Abstract
Study Design
A population-based retrospective cohort study.
Objective
The aim of this study was to examine risk factors for long-term opioid use following lumbar spinal fusion surgery in a nationally representative cohort of commercially insured adults.
Summary of Background Data
Opioid prescription rates for the management of low back pain have more than doubled in the US over the past decade. Although opioids are commonly used for the management of pain following lumbar spinal fusion surgery, to date, no large-scale nationally representative studies have examined the risk factors for long-term opioid use following such surgical intervention.
Methods
Using one of the nation’s largest commercial insurance databases, we conducted a retrospective cohort study of 8,377 adults, aged 21–63 years, who underwent lumbar spinal fusion surgery between January 1, 2009 and December 31, 2012. Long-term opioid use was defined as ≥365 days of filled opioid prescriptions in the 24 months following lumbar fusion. Multivariable logistic regression was used to calculate adjusted odds ratios (ORs) and 95% confidence intervals for the risk of long term opioid use following lumbar fusion.
Results
After adjusting for covariates, the following factors were associated with an increased risk of long term opioid use following surgery: duration of opioid use in the year before lumbar surgery [Referent (0 days); Quartile 1 (1–22 days) OR=2.27, 95% CI=1.48–3.49; Quartile 2(23–72 days): OR=5.94, 95% CI=4.00–8.83; Quartile 3: (73–250 days) OR=25.31, 95% CI=17.26–37.10; Quartile 4 (≥ 250days) OR=219.95, 95% CI=148.53–325.71 )], re-fusion surgery (OR=1.32, 95% CI=1.02–1.72), and diagnosis of depression (OR=1.43, 95% CI=1.18–1.74). Receipt of anterior fusion was associated with a modest decrease in the risk of long-term opioid use (OR=0.79, 95% CI=0.63–0.99).
Conclusions
These findings may provide clinically relevant information to physicians, patients, and their families regarding the risk factors for opioid dependence following lumbar fusion surgery.
Keywords: opioids, lumbar fusion, minimally invasive fusion, low back pain, comorbidity, depression, smoking
Opioid prescription rates for the management of low back pain have more than doubled in the US over the past decade.1 Opioids are frequently prescribed in patients undergoing lumbar spinal fusion surgery,2,3,4,5,6 which is used to treat a broad range of conditions associated with low back pain, including degenerative disk disease, disc herniation, spinal stenosis, spondylolisthesis, spondylolysis, scoliosis, and tumor.7,8,9 Over the past ten years, opioid expenditures for spine-related pain increased by 660%,1,10 and hospital discharge rates associated with spinal fusion surgeries increased by 137%, with the greatest increase attributable to lumbar and cervical fusion surgeries.9 In view of these numbers, there is widespread concern about long-term opioid dependence among patients who undergo spinal fusion surgery.3,11,12 Moreover, the efficacy of long-term opioid use for low back pain following spinal fusion surgery is widely debated.2,11,13
A range of studies have examined the risk of long-term opioid use following spine surgery. Collectively, these investigations have shown that a number of factors may adversely affect post-surgical pain and opioid use. 2,11, 14–17 For example, long-term opioid use before spine surgery has been linked with hyperalgesia, opioid tolerance, and opioid use following surgery.2,11,18 Likewise, type of spine surgery is reported to be associated with a range of post-surgical pain outcomes and opioid dependence.2,14,15,16,18,19 In addition, behavioral risk factors such as diagnosis of depression and smoking have both been associated with high self-rated pain scores and higher risk of long term opioid use after spine surgery.2,11,20,21,22 To date, however, risk factors for long term opioid use after lumbar spinal fusion surgery have not been examined in a large-scale nationally representative study. Ours is the first study to focus on this important healthcare issue using one of the nation’s largest commercial insurance databases.
MATERIALS AND METHODS
Data source
This study used de-identified administrative health data from Clinformatics Data Mart ™ (CDM, Optum Insight, Eden Prairie, MN), a database of one of the nation’s largest commercial health insurance programs. CDM has been used to examine pharmacotherapy and health services in previous studies.23,24,25,26 Individuals are enrolled in this insurance program under either health maintenance organizations, preferred provider organizations, point of services, or exclusive provider organizations. For each of these plans, providers are required to submit complete claims to receive reimbursement. We used a combination of outpatient, inpatient, and pharmacy claims data. The pharmacy database contains eligibility and claims information for medications from retail pharmacies through a member’s pharmacy benefit. For each medication, the database contains medication name, date of fill, formulation (e.g., oral, transdermal, injectable), dose, quantity, and days of supply. This study was approved by the institutional review board of the University of Texas Medical Branch at Galveston.
Study design
We conducted a retrospective cohort study of 8,377 patients who underwent lumbar fusion between January 1, 2009 and December 31, 2012 for degenerative spine conditions, post-laminectomy or as a re-fusion procedure (Table 1). In order to be included in the study, participants were required to: be between 21 and 63 years of age at the time of surgery, be enrolled in commercial insurance plan through the duration of the study and have complete claims data for 12 months preceding and 24 months following the surgery. Because patients ≥ 65 years of age— in this commercial insurance database— are not representative of the general ≥ 65 year US population (the overwhelming majority of whom receive their health insurance through Medicare). We therefore restricted our analyses to persons ≥ 65 years of age. Because inclusion in the study required a two-year look-back period for the date of surgery, we set the exclusion criterion for this ≥ 65 cohort at 63 years of age. Persons with any of the following diagnoses before surgery were excluded from the study: neoplasm (ICD-9=140–239), fracture (ICD-9=733.1, 733.10, 733.13, 733.95, 733.8, 733.81–733.82, 805–806.9, 839–839.59), infection (ICD-9=324.1, 730–730.99) or inflammation (ICD-9=720.0–720.9) involving the spine; history of a major traumatic accident (ICD-9= E800–E849.9) within 12 months prior to surgery or diagnosis of pregnancy (ICD-9=630–676).
Table 1.
Descriptive Statistics of Variables by Fusion Type
| Overall | Posterior (n=5450) | Anterior (n=791) | 360 Fusion (n=1230) | OPMI Fusion (n=906) | p | |
|---|---|---|---|---|---|---|
| Demographic Characteristics | N (%) | N (%) | N (%) | N (%) | N (%) | |
| 20–29 | 227 (2.71) | 141 (2.59%) | 25 (3.16%) | 35 (2.85%) | 26 (2.87%) | |
| 30–39 | 910 (10.86) | 500 (9.17%) | 155 (19.6%) | 158 (12.85%) | 97 (10.71%) | |
| 40–49 | 2173 (25.94) | 1284 (23.56%) | 303 (38.31%) | 347 (28.21%) | 239 (26.38%) | |
| 50–63 | 5067 (60.49) | 3525 (64.68%) | 308 (38.94%) | 690 (56.1%) | 544 (60.04%) | |
| Gender | 0.47 | |||||
| Male | 3675(43.87) | 2410 (44.22%) | 343 (43.36%) | 516 (41.95%) | 406 (44.81%) | |
| Female | 4702 (56.13) | 3040 (55.78%) | 448 (56.64%) | 714 (58.05%) | 500 (55.19%) | |
| Region | 0.003 | |||||
| Midwest | 2306 (27.54) | 1530 (28.08) | 245 (30.97) | 332 (27.06) | 199 (21.96) | |
| Northeast | 484 (5.78) | 299 (5.49) | 41 (5.18) | 84 (6.85) | 60 (6.62) | |
| South | 4410 (52.68) | 2870 (52.68) | 389 (49.18) | 633 (51.59) | 518 (57.17) | |
| West | 1172 (14.00) | 749 (13.75) | 116 (14.66) | 178 (14.51) | 129 (14.24) | |
| Indication | .0001 | |||||
| Degenerative | 7141 (85.25) | 4722 (86.64%) | 629 (79.52%) | 1054 (85.69%) | 736 (81.24%) | |
| Post-Laminectomy | 391 (4.67) | 239 (4.39%) | 41 (5.18%) | 79 (6.42%) | 32 (3.53%) | |
| Re-Fusion | 489 (5.84) | 326 (5.98%) | 32 (4.05%) | 74 (6.02%) | 57 (6.29%) | |
| Other | 356 (4.25) | 163 (2.99%) | 89 (11.25%) | 23 (1.87%) | 81 (8.94%) | |
| Comorbidity | ||||||
| Elixhauser Score | .0001 | |||||
| 0 | 4229 (50.48) | 2655 (48.72) | 467 (59.04) | 625 (50.81) | 482 (53.20) | |
| 1 | 2328 (27.79) | 1549 (28.42) | 199 (25.16) | 347 (28.21) | 233 (25.72) | |
| 2+ | 1820 (21.73) | 1246 (22.86) | 125 (15.80) | 258 (20.98 | 191 (21.08) | |
| Depression | 925 (11.04) | 575 (10.55%) | 109 (13.78%) | 151 (12.28%) | 90 (9.93%) | 0.02 |
| Obesity | 341 (4.07) | 222 (4.07%) | 24 (3.03%) | 50 (4.07%) | 45 (4.97%) | 0.26 |
| Smoking | 390 (4.66) | 251 (4.61%) | 39 (4.93%) | 56 (4.55%) | 44 (4.86%) | 0.96 |
| OPR Pre-Fusion | 0.15 | |||||
| Q1: 0 | 1332 (15.90) | 896 (16.44%) | 112 (14.16) | 189 (15.37) | 135 (14.90) | |
| Q2: 1–22 days | 1765 (21.07) | 1172 (21.50) | 146 (18.46) | 248 (20.16) | 199 (21.96) | |
| Q3: 23–72 days | 1771 (21.14) | 1152 (21.14) | 174 (22.00) | 267 (21.71) | 178 (19.65) | |
| Q4: 73–250 days | 1749 (20.88) | 1136 (20.84) | 178 (22.50) | 243 (19.76) | 192 (21.19) | |
| Q5: 250+ | 1760 (21.01 | 1094 (20.07) | 181 (22.88) | 283 (23.01) | 202 (22.30) | |
| OPR Post-Fusion | ||||||
| Dispensed >=365 days | 2458 (29.34) | 1557 (28.57%) | 235 (29.71%) | 403 (32.76%) | 263 (29.03%) | 0.035 |
Participants were categorized into one of four groups based on surgical approach to lumbar fusion: posterior (ICD-9-CM=81.05–81.08; CPT=22612, 22614, 22630, 22632, 22840, 22842), anterior (ICD-9-CM=81.04, 81.06; CPT= 22558, 22585), 360° fusion (any anterior or posterior code) and outpatient minimally invasive (OPMI) fusion (identified by an outpatient location for the procedure). For this investigation, we included only persons with complete enrollment data and complete diagnostic and procedure codes. No member of the final study had missing values on any of the study variables. Therefore, no members of the study cohort were excluded from any of the analyses.
Variables
The outcome variable, long term opioid use after lumbar fusion, was defined as ≥365 days of opioid prescriptions dispensed in the two years following surgery, which was measured using pharmacy claims data. Opioids belonged to schedules II, III, IV or IV. (Appendix Table 1: Drug Enforcement Administration (DEA) and therapeutic class codes27).
The independent variables included gender, age, geographic region, type of lumbar fusion (posterior, anterior, 360 or OPMI), indication for fusion (degenerative, post-laminectomy or repeat fusion); comorbidities, Elixhauser comorbidty Index28 excluding depression, obesity and smoking), depression (ICD-9-CM=296.2, 296.3, 300.4, 300.5, 309.0, 309.1 309.28, 309.82, 309.83, 309.89, 311), obesity (ICD-9-CM=278.00, 278.01, 278.02), smoking (305.1) and the total number of days of opioids were dispensed within 365 days prior to the date of surgery were examined as independent variables. All comorbidities were assessed using a 365 day look-back period from the date of surgery. Opioid use was assessed using pharmacy claims data, while information regarding all other predictor variables was obtained from physician and facility claims data. Opioid use before lumbar fusion use was grouped into quartiles among those with any opioid use (quartile 1=1–22 days; quartile 2=23–72 days; quartile 3=73–250 days; quartile 4>250 days), with 0 days of use serving as the referent.
We used a 2-year window to measure opioid use after surgery. Although this interval is substantially longer than some reported definitions of long term opioid use,29,30 several studies have reported that a substantial percentage of patients use opioids consistently for up to 2 years following lumbar surgery.2,31,32–34 We examined long-term opioid use as both a binary (≥365 days) and a continuous outcome. Finally, we examined demographic variables including age and gender.
Statistical analysis
Bivariate analyses including chi-square and one-way analyses of variance (ANOVA) were used to examine differences in the study variables across four fusion types (Table 1). A plot was generated to assess cumulative months of opioid use across the 2-year follow-up period. Multivariable logistic regression models— simultaneously adjusting for all of the aforementioned clinical and demographic covariates— were used to assess the adjusted risk of long term opioid use associated with each of the study variables. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
A total of 8,377 patients who underwent lumbar fusion surgery were included in the study. The distribution of each of the covariates according to fusion type is presented in Table 1. The mean age of the study population was 49.6 years (SD=8.8). Of the 8,377 participants, 56.13% were females and 86.43% were above 40 years of age. Participants undergoing anterior fusion were younger (mean age=46.8 years), compared to participants undergoing other fusion types. Posterior fusion (65.06%) was the most common surgical approach and degenerative disease (85.25%) was the most common indication for lumbar fusion, regardless of fusion type. Participants who did not have any of the three major indications for lumbar surgery were categorized as “other” (4.25%). Posterior fusion recipients had the highest proportion of ≥2 comorbid conditions (22.86%). Prevalence of depression in the sample was 11.04% overall. Mean duration of opioid use before lumbar fusion was 173 days (SD=204); 15.9% participants did not use opioids before lumbar fusion. Opioid use before fusion did not vary significantly by fusion type. In the 2 year post fusion follow-up period, opioids were dispensed for a mean duration of 385 days (SD=443). Participants undergoing 360° fusion were more likely to experience long term opioid use after surgery (32.76%), compared to other fusion types. Overall, 29.34% participants received long term opioids after surgery.
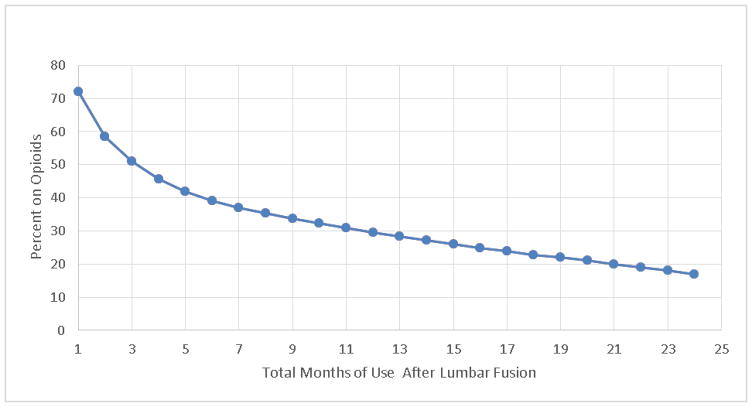
Figure 1 presents the percentage of patients’ cumulative duration of post-surgery opioid use (1 through 24 months). Approximately 50% of lumbar surgery patients used opioids for a total of 3 months after surgery; approximately, 40 % used opioids for 6 months; 30% 12 months; and 17% 24 months.
Figure 1.
Cumulative opioid use after surgery
Results from the multivariable logistic regression are presented in Table 2. After adjusting for covariates, opioid use before lumbar fusion was associated with an increased risk of long term opioid use after lumbar fusion. Compared to the referent (0 days), the odds of long term opioid use increased in a monotonic fashion with quartile of pre-surgery opioid use in the year before lumbar surgery [Quartile 1 (1–22 days) OR=2.27, 95% CI=1.48–3.49; Quartile 2(23–72 days): OR=5.94, 95% CI=4.00–8.83; Quartile 3: (73–250 days) OR=25.31, 95% CI=17.26–37.10; Quartile 4 (≥ 250days) OR=219.95, 95% CI=148.53–325.71 )], indication for re-fusion (OR=1.32, 95% CI=1.02–1.72), and diagnosis of depression (OR=1.43, 95% CI=1.18–1.74). Receipt of anterior fusion was associated with a modest decrease in the risk of long-term opioid use (OR=0.79, 95% CI=0.63–0.99). Neither Elixhauser comorbidity score nor geographic region were associated with an increased risk of long-term opioid use following surgery.
Table 2.
Odds Ratio Estimates for Risk of Being Dispensed Opioid Pain Relievers for 365 days or more in 2-year, Post-Fusion Follow-Up Period
| Variable | % Dispensed > 365 Days OPR | OR Estimate | 95% CI | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (n=2459) | 49.95 (8.92) | 0.99 | 0.98–0.99 | 0.0001 |
| Male | 1025 (27.89%) | 1 | Reference | |
| Female | 1433 (30.48%) | 1.02 | 0.89–1.16 | 0.77 |
| Region | ||||
| South | 1344 (30.48%) | 1 | Reference | |
| Midwest | 613 (26.58%) | 0.88 | 0.75–1.03 | 0.10 |
| Northeast | 145 (29.96%) | 1.04 | 0.79–1.38 | 0.77 |
| West | 354 (30.20%) | 0.98 | 0.80–1.19 | 0.82 |
| Fusion Type | ||||
| Posterior Fusion | 1557 (28.57%) | 1 | Reference | |
| Anterior Fusion | 235 (29.71%) | 0.79 | 0.63–0.99 | 0.04 |
| 360 Fusion | 403 (32.76%) | 1.18 | 0.98–1.41 | 0.09 |
| OPMI Fusion | 263 (29.03%) | 0.87 | 0.70–1.08 | 0.20 |
| Indication | ||||
| Degenerative | 2030 (28.43%) | 1 | Reference | |
| Post-Laminectomy | 153 (39.13%) | 1.14 | 0.85–1.52 | 0.38 |
| Re-Fusion | 189 (38.65%) | 1.32 | 1.02–1.72 | 0.04 |
| Other | 86 (24.16%) | 1.25 | 0.89–1.75 | 0.21 |
| Comorbidity | ||||
| Elixhauser Score | ||||
| 0 | 1036 (24.50%) | 1 | Reference | |
| 1 | 723 (31.06%) | 1.12 | 0.96–1.31 | 0.17 |
| 2+ | 699 (38.41%) | 1.15 | 0.97–1.37 | 0.11 |
| Depression | 458 (49.51%) | 1.43 | 1.18–1.74 | <0.001 |
| Obesity | 130 (38.12%) | 0.91 | 0.67–1.23 | 0.53 |
| Smoking | 189 (48.46%) | 1.12 | 0.91–1.58 | 0.21 |
| OPR Use Pre-Fusion | ||||
| Quintile 1: 0 | 29 (2.18%) | 1 | Reference | |
| Quintile 2: 1–22 days | 86 (4.87%) | 2.274 | 1.48–3.49 | <0.001 |
| Quintile 3: 23–72 days | 213 (12.03%) | 5.939 | 4.00–8.83 | <0.001 |
| Quintile 4: 73–250 days | 651 (37.22%) | 25.305 | 17.26–37.10 | <0.001 |
| Quintile 5: 250+ | 1479 (84.03%) | 219.946 | 148.53–325.71 | <0.001 |
DISCUSSION
In this nationally representative cohort study of 8,377 adults who underwent lumbar spinal surgery, opioid use before surgery—assessed both as a categorical and continuous variable—was the strongest predictor of long term opioid use following surgery. In addition, diagnosis of depression and having re-fusion surgery were associated with an increased risk of long term opioid use whereas anterior fusion was protective against long-term opioid use.
In general, most of our findings were consistent with previous studies that investigated risk factors for long term opioid use after spine surgery. For example, in a retrospective cohort study of 1002 workers’ compensation subjects, Anderson et al reported that long-term opioid use before lumbar fusion was associated with a six-fold higher risk of long-term opioid after lumbar fusion.2 Likewise, in a prospective cohort study of 583 patients, opioid use before spine surgery was associated with increased incidence of opioid dependence at 12 months after spine surgery.11 Another prospective cohort study found a positive association between opioid use before spine surgery and increased surgical site pain after surgery.19 Our findings are also consistent with research showing pre-surgical opioid use as a predictor of various adverse post-surgical outcomes in spine surgery recipients such as poor self-rated pain, disability and overall health.35 In addition, pre surgery opioid use has been associated with increased hospital length of stay, delay in returning to work, surgical complications and other adverse functional outcomes after surgery.13,4,36,37 It is possible that some patients with substantial pre-surgery opioid use might have developed addiction disorders prior to surgery. Compared to other risk factors, the considerably higher risk of long term opioid use associated with opioid use before surgery highlights the need for caution in prescribing opioids to individuals planning to undergo lumbar surgery to treat back pain symptoms.
Our findings were not consistent with prior studies that identified comorbidity as an important predictor of poor pain outcomes and high risk of long term opioid use following major orthopedic surgery, including spine surgery.21,38,39 In addition, the Elixhauser comorbidity index has been linked with increased risk of readmission, various surgical and post-surgical complications, increased length of stay and mortality following spine or other major surgery.40,41 It is possible that our focus on just one type of spine surgery (i.e., lumbar fusion) and a single outcome (i.e. risk of long-term opioid use) failed to capture potential adverse post-surgical events associated with baseline comorbidity. Our finding that depression was associated with long term opioid use is also consistent with previous research. Prior studies have reported clinical depression as a risk factor for long term opioid use in spine surgery recipients.2,21 Preoperative depression has been associated with poor post-surgical pain outcomes, low treatment satisfaction and fewer improvements in symptom severity, disability and walking ability following lumbar surgery.16,20 Further, clinical depression is reported to be associated with poor return to work status following lumbar fusion surgery.21
Our finding that anterior approach to lumbar fusion was associated with a decreased risk of long-term opioid use is in contrast to a recent cohort study of 10,941 participants that reported poorer post-operative outcomes and increased health care utilization following anterior fusion, compared to posterior fusion.15 However few other studies found no major differences in surgical or functional outcomes between the two approaches or reported slightly better surgical outcomes following anterior fusion.42,43 We did not find a statistically significant association between OPMI fusion and risk of long-term opioid use. Previous studies reported favorable pain outcomes and shorter duration of opioid use following minimally invasive spinal fusion compared to more invasive open fusion 17,44–47 In general, however our finding is corroborated by the results of a few prior studies that found similar long-term clinical and functional outcomes for the two groups.48,49
Our finding of increased risk of long-term opioid use associated with re-fusion surgery is consistent with prior evidence suggesting ongoing post-operative low back pain, and continued narcotic use following repeat lumbar fusion surgery.50
Our finding that smoking was not associated with long term opioid use is consistent with several prior studies that reported little or no difference in adverse outcomes following spine surgery.51–53 Some studies, however, have reported that smoking was associated with an increased risk of perioperative complications, non-healing spinal fusion and higher risk of long term opioid use after spine surgery.22,54,55 It is important to acknowledge, however, that because smoking is substantially under-reported in administrative claims data, our findings are subject to possible misclassification bias. Further research should examine the impact of nicotine and smoking exposure on pain, with particular emphasis on duration of this outcome.
Our findings should be considered in light of several limitations. First, we used insurance claims data wherein diagnoses and type of surgery were based on billing codes that may not always be accurate or complete. Second, although we used a nationally representative sample, we included only commercially insured patients. Third, pharmacy claims data only captures medications prescribed by physicians in the insurance plan, and some patients may obtain opioids illegally.56 Fourth, we required 24 month post-fusion insurance enrollment as inclusion criteria, thereby excluding participants who either changed or lost coverage or died during this period. Fifth, our data provide information on the date the prescription was filled but not on the date it was purchased or picked up by the patient. It is possible, therefore, that some of the drug exposure periods used in this study underestimated the true medication exposure period. It is also possible that some patients who did pick up their prescription did not adhere to the full prescribed regimen.
The Centers for Disease Control and Prevention (CDC) reports one death every 19 minutes from prescription drug overdose; 73% of these deaths result from opioid overdose.57 Opioids are commonly used agents for post -surgical pain management in patients undergoing lumbar fusion surgery.2–6 However, evidence regarding risk factors for long term opioid use following lumbar fusion is inconclusive, particularly the potential variation in risk associated with type of surgery, indication for surgery, duration of prior opioid use, or other clinical and behavioral factors.2,7,11,15,20,22,40 The 2016 CDC guidelines emphasized the role of non-drug alternatives and non-opioid pharmacologic therapy as preferred approaches for chronic pain, in part because little evidence supports effectiveness of long-term opioids in improving pain and function in chronic non-cancer pain.58
The strong association between pre-lumbar fusion opioid quartiles and post lumbar fusion opioid use suggests clinicians should closely monitor the use of opioids in the treatment of back pain. In general, opioid use before lumbar surgery has been associated with poor post-surgical outcomes, including poor pain control, longer length of stay, increased risk of infections, and poor wound healing.4,13,19,33,36,37 While a few prior studies investigated the association between pre-operative and post-operative opioid use in patients undergoing lumbar fusion or spine surgery in general, ours is the first study to assess this association in a nationally representative sample of patients undergoing lumbar fusion surgery. In view of this, our findings are generalizable to commercially insured working adult population in the US. Policies for greater regulation of opioid prescription for back pain and more rigorous screening for risk factors such as substance abuse disorder, prior opioid use, refusion surgery, depression and relevant comorbidities may help improve short term and long term health outcomes in patients undergoing spine surgery.
Acknowledgments
The National Institutes of Health (grant: R01DA039192-01A1) and the Clinical and Translational Science Award (UL1TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health funds were received in support of this work.
Relevant financial activities outside the submitted work: consultancy.
References
- 1.Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. 2015;350:g6380. doi: 10.1136/bmj.g6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J, Haas A, BSBA, et al. Chronic Opioid Therapy After Lumbar Fusion Surgery for Degenerative Disc Disease in a Workers’ Compensation Setting. Spine (Phila Pa 1976) 2015;40(22):1775–1784. doi: 10.1097/BRS.0000000000001054. [DOI] [PubMed] [Google Scholar]
- 3.Waits KD, Walid MS, Robinson JS. Preoperative pain intensity and chronicity and postoperative analgesia markers of length of stay in patients undergoing spinal fusion. Perm J. 2013;17(2):41–43. doi: 10.7812/TPP/12-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen TH, Randolph DC, Talmage J, et al. Long-term Outcomes of Lumbar Fusion Among Workers’ Compensation Subjects: An Historical Cohort Study. Spine (Phila Pa 1976) 2010;36(4):320–331. doi: 10.1097/BRS.0b013e3181ccc220. [DOI] [PubMed] [Google Scholar]
- 5.Bajwa SJS, Haldar R. Pain management following spinal surgeries: An appraisal of the available options. J Craniovertebr Junction Spine. 2015;6(3):105–110. doi: 10.4103/0974-8237.161589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raw DA, Beattie JK, Hunter JM. Anaesthesia for spinal surgery in adults. Br J Anaesth. 2003;91(6):886–904. doi: 10.1093/bja/aeg253. [DOI] [PubMed] [Google Scholar]
- 7.Aebi M. Indication for Lumbar Spinal Fusion. In: Szpalski M, et al., editors. Surgery for Low Back Pain. Springer-Verlag; Berlin Heidelberg: 2010. pp. 109–122. [Google Scholar]
- 8.Deyo RA, Gray DT, Kreuter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine (Phila Pa 1976) 2005;30(12):1441–1445. doi: 10.1097/01.brs.0000166503.37969.8a. discussion 1446–1447. [DOI] [PubMed] [Google Scholar]
- 9.Rajaee SS, Bae HW, Kanim LEA, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37(1):67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 10.Martin BI, Turner JA, Mirza SK, et al. Trends in Health Care Expenditures, Utilization, and Health Status Among US Adults With Spine Problems, 1997–2006. Spine (Phila Pa 1976) 2009;34(19):2077–2084. doi: 10.1097/BRS.0b013e3181b1fad1. [DOI] [PubMed] [Google Scholar]
- 11.Armaghani S, Lee D, Bible J, et al. Preoperative Opioid Use and Its Association With Perioperative Opioid Demand and Postoperative Opioid Independence in Patients Undergoing Spine Surgery. Spine (Phila Pa 1976) 2014;39(25):E1524–E1530. doi: 10.1097/BRS.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 12.Walid MS, Hyer L, Ajjan M, et al. Prevalence of opioid dependence in spine surgery patients and correlation with length of stay. J Opioid Manag. 3(3):127–128. 130–132. doi: 10.5055/jom.2007.0050. [DOI] [PubMed] [Google Scholar]
- 13.Armaghani SJ, Lee DS, Bible JE, et al. Increased Preoperative Narcotic Use and its Association with Postoperative Complications and Length of Hospital Stay in Patients Undergoing Spine Surgery. J spinal Disord Tech. 2014;29(615):93–98. doi: 10.1097/BSD.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 14.Rao PJ, Loganathan A, Yeung V, et al. Outcomes of anterior lumbar interbody fusion surgery based on indication: a prospective study. Neurosurgery. 2015;76(1):7–23. doi: 10.1227/NEU.0000000000000561. discussion 23–24. [DOI] [PubMed] [Google Scholar]
- 15.Huang KT, Hazzard M, Thomas S, et al. Differences in the outcomes of anterior versus posterior interbody fusion surgery of the lumbar spine: a propensity score-controlled cohort analysis of 10,941 patients. J Clin Neurosci. 2015;22(5):848–853. doi: 10.1016/j.jocn.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Adogwa O, Parker SL, Shau DN, et al. Preoperative Zung Depression Scale predicts outcome after revision lumbar surgery for adjacent segment disease, recurrent stenosis, and pseudarthrosis. Spine J. 2012;12(3):179–185. doi: 10.1016/j.spinee.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Adogwa O, Parker SL, Bydon A, et al. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24(8):479–484. doi: 10.1097/BSD.0b013e3182055cac. [DOI] [PubMed] [Google Scholar]
- 18.Krebs EE, Lurie JD, Fanciullo G, et al. Predictors of long-term opioid use among patients with painful lumbar spine conditions. J Pain. 2011;11(1):1–15. doi: 10.1016/j.jpain.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armaghani SJ, Even JL, Zern EK, et al. The Evaluation of Donor Site Pain After Harvest of Tricortical Anterior Iliac Crest Bone Graft for Spinal Surgery: A Prospective Study. Spine (Phila Pa 1976) 2016;41(4):E191–E196. doi: 10.1097/BRS.0000000000001201. [DOI] [PubMed] [Google Scholar]
- 20.Sinikallio S, Aalto T, Airaksinen O, et al. Depression is associated with poorer outcome of lumbar spinal stenosis surgery. Eur Spine J. 2007;16(7):905–912. doi: 10.1007/s00586-007-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JT, Haas AR, Percy R, et al. Clinical depression is a strong predictor of poor lumbar fusion outcomes among workers’ compensation subjects. Spine (Phila Pa 1976) 2015;40(10):748–756. doi: 10.1097/BRS.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 22.Sandén B, Försth P, Michaëlsson K. Smokers show less improvement than nonsmokers two years after surgery for lumbar spinal stenosis: a study of 4555 patients from the Swedish spine register. Spine (Phila Pa 1976) 2011;36(13):1059–1064. doi: 10.1097/BRS.0b013e3181e92b36. [DOI] [PubMed] [Google Scholar]
- 23.Voss EA, Ma Q, Ryan PB, et al. The impact of standardizing the definition of visits on the consistency of multi-database observational health research. BMC Med Res Methodol. 2015;15(1):13. doi: 10.1186/s12874-015-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Defalco FJ, Ryan PB, Soledad Cepeda M. Applying standardized drug terminologies to observational healthcare databases: A case study on opioid exposure. Health Serv Outcomes Res Methodol. 2013;13(1):58–67. doi: 10.1007/s10742-012-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Modi A, Siris ES, Chun-Po SF, et al. Gastrointestinal Events Among Patients Initiating Osteoporosis Therapy: A Retrospective Administrative Claims Database Analysis. Clin Ther. 2015;37(6):1228–1234. doi: 10.1016/j.clinthera.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Oleen-Burkey M, Cyhaniuk A, Swallow E. Retrospective US database analysis of persistence with glatiramer acetate vs. available disease-modifying therapies for multiple sclerosis: 2001–2010. BMC Neurol. 2014;14:11. doi: 10.1186/1471-2377-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Drug Enforcement Administration. [Accessed July 15,2016];DEA/US Department of Justice web site. Available at: https://www.dea.gov/druginfo/ds.shtml.
- 28.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Svendsen K, Skurtveit S, Romundstad P, et al. Differential patterns of opioid use: Defining persistent opioid use in a prescription database. Eur J Pain. 2012;16(3):359–369. doi: 10.1002/j.1532-2149.2011.00018.x. [DOI] [PubMed] [Google Scholar]
- 30.Chou R, Fanciullo GJ, Fine PG, et al. Clinical Guidelines for the Use of Chronic Opioid Therapy in Chronic Noncancer Pain. J Pain. 2014;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carragee EJ, Cheng I. Minimum acceptable outcomes after lumbar spinal fusion. Spine J. 2010;10(4):313–320. doi: 10.1016/j.spinee.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence JTR, London N, Bohlman HH, et al. Preoperative narcotic use as a predictor of clinical outcome: results following anterior cervical arthrodesis. Spine (Phila Pa 1976) 2008;33(19):2074–2078. doi: 10.1097/BRS.0b013e3181809f07. [DOI] [PubMed] [Google Scholar]
- 33.Moore KR, Pinto MR, Butler LM. Degenerative disc disease treated with combined anterior and posterior arthrodesis and posterior instrumentation. Spine (Phila Pa 1976) 2002;27(15):1680–1686. doi: 10.1097/00007632-200208010-00018. [DOI] [PubMed] [Google Scholar]
- 34.Thavaneswaran P, Vandepeer M. Lumbar artificial intervertebral disc replacement: a systematic review. ANZ J Surg. 2014;84(3):121–127. doi: 10.1111/ans.12315. [DOI] [PubMed] [Google Scholar]
- 35.Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(e89):1–8. doi: 10.2106/JBJS.M.00865. [DOI] [PubMed] [Google Scholar]
- 36.Albert TJ, Pinto M, Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion. A functional and radiographic outcome study. Spine (Phila Pa 1976) 2000;25(1):123–129. doi: 10.1097/00007632-200001010-00021. discussion 130. [DOI] [PubMed] [Google Scholar]
- 37.Smith JS, Shaffrey CI, Glassman SD, et al. Clinical and radiographic parameters that distinguish between the best and worst outcomes of scoliosis surgery for adults. Eur Spine J. 2013;22(2):402–410. doi: 10.1007/s00586-012-2547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armaghani SJ, Archer KR, Rolfe RC, et al. Diabetes Is Related to Worse Patient-Reported Outcomes at Two Years Following Spine Surgery. J Bone Joint Surg Am. 2016;98:15–22. doi: 10.2106/JBJS.O.00297. [DOI] [PubMed] [Google Scholar]
- 39.Singh JA, Lewallen DG. Medical and psychological comorbidity predicts poor pain outcomes after total knee arthroplasty. Rheumatology (Oxford) 2013;52(5):916–923. doi: 10.1093/rheumatology/kes402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta HB, Dimou F, Adhikari D, et al. Comparison of Comorbidity Scores in Predicting Surgical Outcomes. Med Care. 2016;54(2):180–187. doi: 10.1097/MLR.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen Y, Silverstein JC, Roth S. In-hospital complications and mortality after elective spinal fusion surgery in the united states: a study of the nationwide inpatient sample from 2001 to 2005. J Neurosurg Anesthesiol. 2009;21(1):21–30. doi: 10.1097/ANA.0b013e31818b47e9. [DOI] [PubMed] [Google Scholar]
- 42.Min J-H, Jang J-S, Lee S-H. Comparison of anterior- and posterior-approach instrumented lumbar interbody fusion for spondylolisthesis. J Neurosurg Spine. 2007;7(1):21–26. doi: 10.3171/SPI-07/07/021. [DOI] [PubMed] [Google Scholar]
- 43.Freudenberger C, Lindley EM, Beard DW, et al. Posterior Versus Anterior Lumbar Interbody Fusion with Anterior Tension Band Plating: Retrospective Analysis. Orthopedics. 2009;32(7):492–496. doi: 10.3928/01477447-20090527-12. [DOI] [PubMed] [Google Scholar]
- 44.Lee KH, Yue WM, Yeo W, et al. Clinical and radiological outcomes of open versus minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2012;21:2265–70. doi: 10.1007/s00586-012-2281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng CW, Yue WM, Poh SW, et al. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (03622436) 2009;34(13):1385–1389. doi: 10.1097/BRS.0b013e3181a4e3be. [DOI] [PubMed] [Google Scholar]
- 46.Tian NF, Wu Y-S, Zhang X-L, et al. Minimally invasive versus open transforaminal lumbar interbody fusion: a meta-analysis based on the current evidence. Eur Spine J. 2013;22(8):1741–1749. doi: 10.1007/s00586-013-2747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hudak EM, Perry MW. Outpatient minimally invasive spine surgery using endoscopy for the treatment of lumbar spinal stenosis among obese patients. J Orthop. 2015;12(3):156–159. doi: 10.1016/j.jor.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Comparison of one-level minimally invasive and open transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Eur Spine J. 2010;19(10):1780–1784. doi: 10.1007/s00586-010-1404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Zhou Y, Zhang ZF, Li CQ, Zheng WJ, Liu J. Minimally invasive or open transforaminal lumbar interbody fusion as revision surgery for patients previously treated by open discectomy and decompression of the lumbar spine. Eur Spine J. 2011;20(4):623–628. doi: 10.1007/s00586-010-1578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phillips FM, Carlson GD, Bohlman HH, et al. Results of surgery for spinal stenosis adjacent to previous lumbar fusion. J Spinal Disord. 2000;13(5):432–437. doi: 10.1097/00002517-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Stienen MN, Joswig H, Smoll NR, et al. Short- and long-term effects of smoking on pain and health-related quality of life after non-instrumented lumbar spine surgery. Clin Neurol Neurosurg. 2016;142:87–92. doi: 10.1016/j.clineuro.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 52.Gulati S, Nordseth T, Nerland US, et al. Does daily tobacco smoking affect outcomes after microdecompression for degenerative central lumbar spinal stenosis? – A multicenter observational registry-based study. Acta Neurochir (Wien) 2015;157(7):1157–1164. doi: 10.1007/s00701-015-2437-1. [DOI] [PubMed] [Google Scholar]
- 53.Luszczyk M, Smith JS, Fischgrund JS, et al. Does smoking have an impact on fusion rate in single-level anterior cervical discectomy and fusion with allograft and rigid plate fixation? J Neurosurg Spine. 2013;19(5):527–531. doi: 10.3171/2013.7.SPINE13208. [DOI] [PubMed] [Google Scholar]
- 54.Schroeder GD, Kepler CK, Hilibrand AS. The effect of smoking on patients having spinal surgery. Curr Orthop Pract. 2016;27(2):140–145. [Google Scholar]
- 55.Kaye AD, Prabhakar AP, Fitzmaurice ME, et al. Smoking cessation in pain patients. Ochsner J. 2012;12(1):17–20. [PMC free article] [PubMed] [Google Scholar]
- 56.Gross DP, Stephens B, Bhambhani Y, et al. Opioid prescriptions in canadian workers’ compensation claimants: prescription trends and associations between early prescription and future recovery. Spine (Phila Pa 1976) 2009;34(5):525–531. doi: 10.1097/BRS.0b013e3181971dea. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. [Accessed July 10,2016];CDC Grand Rounds: Prescription Drug Overdoses — a U.S. Epidemic [CDC web site] Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6101a3.html.
- 58.Centers for Disease Control and Prevention. [Accessed July 10, 2016];Injury Prevention & Control: Opioid Overdose: CDC Guideline for Prescribing Opioids for Chronic Pain. Available at http://www.cdc.gov/drugoverdose/prescribing/guideline.html.