Abstract
Hemophilia A is a bleeding disorder caused by mutations in the gene encoding factor VIII (FVIII), a cofactor protein that is essential for normal blood clotting. Approximately one in three patients with severe hemophilia A produce neutralizing antibodies (inhibitors) that block its biologic function in the clotting cascade. Current efforts to eliminate inhibitors consist of repeated FVIII injections under what is termed an “ITI” protocol (Immune Tolerance Induction). However, this method is extremely costly and approximately 30% of patients undergoing ITI do not achieve peripheral tolerance. Human T regulatory cells (Tregs) have been proposed as a new strategy to treat this anti-drug antibody response, as well as other diseases. Polyclonal Tregs are nonspecific and could potentially cause general immunosuppression. Novel approaches to induce tolerance to FVIII include the use of engineered human and mouse antigen-specific Tregs, or alternatively antigen-specific cytotoxic cells, to delete, anergize or kill FVIII-specific lymphocytes. In this review, we discuss the current state of engineered T-cell therapies, and we describe recent progress in applying these therapies to induce FVIII-specific tolerance.
Keywords: B cell antibody receptor (BAR), Chimeric antigen receptor (CAR), Cytotoxic T cells, Factor VIII, Hemophilia, Inhibitors, Regulatory T cells (Tregs), T cell therapy
Introduction
Hemophilia A is an X-linked bleeding disorder due to mutations in the F8 gene encoding coagulation protein factor VIII (FVIII) [1, 2]. The incidence of hemophilia A is 1 in 5000 male births [3]. FVIII is a critical component of the blood coagulation cascade. It serves, as a co-factor in the intrinsic tenase complex, in which FVIII greatly accelerates the activation of the serine protease factor X by factor IXa. These reactions result in a burst of thrombin, which converts fibrinogen to fibrin and further activates platelets, resulting in a fibrin mesh and platelet plug that staunch bleeding [4]. FVIII is 265kDa protein comprised of domains A1-A2-B-A3-C1-C2 [5]. Severe hemophilia A is defined as <1% FVIII clotting activity [6], and the causative mutations are F8 intron 22 inversions (~45% of cases), intron 1 inversions (3–5% of cases) [7], and F8 deletions, splice site and missense mutations. Importantly, FVIII antigen in plasma/serum is generally undetectable in severe hemophilia A. Patients with mild or moderate hemophilia A have 1–5% or 5–45% normal clotting factor activity, respectively [6] and their incidence of inhibitors is lower than in severe hemophilia A. These patients circulate a dysfunctional, hemophilic FVIII protein; however even missense mutations can provoke inhibitor development in patients infused with wild-type FVIII protein [8–10]. Current standard of care for hemophilia A is regular infusions of either plasma-derived or recombinant FVIII for prophylaxis or for bleeding episodes (“on demand”). Unfortunately, a major problem is that a significant fraction of patients develop neutralizing antibodies, referred to as inhibitors, towards the infused FVIII due to a lack of central immune tolerance. Inhibitors occur in approximately 25–30% of the patients with severe hemophilia A, and in about 5% of patients with mild hemophilia A [11]. Many of these inhibitors neutralize or delay clotting by binding to functionally important FVIII surfaces, e.g. the A2, C1 and C2-domains [12], which interact with the FVIII chaperone protein von Willebrand factor and/or phospholipids and protein components of the intrinsic tenase complex.
The most prevalent current treatment to eliminate inhibitors is recurrent high-dose administration of FVIII, termed Immune Tolerance Induction (ITI) [13]. Drawbacks to ITI therapy, which is administered until titers subside or for 2–4 years, are its high cost and the fact that 20–40% of patients ultimately fail this therapy, making their clinical management challenging [14, 15]. In addition, the mechanism by which tolerance to FVIII is established following successful ITI treatment is still not understood. Less expensive and more effective immune tolerance protocols remain an unmet need. Cellular immunotherapy has been considered a promising approach to treat diseases including cancer and autoimmunity [16]. Understanding and applying immunologic tolerance induction to prevent and/or eliminate hemophilic inhibitors is the primary focus of research in our lab and others. Recent advances in chimeric antigen receptor (CAR) immunotherapies for cancer, and recent success in the treatment of resistant leukemia using CAR transduced cytotoxic T cells [17] [18], have suggested that related approaches may prove fruitful in “engineering” tolerance to protein antigens such as FVIII. The use of engineered T cells to express modified T cell or B cell receptors can be advantageous; these T cells will have enhanced specificity, as they can more effectively target T effector cells and/or antibody-secreting cells, and could thereby overcome significant fundamental limitations of polyclonal cellular immunotherapies.
Discovery of CAR T Cell Therapy
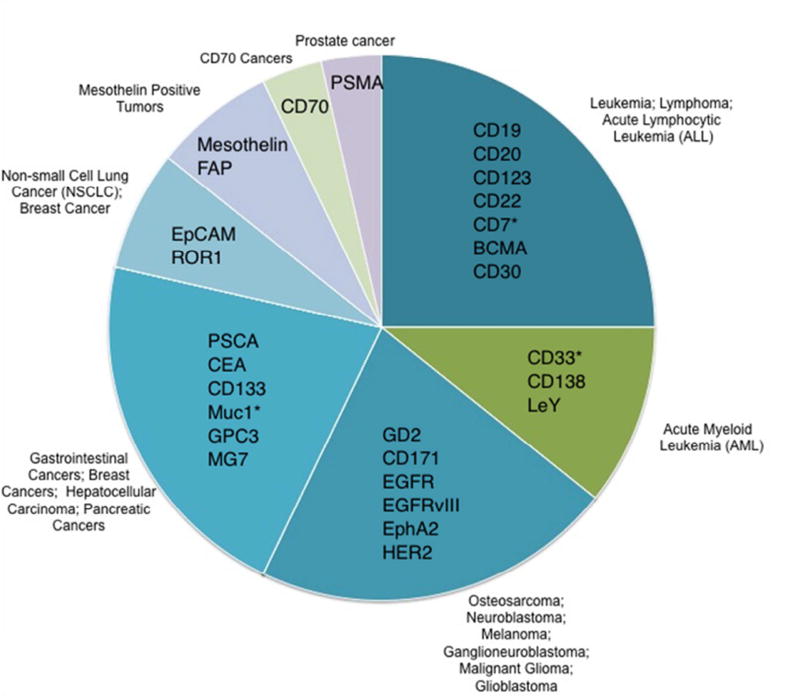
In 1989, Eshhar and colleagues designed the concept of redirecting T-cells with antibody specificity in an MHC independent manner [19, 20]. An anti-TNP (2,4,6-trinitrophenyl) immunoglobulin variable region replaced the variable region of a standard T cell receptor (TCR). This created “T bodies” that were non-MHC-restricted, genetically engineered T cells specific for a single, defined antigen [19, 21]. This represented the first generation of “CAR” T cells. The second assembly of CARs consisted of an antibody single chain variable fragment (scFv) comprising the variable heavy VH and variable light VL coupled to the endogenous immunoreceptor tyrosine-based activation motifs (ITAMs) of the CD3ζ chain or immunoglobulin Fc receptor γ-chain [19, 21, 22]. This generation of chimeric receptors served as the template for today’s CARs. A single gene product consisting of an scFv against CD33, a transmembrane receptor of myeloid lineage, and the signaling moieties CD28 and CD3ζ was generated [23]. In 2004, CD19-CARs were generated with the co-stimulatory molecule 41BB (CD137), which enhanced and diversified the robust performance of T cells [24]. 41BB has been shown to reduce the T cell exhaustion triggered by prolonged CAR signaling [25], and successful clinical trials ensued [17]. Major changes in the field of CAR-mediated immune therapy were facilitated by early clinical trials using CD19-CAR T cells to treat patients with relapsed or refractory hematological malignancies, with promising clinical outcomes [26–31]. Understanding and applying CAR therapy has been a notable achievement in the last decade in both oncology and hematology. Increasing research into T cell signaling, function, persistence and the importance of co-stimulation, has led to significant improvements in CAR T cell design. The third generation of CARs combines different intracellular signaling domains (e.g., CD28, OX40 or 41BB with CD3ζ) to ensure that CAR T cells are fully activated and effective after encountering their specific targets [32]. Currently, about 30 different CARs are in clinical trials (Figure 1). The majority of current CAR therapies are directed against hematological malignancies. Most target antigens are not restricted to tumor cells and can also be found on selected normal cells. Research in CAR tumor immunology and cancer therapy is now expanding to include safety, efficacy, and implementation of GMP standards to prepare T cell infusion products, sometimes referred to as “living drugs”.
Figure 1. Ongoing CAR Clinical Trials in United States.
CAR T cells targeting various antigens under clinical trials [61]. * Represents NK cells in clinical trials.
Engineered T Cells to Modulate Responses in Transplantation and Autoimmunity
Adoptive T cell transfer therapy has been proposed to treat a variety of autoimmune disorders [33–37] and to prevent the immune responses to therapeutic proteins [38]. However, polyclonal T regulatory cells (Tregs) manifest a repertoire of specificities and could lead to non-specific immunosuppression [39]. Moreover, the frequency of the specific Tregs that we might wish to use would likely be very low [40]. To increase the number of specific Tregs, one would have to isolate and expand such rare cells. As an alternative approach, several labs have engineered Tregs to express either scFv or TCRs specific for target peptide antigens [41]. Currently, there are multiple publications utilizing CAR Tregs in studies of transplantation and autoimmunity [39, 42–45]. For example, MacDonald et al., transduced human Tregs with an scFv specific for an HLA class I antigen and showed that these engineered Tregs suppress rejection in a xenotransplant model [39].
Engineered Specific Tregs in Hemophilia A
The antibody response to FVIII is known to be CD4+ T-cell dependent [2, 46–48]. Studies of HIV infected hemophilia patients with inhibitors demonstrated that as T cells numbers diminished the inhibitor titers also decreased, but as T cell numbers recovered with anti-retroviral therapy, so did the inhibitor titers [46, 47]. In addition, experiments with F8 knockout mice demonstrated that blocking co-stimulatory B7/CD28 or CD40/CD40L interactions required for T-cell activation and antibody response can prevent anti-FVIII antibody formation [2, 48, 49]. Thus, we hypothesized that Tregs targeting T-cell helper activity might be able to suppress inhibitor formation. We also hypothesized that FVIII-specific memory B cells or antibody-secreting cells (ASCs) could be targeted for suppression/elimination.
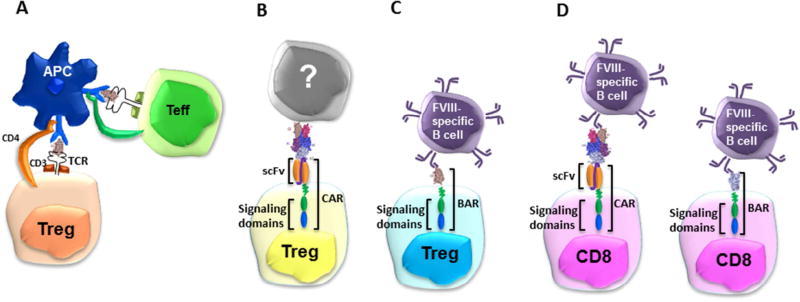
We have recently developed multiple alternative approaches to develop potential T-cell therapies by engineering recombinant FVIII-specific TCRs [50] or CARs [51], as well as FVIII specific B-cell antibody receptors (BARs) on expanded polyclonal Tregs or CD8+ antigen-specific T-effectors, respectively (Figure 2) [52, 53].
Figure 2. Strategies for generating engineered T cells specific to FVIII protein or peptides.
(A) Recombinant TCR: TCR-transduced FVIII-C2 domain-specific Tregs restricted to HLA-DRB1* 01:01. (B) CARs: Single chain variable domain (scFv) transduced Tregs specific to FVIII-A2. (C) BARs: FVIII C2 or FVIII A2 domain-linked transduced Tregs with transmembrane and signal transduction domains CD28-CD3ζ. (D) CARs CD8+ and BARs CD8+ T Cells: Cytotoxicity to target cells by indirect engagement of FVIII-A2 CAR expressing CTLs or by direct engagement through FVIII-A2 BAR CTLs.
TCR engineered Tregs in Hemophilia A
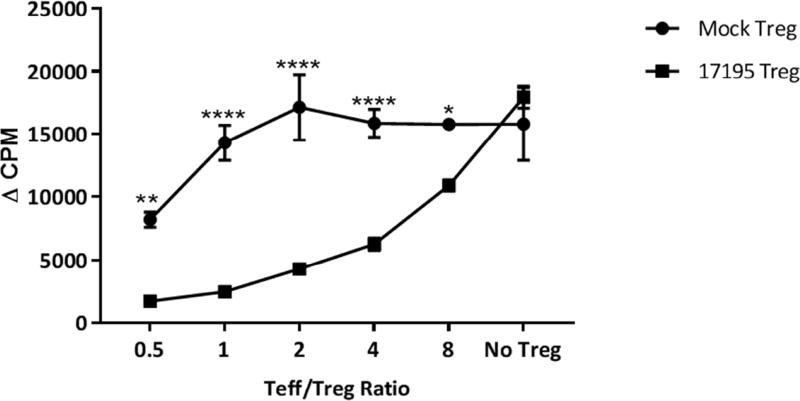
The first category of engineered Tregs generated in our lab by Kim et al. [50] express a recombinant T cell receptor (called 17195) that recognizes a peptide from the FVIII C2 domain (Figure 2A). The variable regions of this TCR α and β chain were cloned from a well-characterized T cell clone isolated from a hemophilia A subject [54] and combined with the human TCR constant regions, connected by a P2A peptide linker [54]. Expression of this TCR was under control of the 5’ MMLV LTR, with the green fluorescent protein (GFP) inserted downstream of the TCR coding region separated by an internal ribosomal entry site (IRES) [50]. The original T cell clone recognizes the FVIII-C2 domain peptide (residues FVIII-2194-2210) restricted by HLA-DRB1*01:01 (abbreviated DR1). Human CD4+ effector T cells transduced to express this TCR, proliferated and produced cytokines (e.g., IL-2 and IFNγ) when stimulated with the cognate peptide. When sorted human Tregs (CD25+, CD127lo) were transduced with a retroviral vector to express this TCR (17195), these cells expressed and maintained the Treg markers forkhead box P3 (FoxP3), glycoprotein-A repetitions predominant (GARP), latency-associated peptide (LAP) and glucocorticoid-induced TNF receptor-related ligand (GITR). Stimulation with the C2 peptide (FVIII-2194-2210) increased the expression of Treg markers. These 17195 TCR-Tregs proliferated, but did not secrete IFNγ and IL-2, in response to the C2 peptide. When 17195 transduced T effector and T regulatory cells were mixed in different ratios, 17195 TCR-Tregs suppressed proliferation and cytokine secretion of T cells from the parent clone or from 17195-T effector cells specific for the C2 peptide (Figure 3). These 17195-Tregs were also effective in suppressing a FVIII-specific mouse B-cell response in vitro. The observed effect could be due to direct suppression of FVIII-specific memory B cells (as antigen presenting cells, APC) or suppression of T helper cells, or both [50]. Importantly, 17195 TCR-Tregs also effectively suppressed the anti-FVIII antibody response in vivo across a xenogeneic barrier [51]. It is important to note that the 17195 TCR-Tregs, while specific for a single peptide epitope, were able to suppress the antibody response to the entire FVIII molecule; that is, there must have been bystander suppression to other FVIII epitopes. However, it is worth noting that because the 17195 TCR is HLA-DR1 restricted, consequently clinical application would require the patients to express the same HLA allele. Cloning FVIII-specific TCR variable regions from additional patients representing the most-common HLA class II alleles could overcome this issue [41, 55].
Figure 3. FVIII-selective immunosuppression by 17195TCRTregs.
Human CD4+ effector T cells mixed in different ratios of Teff/Treg were stimulated with rFVIII (0.5 µg/mL). Immunosuppression was evaluated using a 3H-thymidine incorporation assay. Representative data from 1 of 3 experiments are shown [50]. Statistical analysis based on 2 way Anova. (* 0.169; **0.019; **** <0.0001)
CAR-Tregs in Hemophilia A
Engineered Tregs generated by Yoon, Schmidt and co-workers [51] were transduced to express a CAR containing an scFv specific to the FVIII A2 domain (termed ANS8 CAR). This scFv was isolated from a phage library and cloned into the human IgG1 heavy chain [56, 57], together with standard components for CAR design, including the CD28 transmembrane and intracellular domains and the intracellular domain of the CD3ζ chain (Figure 2B). Retroviral vectors encoding the ANS8 CAR were used to transduce human T effectors or human Treg cells [51]. In contrast to the recombinant TCR strategy, the ANS8 CAR transduced Tregs are not MHC-restricted and could be used to treat any patient regardless of the MHC haplotype. These ANS8 CAR-Tregs in vitro abrogated the proliferation of T effector cells specific for the FVIII C2 domain (17195 T effectors) in the presence of FVIII, as well as of T effector cells specific to MBP in presence of both MBP and FVIII. This demonstrated both specificity and a bystander effect when both antigenic epitopes were present locally. In vitro, ANS8 CAR-Tregs were similarly effective in suppressing secondary anti-FVIII antibody production from FVIII-primed murine splenocytes. The results obtained using ANS8 CARs suggest that CAR-Tregs that recognize a single FVIII epitope may be able to effectively control inhibitors formation against multiple epitopes of FVIII. Indeed, those ANS8 CAR-Tregs remain specific, since their activation and suppression activity occur in the local milieu where the anti-FVIII immune response is ongoing [51]. A similar CAR approach was adopted by Fu et al when they transduced murine CD4+ T cells with a lentiviral vector encoding a high-affinity anti-FVIII ScFv linked to the CAR signaling domain, fused with Foxp3 cDNA named F8CAR-Foxp3-LV. Those cells significantly suppressed the antibody response to FVIII [58].
BAR-Tregs in Hemophilia A
FVIII-specific B cells express immunoglobulin receptors that recognize B-cell epitopes on the FVIII surface; thus, they are required for high-titer inhibitor responses. With the goal of targeting FVIII-specific B cells directly, we designed a new receptor called BAR (B-cell-targeting Antibody Receptor) [52, 53]. The extracellular component of BAR is comprised of antigen domains (either FVIII A2 or C2 domains) linked to transmembrane and signal domains CD28-CD3ζ. Thus, A2-BAR or C2-BAR expressing Tregs could be activated by antibodies recognizing FVIII or by interactions with FVIII-specific B cells. Thus, BAR immunosuppression could occur through interactions with FVIII A2 or C2-specific B cells, respectively (Figure 2C). In vivo, FVIII-A2 BAR and FVIII-C2 BAR human Tregs effectively and specifically prevented the antibody response to FVIII immunizations in hemophilic mice for as long as eight weeks [53]. The mechanism of BAR Treg suppression remains to be further investigated, but preliminary in vitro analysis of B and T cells from “tolerant” mice recipients suggests that FVIII-specific B cells may be directly suppressed, whereas the helper T cells were not (Yoon et al., unpublished).
Approaches Using Cytotoxic T Cells in Hemophilia A
Cytotoxic T lymphocytes (CTLs) are equipped to kill virus-infected or mutated tumor cells with remarkable specificity. Upon TCR recognition, CTLs form an immune synapse with their target cell, and deliver cytolytic granules containing perforin and granzyme B to the synapse through marked reorganization of both the actin and the microtubule cytoskeletons. Alternatively, FasL expression on CTLs enables Fas mediated cell death. Theoretically, one can express a single chain antibody specific for a FVIII domain (e.g. FVIII-A2) to create specific CAR- expressing CTLs. These should be able to kill FVIII-specific B cells when loaded with FVIII protein, which would then be recognized by the FVIII-specific B cell receptor. Currently, these types of CAR-CTLs are being developed in our lab. This indirect approach requires that CAR-CTLs, antigen and specific B cell or antibody secreting cell be in the same local environment. These CTLs are FVIII–specific, and further studies are required to understand their off-site activation potential in the presence of circulating FVIII. As FVIII is bound to von Willebrand factor in the circulation, some FVIII B-cell epitopes may not be recognized as efficiently by CAR-CTLs. This possibility is, however, testable.
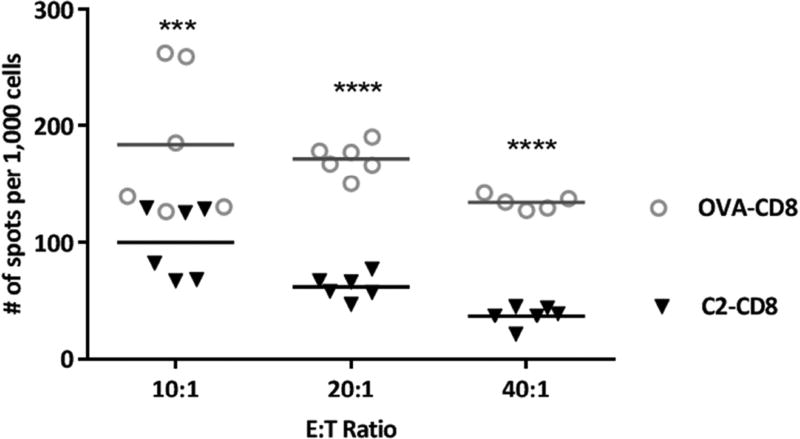
For a more direct approach to target and kill FVIII-specific B cells, we reasoned that expression of FVIII domains on a CTL cell would enable them to be recognized by surface IgM+ve FVIII-specific B cells through the BCR. These specific B cells would then be successfully eliminated in an MHC-independent manner. We refer to these cells as BAR-CD8+. To test their potential efficacy, FVIII-C2 or A2 BAR-expressing CTLs derived from healthy donor CD8+ T cells were mixed with a FVIII C2 domain specific hybridoma BO2C11 or 3G6). These FVIII-C2 BAR CD8+ were effective in killing all the hybridoma cells (Figure 4). Hybridoma cell functionality and death rates were measured by an ELISPOT assay to detect antibody [52]. We further demonstrated that BAR CD8+ expressing the FVIII A2 domain could kill A2-specific hybridomas (413) (Parvathaneni et al. in preparation). These results were obtained with both human and murine CD8+ cells transduced with FVIII A2 or C2 domains and with murine or human target cells. Similar studies have been published in an autoimmune pemphigus model, where Ellebrecht et al. has demonstrated the ability of engineered CD8+ T cells expressing domains of desmoglein 3 (Dsg3), to eliminate anti-Dsg3 BCR expressing hybridomas in vitro and in vivo [59]. Further experiments to test the efficiency of FVIII BAR-CTLs in eliminating an ongoing humoral response to FVIII immunized mice are under way. Based on our data and that of Ellebrecht et al., it should be relatively straightforward to adapt the BAR-CD8+ T cell approach to kill/suppress antigen-specific B cells involved in other adverse immune responses, in addition to anti-FVIII inhibitor formation in hemophilia A.
Figure 4. FVIII ELISPOT to measure antibody secretion from surviving BO2C11 cells.
FVIII-C2 BAR cytotoxic T cells were co-cultured with BO2C11 cells for 5hrs at various E:T ratios and the cells were plated on FVIII (2 µg/mL) coated ELISPOT plates overnight. The number of spots per million cells was calculated by imaging using a CTL plate reader™. Statistical analysis based on 2 way Anova. (*** 0.0001; **** <0.0001)
From the bench to the clinic
In summary, to prevent and reverse inhibitor formation in hemophilia A, our lab has developed a series of strategies using engineered T cells. Human Tregs transduced with a FVIII-specific TCR (17195), an scFv CAR (ANS8), or A2/C2 BARs were shown to maintain Treg phenotypic markers after long-term (> 3–6 weeks) in vitro expansion, and they were demonstrated to be effective both in vitro and in vivo. These data verify the basic principles of these approaches and support translation to clinical trials. These antigen-specific Tregs are highly efficient in activation and suppression compared to polyclonal Tregs, which should considerably reduce the number of transfused Tregs required for immunotherapies, and their specificity should also help to prevent potential global immune suppression. The use of these antigen-specific T-cell populations is highly recommended for the generation of novel therapeutic strategies as the potential safety profiles of such therapeutics appear, at this point, to be promising (figure 5). In addition, the TCR-, CAR-, or BAR-Tregs can be further engineered to include components such as suicide genes, additional signaling motifs for enhanced function, or advanced designs such as ON/OFF switches. The CD19 CARs have shown potential to establish a long-term memory responses and have been shown to be successful in preventing relapses [60]. We have focused so far on engineered human Tregs, which we know will be rejected in mouse recipients. In order to establish the survival and long-term in vivo effect of these engineered Treg cells, we are now engineering mouse Treg cells to be tested in syngeneic mouse models. The potential of mouse CTLs to provide long-term survival in vivo is currently being investigated. Ultimately, if animal models continue to generate results with translational potential, the therapeutic potential of these engineered T cells, whether Tregs or CTLs, must be tested in human clinical trials.
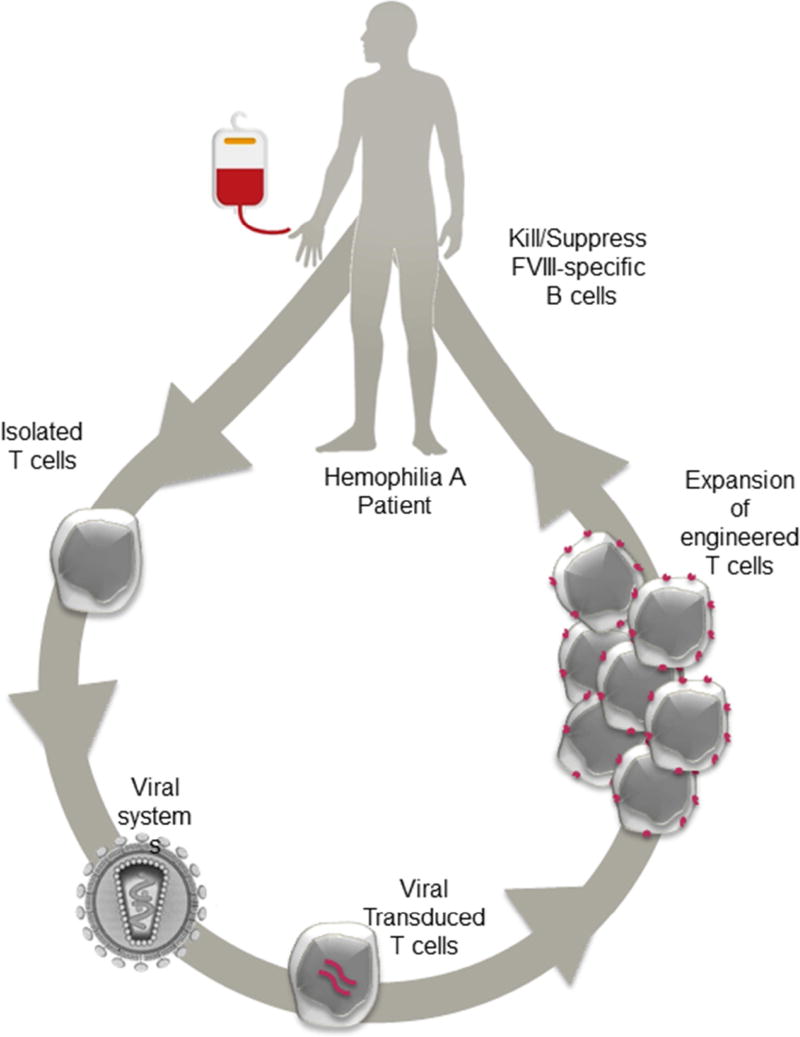
Figure 5. Schematic flow chart of potential T cell therapy for hemophilia A inhibitor patients.
Lymphocytes are collected from patients by leukopheresis and T lymphocytes (Tregs or CD8+ T cells) are enriched. T lymphocytes are transduced to express the chimeric receptors and then undergo ex vivo rapid expansion. Prior to infusion, patients may undergo plasmapheresis to remove circulating high-titer inhibitors. Lastly, the patients are infused with the engineered T cell product.
Acknowledgments
We would like to thank all current and past members of the Scott lab for their contributions to this review, namely DRs Yongchan Kim, Jeongheon Yoon, Aihong Zhang, Patrick Adair and Anja Scmidt for their research contributions. We also appreciate Drs Kim, Yoon, Zhang and Adair for critical reading of this review. The work in the Scott lab has been supported by NIH grants RO1 HL126727 and R21 HL127495, as well as Pfizer ASPIRE grant.
All authors have read the journal’s authorship agreement and the manuscript has been reviewed and approved by all authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no conflicts of interest.
References
- 1.Adair P, Su Y, Scott DW. Tolerance induction in hemophilia A animal models: battling inhibitors with antigen-specific immunotherapies. Discov Med. 2013;15(84):275–82. [PubMed] [Google Scholar]
- 2.Scott DW, Pratt KP, Miao CH. Progress toward inducing immunologic tolerance to factor VIII. Blood. 2013;121(22):4449–56. doi: 10.1182/blood-2013-01-478669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. [cited 2017 January 11];World Federation of Hemophilia. Available from: https://www.wfh.org/
- 4.Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515–23. doi: 10.4103/0019-5049.144643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fay PJ. Factor VIII structure and function. Int J Hematol. 2006;83(2):103–8. doi: 10.1532/IJH97.05113. [DOI] [PubMed] [Google Scholar]
- 6.Osooli M, Berntorp E. Inhibitors in haemophilia: what have we learned from registries? A systematic review. J Intern Med. 2015;277(1):1–15. doi: 10.1111/joim.12301. [DOI] [PubMed] [Google Scholar]
- 7.Gouw SC, et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. 2012;119(12):2922–34. doi: 10.1182/blood-2011-09-379453. [DOI] [PubMed] [Google Scholar]
- 8.d'Oiron R, et al. Deletion of alanine 2201 in the FVIII C2 domain results in mild hemophilia A by impairing FVIII binding to VWF and phospholipids and destroys a major FVIII antigenic determinant involved in inhibitor development. Blood. 2004;103(1):155–7. doi: 10.1182/blood-2003-04-1321. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger RA, et al. HLA-DR-restricted T-cell responses to factor VIII epitopes in a mild haemophilia A family with missense substitution A2201P. Haemophilia. 2010;16(102):44–55. doi: 10.1111/j.1365-2516.2008.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James EA, et al. T-cell responses over time in a mild hemophilia A inhibitor subject: epitope identification and transient immunogenicity of the corresponding self-peptide. J Thromb Haemost. 2007;5(12):2399–407. doi: 10.1111/j.1538-7836.2007.02762.x. [DOI] [PubMed] [Google Scholar]
- 11.Scott DW. Why do immunology research in hemophilia? Cell Immunol. 2016;301:1. doi: 10.1016/j.cellimm.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Spiegel PC, Jr, et al. Structure of a factor VIII C2 domain-immunoglobulin G4kappa Fab complex: identification of an inhibitory antibody epitope on the surface of factor VIII. Blood. 2001;98(1):13–9. doi: 10.1182/blood.v98.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Kasper CK, Pool JG. Letter: Measurement of mild factor VIII inhibitors in Bethesda units. Thromb Diath Haemorrh. 1975;34(3):875–6. [PubMed] [Google Scholar]
- 14.Guelcher CJ. Evolution of the Treatments for Hemophilia. J Infus Nurs. 2016;39(4):218–24. doi: 10.1097/NAN.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 15.Meeks SL, Batsuli G. Hemophilia and inhibitors: current treatment options and potential new therapeutic approaches. Hematology Am Soc Hematol Educ Program. 2016;2016(1):657–662. doi: 10.1182/asheducation-2016.1.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caspi R. Autoimmunity in the immune privileged eye: pathogenic and regulatory T cells. Immunol Res. 2008;42(1–3):41–50. doi: 10.1007/s12026-008-8031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter DL, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochenderfer JN, et al. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116(19):3875–86. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86(24):10024–8. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross G, et al. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc. 1989;21(1 Pt 1):127–30. [PubMed] [Google Scholar]
- 21.Eshhar Z. Tumor-specific T-bodies: towards clinical application. Cancer Immunol Immunother. 1997;45(3–4):131–6. doi: 10.1007/s002620050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshhar Z. The T-body approach: redirecting T cells with antibody specificity. Handb Exp Pharmacol. 2008;(181):329–42. doi: 10.1007/978-3-540-73259-4_14. [DOI] [PubMed] [Google Scholar]
- 23.Finney HM, et al. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998;161(6):2791–7. [PubMed] [Google Scholar]
- 24.Imai C, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 25.Long AH, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–90. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter DL, et al. Randomized, Phase II Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) in Patients with Relapsed, Refractory CLL. Blood. 2014;124(21):1982–1982. [Google Scholar]
- 27.Scholler J, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4(132):132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochenderfer JN, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster SJ, et al. Sustained Remissions Following Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) in Patients with Relapsed or Refractory CD19+ Lymphomas. Blood. 2015;126(23):183–183. [Google Scholar]
- 32.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566–81. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arellano B, Graber DJ, Sentman CL. Regulatory T cell-based therapies for autoimmunity. Discov Med. 2016;22(119):73–80. [PMC free article] [PubMed] [Google Scholar]
- 34.Hori S, et al. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci U S A. 2002;99(12):8213–8. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masteller EL, et al. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol. 2005;175(5):3053–9. doi: 10.4049/jimmunol.175.5.3053. [DOI] [PubMed] [Google Scholar]
- 36.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7(8):585–98. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 37.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73(23):6900–12. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacDonald KG, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126(4):1413–24. doi: 10.1172/JCI82771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkar D, et al. Ex Vivo Expanded Autologous Polyclonal Regulatory T Cells Suppress Inhibitor Formation in Hemophilia. Mol Ther Methods Clin Dev. 2014;1 doi: 10.1038/mtm.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hull CM, et al. Generation of human islet-specific regulatory T cells by TCR gene transfer. J Autoimmun. 2017 doi: 10.1016/j.jaut.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Boardman DA, et al. Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am J Transplant. 2016 doi: 10.1111/ajt.14185. [DOI] [PubMed] [Google Scholar]
- 43.Braza F, et al. Regulatory T Cells in Kidney Transplantation: New Directions? Am J Transplant. 2015;15(9):2288–300. doi: 10.1111/ajt.13395. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira MC, et al. Involvement of regulatory T cells in the immunosuppression characteristic of patients with paracoccidioidomycosis. Infect Immun. 2010;78(10):4392–401. doi: 10.1128/IAI.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belmonte N, Gertner-dardenne J, Foussat A. Regulatory T Cell Engineered with Chimeric Antigen Receptor (CAR-Treg) for Inflammatory and Autoimmune Diseases. Cytotherapy. 2016;18(6):S95. [Google Scholar]
- 46.Aledort LM, et al. HIV and hemophilia. J Thromb Haemost. 2007;5(3):607–10. doi: 10.1111/j.1538-7836.2007.02371.x. [DOI] [PubMed] [Google Scholar]
- 47.Bray GL, et al. Loss of high-responder inhibitors in patients with severe hemophilia A and human immunodeficiency virus type 1 infection: a report from the Multi-Center Hemophilia Cohort Study. Am J Hematol. 1993;42(4):375–9. doi: 10.1002/ajh.2830420408. [DOI] [PubMed] [Google Scholar]
- 48.Qian J, et al. Prevention and treatment of factor VIII inhibitors in murine hemophilia A. Blood. 2000;95(4):1324–9. [PubMed] [Google Scholar]
- 49.Qian J, et al. Role of CD154 in the secondary immune response: the reduction of pre-existing splenic germinal centers and anti-factor VIII inhibitor titer. Eur J Immunol. 2000;30(9):2548–54. doi: 10.1002/1521-4141(200009)30:9<2548::AID-IMMU2548>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 50.Kim YC, et al. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood. 2015;125(7):1107–15. doi: 10.1182/blood-2014-04-566786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon J, et al. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood. 2017;129(2):238–245. doi: 10.1182/blood-2016-07-727834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parvathaneni K, et al. BAR-CD8 T-Cell Mediated Targeted Killing of Inhibitor Producing FVIII-Specific B Cells. Blood. 2015;126(23):294–294. [Google Scholar]
- 53.Zhang A-H, et al. Targeting antigen-specific B cells using BAR-transduced cytotoxic and regulatory T cells. The Journal of Immunology. 2016;196(1 Supplement):70.7–70.7. [Google Scholar]
- 54.Ettinger RA, et al. Lineages of human T-cell clones, including T helper 17/T helper 1 cells, isolated at different stages of anti-factor VIII immune responses. Blood. 2009;114(7):1423–8. doi: 10.1182/blood-2009-01-200725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theaker SM, et al. T-cell libraries allow simple parallel generation of multiple peptide-specific human T-cell clones. J Immunol Methods. 2016;430:43–50. doi: 10.1016/j.jim.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kahle J, et al. Epitope mapping via selection of anti-FVIII antibody-specific phage-presented peptide ligands that mimic the antibody binding sites. Thromb Haemost. 2015;113(2):396–405. doi: 10.1160/TH14-01-0101. [DOI] [PubMed] [Google Scholar]
- 57.Naumann A, et al. Selection and characterisation of FVIII-specific single chain variable fragments. Hamostaseologie. 2013;33(Suppl 1):S39–45. [PubMed] [Google Scholar]
- 58.Fu R, et al. Factor VIII-specific CAR regulatory T cells modulate murine anti-factor VIII immune responses. The Journal of Immunology. 2016;196(1 Supplement):126.13–126.13. [Google Scholar]
- 59.Ellebrecht CT, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353(6295):179–84. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. [cited 2016 December 8];2016 Ongoing Clinical Trials in United States. NCT02937103 (CD123); NCT02935153 (CD22); NCT02742727(CD7); NCT02954445 (BCMA); NCT02958410 (CD30); NCT02958397 (CD33); NCT01886976 (CD138); NCT02958384 (LeY); NCT02919046 (GD2); NCT02311621(CD171); NCT02844062 (EGFRvIII); NCT02575261(EphA2); NCT02713984 (HER2); NCT02744287 (PSCA); NCT02349724(CEA); NCT02541370 (CD133); NCT02617134 (Muc1); NCT02876978 (GPC3); NCT02862704 (MG7); NCT02915445 (EpCAM); NCT02706392 (ROR1); NCT01583686 (Mesothelin); NCT01722149 (FAP); NCT02830724 (CD70); NCT01140373 (PSMA)]. Available from: www.clinicaltrials.gov.