 20%, difference = −10%, 95% CI = −14%, −5%). The mean target FEV1 was not significantly different from the highest recorded FEV1 in the 12 months prior to admission (difference = −0.7% [−2.7%, 1.3%], but was greater than the best FEV1 in the previous 6 months (difference = 4.5%, [2.9%, 6.0%]).
Figure 3.
Absolute difference between specified target FEV1 (% predicted) and admission FEV1 (% predicted), and absolute difference between specified target FEV1 (% predicted) and best FEV1 (% predicted) 6 months prior. Numbers adjacent indicate the number of patients included.
Table 3.
Target lung function goal as stated by the clinician, compared to lung function at different points in time.
| N | Mean | SD | |
|---|---|---|---|
| Target FEV1 (% predicted) | 116 | 65.3 | 23.7 |
| Admission FEV1 (% predicted) | 203 | 51.1 | 21.6 |
| Absolute difference in target - admission FEV1 (% predicted) | 106 | 16.3 | 12.8 |
| Best FEV1 in 6 months prior (% predicted) | 200 | 60.6 | 22.8 |
| Absolute difference in target- best FEV1 in 6 months prior (% predicted) | 109 | 4.5 | 8.0 |
| Best FEV1 in 12 months prior (% predicted) | 215 | 64.0 | 22.8 |
| Absolute difference in target - best FEV1 in 12 months prior (% predicted) | 114 | −0.7 | 10.7 |
No factors predicted whether the clinicians’ goal for therapy was symptom improvement or recovery of baseline FEV1. Specifically, there were no differences in duration of symptoms, percentage of patients who received oral and/or inhaled antibiotics prior to admission, disease stage, percent whose FEV1 dropped ≥ 10% on admission, or percentage of individuals needing IV antibiotics in the previous 6 months between groups of patients categorized by goal of therapy.
Physicians completed the survey at the time of admission to report their willingness to enroll each patient in a variety of study proposals (Table 4). Most centers reported a general willingness to enroll subjects in several study designs, but one center was consistently different from the others. The willingness to enroll in trials was similar for adolescents and adults. For studies of antibiotic treatment durations, there was low enthusiasm for only 7 days (only 29% of physicians were willing to enroll patients in a study that included a duration of 7 days), but greater enthusiasm for durations of 10 (72%) and 14 (87%) days. There was similar enthusiasm for studies of specific antibiotics (87%) and corticosteroids (84%).
Table 4.
Physician willingness to enroll in various potential study designs
| Adolescent patients (N=42) |
Adult patients (N=178) |
Total patients (N=220) |
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Study of fixed duration | |||||||
| 7 days | 13 | 31 | 49 | 28 | 62 | 29 | |
| 10 days | 30 | 74 | 124 | 72 | 155 | 72 | |
| 14 days | 36 | 86 | 154 | 88 | 190 | 87 | |
| Study of specified antibiotics (e.g., fixed vs pathogen specific) | 41 | 98 | 149 | 86 | 190 | 85 | |
| Study of other interventions (e.g., steroids) | 37 | 88 | 142 | 83 | 179 | 84 | |
4. Discussion
There is no established definition of PEx, and there is great variability in current treatment practices (8). We performed an observational study to understand the rationale for current treatment practices and measures of treatment success. In the STOP study, we have identified some key observations that must be accounted for in future interventional studies of treatment of PEx. First, we found that nearly half of patients were treated with IV antibiotics in the 6 months prior to enrolling in STOP, confirming previous reports that these are recurring events (19). As the number of previous PEx may affect treatment outcomes (19), a patient’s PEx history will need to be accounted for in any randomization process. Second, nearly half of all patients were treated as an outpatient with oral and/or inhaled antibiotics prior to the initiation of IV antibiotics. We did not collect additional details about this outpatient therapy, so it is not clear if the admission for IV treatment represented a failure of outpatient treatment or if outpatient therapy was merely a temporizing measure before the planned admission. Adolescent patients were more likely to have been treated with oral and/or inhaled antibiotics prior to the initiation of IV antibiotics. Whether an interventional study of PEx treated with IV antibiotics is relevant in the adolescent population is unclear, as it is likely that the number of PEx treated with oral and/or inhaled antibiotics is much greater (14).
In addition, we captured physician goals of therapy and key clinical features not routinely included in other studies of PEx including minor hemoptysis, wheezing, and chest pain. We did not find that physician goals of therapy correlated with any patient characteristics at the time of admission. More typical symptoms such as cough and sputum production have not shown sufficient discrimination in predicting clinical outcomes (20). Hemoptysis is often thought to be a manifestation of a PEx (21). Although we specifically excluded patients with massive hemoptysis, the prevalence of milder hemoptysis in our study was comparable to a recent report (22).
The decision to treat a PEx with IV antibiotics versus oral and/or inhaled antibiotics is likely affected by a patient’s baseline severity of lung disease, the change from baseline spirometry, respiratory culture results, and their experience with previous treatment regimens (14, 19). Surprisingly, a large proportion of patients were admitted for IV antibiotic treatment with their best recorded FEV1 % predicted within the prior 6 and prior 12 months (20% and 12%, respectively). This would suggest that FEV1 loss/recovery was not a primary motivating factor in their treatment, though we did not record whether other factors (e.g., worsening symptoms, new auscultatory findings) were driving the decision to treat. A recent report on PEx treated with oral antibiotics also noted that a similar proportion of patients were treated for a PEx despite having FEV1 at baseline (23). Alternatively, this observation highlights a limitation of relying on intermittent measurements of FEV1, to determine the “baseline” lung function, as has been done in previous registry analyses (6, 7, 24).
Our study has several limitations. First, as there is no standard definition of PEx, we enrolled patients diagnosed with PEx defined by the clinician, making this a pragmatic study that reflects actual clinical practice. It is important to note that previous epidemiologic analyses of risks and outcomes associated with PEx have used clinician decision to treat with IV antibiotics as the definition for exacerbation (2, 6, 7, 25) and none of the available PEx scores have undergone formal validation. We excluded patients whose IV treatment was initiated at home, who anticipated spending <5 days in the hospital, and patients successfully treated with oral and/or inhaled antibiotics, so we cannot know if those patients are truly different from the patients in our cohort, thus our results may not generalize to all PEx seen in CF. In fact, although our cohort is similar in many respects to a recent report from the CFFPR, our patients may be older and have more severe lung disease in comparison to all patients treated with IV antibiotics for a PEx (9). It is possible clinicians were unwilling to enroll these patients in a theoretical study that included a 7-day treatment arm because these patients were deemed to need the most aggressive PEx treatments. We did not track patients treated at participating centers that were not enrolled in STOP. We selected this cohort for STOP to address the primary goal of defining clinical endpoints in response to IV antibiotic treatment, which required more frequent data measurement. To improve the generalizability of future studies, including patients who receive IV treatment at home is necessary. We did not survey participating patients, and so it is unknown whether they share their clinicians’ willingness to enroll in various studies, or what their goals of therapy were. Various etiologies for PEx have been postulated, including acute events (e.g. viral infections (26), clonal shifts of colonizing bacteria (27), acute environmental exposures (28)), progression of underlying disease associated with medical non-adherence (29), increasing infection burden (30), and others (31, 32). We did not collect data on these factors, although whether therapeutic decisions or PEx outcomes differ according to PEx etiology has not been well-studied. Our findings may not be applicable outside of the US, where care practices may differ, or even at other US CF Centers that did not participate.
In preparing for a study of a treatment intervention, the feasibility of, and clinician’s willingness to participate in that study is of great importance. Ensuring participation and enthusiasm for PEx research at US CF Centers that did not participate in STOP is critical; CF Center directors and patient/family representatives must be consulted or surveyed before moving forward with PEx research. We chose a pragmatic design with a simple schedule of visits and study measures to understand the feasibility of future interventional studies, which could be large and unblinded. We were concerned that patients and clinicians would be reluctant to participate in a study that included a predetermined duration of therapy. It is evident that patients with slower FEV1 improvement during PEx tend to be treated longer (33, 34), and they may derive some benefit from a longer duration of treatment (33). Thus, clinicians may not be willing to enroll patients in a study that precludes longer IV courses. However, it appears that the majority of clinicians in this study would be willing to enroll their patients in all but the shortest treatment durations.
Data from the STOP study will be used to design comparative effectiveness research studies to optimize the treatment of, and outcomes after, PEx. We have determined that clinicians would be willing to enroll their patients in several potential study designs. We have identified strengths and weaknesses of the initial STOP study design that will inform future studies. The ultimate goal of these studies will be to standardize treatment of PEx as a means of optimizing outcomes and limiting adverse events in our patients.
Acknowledgments
We thank the patients and their families who participated in the clinical study, as well as the participating sites (investigator, lead research coordinator): Arizona Health Services (Cori Daines, Osmara Molina de Rodriguez); Case Western Reserve University (Elliott Dasenbrook, David Weaver and Bobbi Ksenich); Children’s Hospital of Pittsburgh (Jonathan Spahr, Elizabeth Hartigan); Johns Hopkins University (Natalie West, Abigail Thaxton); Medical University of South Carolina (Patrick Flume, Elizabeth Poindexter); Seattle Children’s Hospital (Ron Gibson, Sharon McNamara); University of Alabama, Birmingham (George M. Solomon, Heather Hathorne and Katie Brand); National Jewish Health, Denver (Jerry Nick, Katie Poch); University of Texas Southwestern (Raksha Jain, Ashley Keller); University of Washington (Christopher Goss, Ellen Wilhelm); University of Wisconsin, Madison (Don Sanders, Linda Makholm).
We thank the Cystic Fibrosis Foundation for the coordinated efforts to collect study data through the CF Foundation Patient Registry (Alex Elbert and Christopher Dowd). We also recognize three anonymous peer reviewers from the CF Therapeutics Development Network Publication Committee for their contributions in strengthening this manuscript.
The study was supported by grants from Cystic Fibrosis Foundation Therapeutics (SANDERS14A0, HELTSH13A1, GOSS13A0, FLUME13A1, CLANCY09Y0, SORSCH15RO, ORENST14Y0, NICKR0, DAINES14Y0), the National Institutes of Health (KL2 TR000428 and P30 DK089507), and the University of Wisconsin-Madison ICTR (NIH UL1 TR000427). This project was also supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina through National Institutes of Health grant UL1TR001450. The study sponsors had no role in study design, analysis, the writing of this manuscript, or the decision to submit for publication.
Abbreviations
- ABPA
Allergic bronchopulmonary aspergillosis
- CF
Cystic fibrosis
- CFFPR
CF Foundation Patient Registry
- CFRSD-CRISS
CF Respiratory Symptom Diary - Chronic Respiratory Infection Symptom Score Questionnaire
- CI
Confidence Interval
- FEV1
Forced expiratory volume at 1 second
- IV
Intravenous
- NTM
Non-tuberculous mycobacteria
- PEx
Pulmonary exacerbation
- STOP
Standardized Treatment of Pulmonary exacerbations
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: PAF, CHG, DBS, SLH, VVT and DRV contributed to the study design. All authors participated in data analysis/interpretation and critical review and revision of the manuscript. PAF and CHG participated in project management. SLH and VVT participated in data management and statistical analysis. All authors approved the final draft for submission.
References
- 1.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148:259–64. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Liou T, Adler F, Fitzsimmons S, Cahill B, Hibbs J, Marshall B. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345–52. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstan M, Morgan W, Butler S, Pasta D, Craib M, Silva S, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151(2):134–9. 9.e1. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Britto M, Kotagal U, Hornung R, Atherton H, Tsevat J, Wilmott R. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121(1):64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 5.Lieu T, Ray G, Farmer G, Shay G. The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics. 1999;103(6):e72. doi: 10.1542/peds.103.6.e72. [DOI] [PubMed] [Google Scholar]
- 6.Sanders D, Bittner R, Rosenfeld M, Hoffman L, Redding G, Goss C. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182(5):627–32. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkins MD, Rendall JC, Elborn JS. Incidence and Risk Factors for Pulmonary Exacerbation Treatment Failures in Patients With Cystic Fibrosis Chronically Infected With Pseudomonas aeruginosa. Chest. 2012;141(2):485–93. doi: 10.1378/chest.11-0917. [DOI] [PubMed] [Google Scholar]
- 8.Heltshe SL, Goss CH, Thompson V, Sagel SD, Sanders DB, Marshall BC, et al. Short-term and long-term response to pulmonary exacerbation treatment in cystic fibrosis. Thorax. 2016;71(3):223–9. doi: 10.1136/thoraxjnl-2014-206750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanDevanter DR, Flume PA, Morris N, Konstan MW. Probability of IV antibiotic retreatment within thirty days is associated with duration and location of IV antibiotic treatment for pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2016;15(6):783–90. doi: 10.1016/j.jcf.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cystic Fibrosis Foundation Patient Registry 2014 Annual Report. Bethesda: 2015. [Google Scholar]
- 11.Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180(9):802–8. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 12.Kraynack NC, Gothard MD, Falletta LM, McBride JT. Approach to treating cystic fibrosis pulmonary exacerbations varies widely across US CF care centers. Pediatr Pulmonol. 2011;46(9):870–81. doi: 10.1002/ppul.21442. [DOI] [PubMed] [Google Scholar]
- 13.Schechter MS, Regelmann WE, Sawicki GS, Rasouliyan L, VanDevanter DR, Rosenfeld M, et al. Antibiotic treatment of signs and symptoms of pulmonary exacerbations: a comparison by care site. Pediatr Pulmonol. 2015;50(5):431–40. doi: 10.1002/ppul.23147. [DOI] [PubMed] [Google Scholar]
- 14.Wagener JS, Rasouliyan L, VanDevanter DR, Pasta DJ, Regelmann WE, Morgan WJ, et al. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013;48(7):666–73. doi: 10.1002/ppul.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collaco JM, Green DM, Cutting GR, Naughton KM, Mogayzel PJ., Jr Location and duration of treatment of cystic fibrosis respiratory exacerbations do not affect outcomes. Am J Respir Crit Care Med. 2010;182(9):1137–43. doi: 10.1164/rccm.201001-0057OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. 2009;8(4):245–52. doi: 10.1016/j.jcf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Goss CH, Caldwell E, Gries KC, Leidy NK, Edwards T, Flume PA, et al. Validation of a novel patient-reported respiratory symptoms instrument in cystic fibrosis: CFRSD-CRISS. Pediatr Pulmonol. 2013;48(S36):A251. [Google Scholar]
- 19.VanDevanter DR, Morris NJ, Konstan MW. IV-treated pulmonary exacerbations in the prior year: An important independent risk factor for future pulmonary exacerbation in cystic fibrosis. J Cyst Fibros. 2016;15(3):372–9. doi: 10.1016/j.jcf.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanDevanter DR, Wagener JS, Pasta DJ, Elkin E, Jacobs JR, Morgan WJ, et al. Pulmonary outcome prediction (POP) tools for cystic fibrosis patients. Pediatr Pulmonol. 2010;45(12):1156–66. doi: 10.1002/ppul.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flume PA, Mogayzel PJ, Jr, Robinson KA, Rosenblatt RL, Quittell L, Marshall BC, et al. Cystic fibrosis pulmonary guidelines: pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med. 2010;182(3):298–306. doi: 10.1164/rccm.201002-0157OC. [DOI] [PubMed] [Google Scholar]
- 22.Thompson V, Mayer-Hamblett N, Kloster M, Bilton D, Flume PA. Risk of hemoptysis in cystic fibrosis clinical trials: A retrospective cohort study. J Cyst Fibros. 2015;14(5):632–8. doi: 10.1016/j.jcf.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.West NE, Beckett VV, Jain R, Sanders DB, Nick JA, Heltshe SL, et al. Standardized Treatment of Pulmonary Exacerbations (STOP) Study: Physician Treatment Goals and Practices for Individuals with Cystic Fibrosis with Pulmonary Exacerbations. J Cyst Fibros. doi: 10.1016/j.jcf.2017.04.003. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanojevic S, McDonald A, Waters V, MacDonald S, Horton E, Tullis E, et al. Effect of pulmonary exacerbations treated with oral antibiotics on clinical outcomes in cystic fibrosis. Thorax. 2016 doi: 10.1136/thoraxjnl-2016-208450. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Waters VJ, Stanojevic S, Sonneveld N, Klingel M, Grasemann H, Yau YC, et al. Factors associated with response to treatment of pulmonary exacerbations in cystic fibrosis patients. J Cyst Fibros. 2015;14(6):755–62. doi: 10.1016/j.jcf.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss C, Aitken M. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166(12 Pt 1):1550–5. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 27.Esther CR, Jr, Lin FC, Kerr A, Miller MB, Gilligan PH. Respiratory viruses are associated with common respiratory pathogens in cystic fibrosis. Pediatr Pulmonol. 2014;49(9):926–31. doi: 10.1002/ppul.22917. [DOI] [PubMed] [Google Scholar]
- 28.Aaron SD, Ramotar K, Ferris W, Vandemheen K, Saginur R, Tullis E, et al. Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2004;169(7):811–5. doi: 10.1164/rccm.200309-1306OC. [DOI] [PubMed] [Google Scholar]
- 29.Goss C, Newsom S, Schildcrout J, Sheppard L, Kaufman J. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–21. doi: 10.1164/rccm.200306-779OC. [DOI] [PubMed] [Google Scholar]
- 30.Eakin MN, Bilderback A, Boyle MP, Mogayzel PJ, Riekert KA. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros. 2011;10(4):258–64. doi: 10.1016/j.jcf.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goss C, Burns J. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62(4):360–7. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmody LA, Zhao J, Schloss PD, Petrosino JF, Murray S, Young VB, et al. Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. Ann Am Thorac Soc. 2013;10(3):179–87. doi: 10.1513/AnnalsATS.201211-107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraemer R, Deloséa N, Ballinari P, Gallati S, Crameri R. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2006;174(11):1211–20. doi: 10.1164/rccm.200603-423OC. [DOI] [PubMed] [Google Scholar]
- 34.Waters V, Stanojevic S, Klingel M, Chiang J, Sonneveld N, Kukkar R, et al. Prolongation of antibiotic treatment for cystic fibrosis pulmonary exacerbations. J Cyst Fibros. 2015;14(6):770–6. doi: 10.1016/j.jcf.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 35.VanDevanter DR, O'Riordan MA, Blumer JL, Konstan MW. Assessing time to pulmonary function benefit following antibiotic treatment of acute cystic fibrosis exacerbations. Respir Res. 2010;11:137. doi: 10.1186/1465-9921-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]