Abstract
Severe cutaneous adverse reactions (SCARs) encompass a heterogeneous group of delayed hypersensitivity reactions, which are most frequently caused by drugs. Our understanding of several aspects of SCAR syndromes has evolved considerably over the previous decade. This review explores evolving knowledge on the immunopathogenic mechanisms, pharmacogenomic associations, in-vivo and ex-vivo diagnostics for causality assessment and medication cross-reactivity data related to SCAR syndromes. Given the rarity and severity of these diseases, multidisciplinary collaboration through large international, national and/or multicentre networks to collect prospective data on patients with SCAR syndromes should be prioritized. This will further enhance a systematised framework for translating epidemiological, clinical, and immunopathogenetic advances into preventive efforts and improved outcomes for patients.
Introduction
Severe cutaneous adverse reactions (SCARs) encompass a heterogeneous group of delayed hypersensitivity reactions, most frequently caused by drugs, which are associated with significant morbidity and mortality.1,2 SCARs include Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS or HSS) and acute generalised exanthematous pustulosis (AGEP).3 The clinical, biochemical and histological characteristics of these syndromes are summarised in Table 1.
Table 1.
Summary of the clinical manifestations and histopathological features of SCAR syndromes.
| SCAR syndrome | Effector mechanisms | Clinical manifestations | Investigation findings | Histopathological features | Latency period | Common causal drugs |
|---|---|---|---|---|---|---|
| SJS/TEN | CD8+ cytotoxic T lymphocyte mediated Fas-FasL and granulysin-mediated apoptosis.4,8,155 | SJS and TEN are a disease continuum; the differentiation is based upon the percentage of body surface area of skin detachment.156–158 Acute onset of blisters and erosions affecting the skin, and mucous membranes; often associated severe systemic complications with significant morbidity and long-term sequelae.159 |
Abnormal liver, renal and respiratory function. Haematological, metabolic, fluid & electrolyte complications. | Subepidermal blister; spectrum of changes ranging from lichenoid reaction pattern with apoptotic keratinocytes, partial to full thickness epidermal necrosis.158 | 1–4 weeks.156,159 | Carbamazepine Phenytoin Lamotrigine Allopurinol Nevirapine NSAID* Sulfonamides Sulfasalazine159 |
| DRESS | T-cell mediated perforin-granzyme B as well as Fas/Fas L-dependent cell death.7,160 | Clinical presentation is heterogeneous: widespread exanthematous eruption, facial oedema, fever and lymphadenopathy.18,160,161
High variability in disease severity; some patients have modest systemic symptoms, while others develop significant morbidity due to internal involvement.160 |
Haematological abnormalities, most commonly eosinophilia and atypical lymphocytes. Abnormal liver, renal, respiratory and other organ function.18,160,161 |
Multiple histological patterns including: interface reaction, apoptotic keratinocytes, parakeratosis, spongiosis.161 | 2–6 weeks.79 | Carbamazepine Phenytoin Lamotrigine Allopurinol Sulfonamides Vancomycin Minocycline Amoxicillin79 |
| AGEP | Activation and proliferation of specific CD4 and CD8 T-cells, perforin/granzyme B and Fas ligand mechanisms to induce apoptosis.162 | Acute onset of widespread non-follicular sterile pustules overlying erythematous oedematous skin, starting in the intertriginous areas, often associated with fever.162–164 | Neutrophilia +/− eosinophilia, abnormal renal/liver function, hypocalcaemia.162–164 | Spongiform subcorneal and/or intradermal pustules with marked oedema of the papillary dermis and polymorphous perivascular infiltrate.165 | 1–5 days.163 | Amoxicillin Quinolones Sulfonamides Terbinafine Hydroxychloroquine Diltiazem166 |
AGEP: acute generalised exanthematous pustulosis, CD: cluster of differentiation, DRESS: drug rash with eosinophilia and systemic symptoms, NSAID: Non-steroidal anti-inflammatory drug, SJS: Stevens-Johnson syndrome, TEN: toxic epidermal necrolysis.
Our understanding of several aspects of SCAR syndromes has evolved considerably over the previous decade. The recent 2016 UK guidelines on the management of SJS/TEN in adults highlighted many areas of evolving research.4 The aim of this review article is to provide a complementary review of emerging immunopathogenic mechanisms, established pharmacogenomic associations, in-vivo and ex-vivo causality assessment tools and medication cross-reactivity data related to SCAR syndromes.
Immunopathogenesis of SCAR
Medications are the causative agents in greater than 85% of SCARs in adults,5 with frequently implicated drugs being antimicrobials, aromatic antiepileptic drugs and antimetabolite agents, particularly, allopurinol and its derivatives.4,5 Regardless of the causal medications, T-cell mediated delayed hypersensitivity reactions, triggered by interactions between small molecule drugs, human leucocyte antigen (HLA) Class I molecules and T-cell receptors (TCR), underlie the pathogenesis of most SCARs. Increasing knowledge suggests that carriage of specific HLA risk allele(s) are necessary but not sufficient factors in initiating the immunopathogenesis cascade.6 Currently three non-mutually exclusive models have been proposed: the hapten/pro-hapten, the pharmacologic interaction (PI) and the altered peptide repertoire models (Fig. 1). The resultant effector immune mechanisms (e.g., eosinophil-mediated injury in DRESS7, CD8+ cytotoxic T-cell mediated injury in SJS/TEN4 and the cytotoxic peptide 15kdal granulysin that has been identified as a key molecule produced by CD8+ T cells, natural killer (NK) T cells and NK cells that is responsible for the disseminated keratinocyte death in SJS/TEN8) in turn contribute to characteristic clinical manifestations of each condition (Table 1). Of note, Bellon et al.’s study suggests that the overexpression of endogenous damage-associate molecular patterns (DAMPs) or alarmins in SJS/TEN support the involvement of the innate immune system in the pathogenesis of delayed hypersensitivity reactions, suggesting an extension of the T-cell mediated hypothesis.9 Indeed, several innate immune components have been investigated in the aetiopathogenesis of SJS/TEN. Morel and colleagues’ study revealed that the innate receptor CD94/NKG2C is expressed by NK cells and cytotoxic T lymphocytes and might be involved in triggering degranulation in response to HLA-E in patients with SJS/TEN.10 A further study by the same authors determined that upregulation of the innate immune molecules, α-defensins 1–3 in T cells, may be involved in the pathogenesis of SJS/TEN.11 There is accumulating data to suggest that humoral and cellular components of the innate immune response may be involved in the pathogenesis of delayed cutaneous hypersensitivity reactions.12
Figure 1.
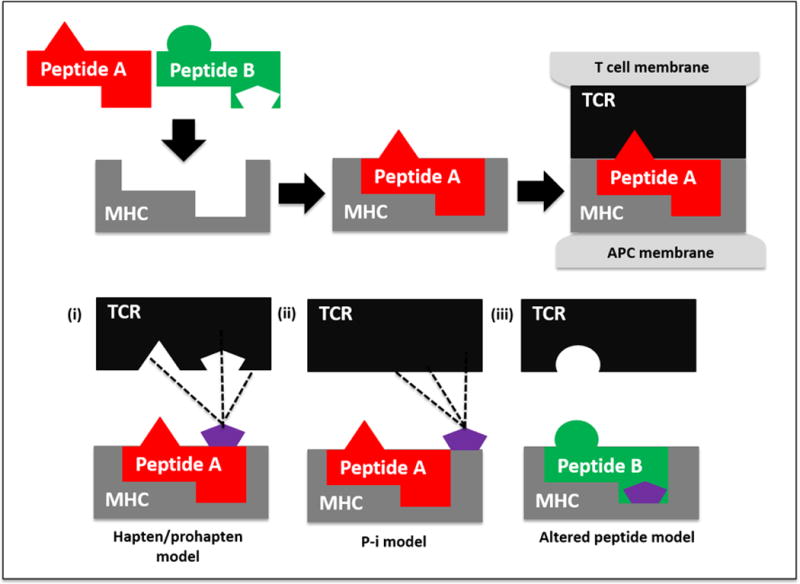
Proposed models of T-cell receptor (TCR), major histocompatability complex (MHC), drug interactions: In the hapten/prohapten model (i) a drug (e.g., penicillin) binds covalently to an endogenous peptide (e.g., albumin), forming a new molecule. Antigen presenting cells process and present it as short peptide fragments within the MHC binding cleft, some of which (peptide A) include drug epitopes (purple pentagon). If recognized by a TCR, a drug-specific immune response can ensue. In the pharmacological-interaction (P-I) model (ii) the drug binds non-covalently to certain MHC molecules or TCRs, stimulating specific TCR and thus generating drug-reactive T-cells. In the altered peptide repertoire model (iii) a drug (e.g., abacavir) binds non-covalently to the binding pocket of a MHC molecule (e.g. HLA-B*57:01), altering its conformation and allowing a new array of self-peptides (peptide B) to stably occupy it and stimulate T-cells. This can lead to drug-induced activation of autoimmunity (e.g., abacavir hypersensitivity reaction.) Adapted from Pavlos et al.175
Higher plasma concentrations of the drug and/or its metabolites, caused by the individual’s in-vivo absorption, distribution, metabolism and elimination enzyme (ADME) activities, or by way of drug-drug interactions, increase the risk for many hypersensitivity reactions.13,14 This apparent dose-dependency seen in severe T-cell mediated adverse drug reactions (ADRs) supports that small molecules are non-covlaently interacting with an immune receptor. For instance, elevated serum levels of oxypurinol, an active metabolite of allopurinol, which has a long plasma half-life, increases the risk of allopurinol hypersensitivity.14 Impaired renal function leading to high plasma concentrations of oxypurinol is also directly correlated with disease severity and mortality.14 Historically, certain types of trimethoprim-sulfamethoxazole hypersensitivity reactions were more likely in those with N-acetyl transferase (NAT) 2 slow-acetylator genotypes.15 Collectively, the paradigm has been shifting towards an interplay between ADME enzymatic activities and immunologic mechanisms being responsible for the initiation of hypersensitivity responses,16 further triggered by yet-to-be-determined insults (such as viral infections), leading to polarisation toward distinct cytokine profiles and effector pathways. Further studies are required to explore this evolving concept of hypersensitivity and drug concentration-dependent relationships.
The role of herpes virus reactivation
Heterologous immunity is a longstanding concept that has recently gained renewed interest to explain both individual susceptibility and tissue specificity of SCAR. In this model, the effector memory T-cells generated during the course of a remote infection and maintained by latency or re-exposure to the infectious agent cross-react with drug modified proteins, thereby highlighting the role of infectious agents, such as chronic persistent DNA viruses including Human Herpes viruses (HHV), in SCAR pathogenesis.16
The concept of heterologous immunity in the immunopathogenesis of SCAR should not be confused with the reactivation of HHV, in particular human herpes virus 6 (HHV-6), which is known to be associated with DRESS.17–20 Reactivation of Epstein-Barr virus (EBV), cytomegalovirus (CMV), HHV-6 and human herpes virus 7 (HHV-7) has been reported to occur in DRESS syndrome typically 2–3 weeks following the original syndrome and in the absence of re-exposure to the drug. It appears to correlate with the immune dysregulation occurring during DRESS syndrome and in particular, regulatory T-cell dysfunction. The reported proportion of patients with HHV-6 reactivation in DRESS varies according to the specific implicated drug and is between 36% and 62%.18,21 HHV-6 reactivation, as measured by a rise in HHV-6 IgG titres and plasma HHV-6 DNA levels, typically occurs 2–3 weeks after the onset of the rash.22 This temporal association suggests a complex interaction between HHV and the immunopathogenesis of DRESS.22 Furthermore, reactivation of HHV have also been associated with the development of more severe disease.19,21–24 The development of autoimmune diseases, such as systemic lupus erythematosus, type 1 diabetes mellitus and autoimmune thyroiditis, is a late complications of DRESS that has been associated with herpes virus reactivation.20,25–27
Reactivation of the other herpes viruses, which include HHV-7, EBV and CMV have also been reported to occur in association with DRESS.22,28,29 Indeed, sequential reactivation of herpes viruses during the course of DRESS has been described in a similar sequence to that in graft-versus-host disease (GVHD): HHV-6 and/or EBV, followed by HHV-7 and subsequently by CMV.29 Viral reactivation may also explain the prolonged clinical symptoms, multi-organ involvement and systemic inflammation following discontinuation of the offending drug.22,29–31
DRESS has been reported in the setting of immune reconstitution inflammatory syndrome (IRIS). IRIS describes an inflammatory processes that occurs soon after the initiation of highly active antiretroviral therapy (HAART) in patients with Human Immunodeficiency Virus (HIV) and is associated with an increase in CD4+ cell count and/or decrease in HIV viral load.32 IRIS occurs as a result of immune recovery and it results in the host recognising pre-existing or latent infections.33 DRESS may be considered a form of immune constitution whereby unregulated immune activation occurs against reactivated herpes viruses.32
For SJS/TEN however, there is weaker evidence, only at case report level, for its association with HHV-6 reactivation and this could also be secondary to phenotype misattribution of viral reactivation associated with the profound immunosuppression secondary to the protracted clinical course and significant courses of immunosuppressants, such as ciclosporin used in SJS/TEN.34,35 The role of CMV has been proposed in the development of AGEP,36 however evidence from European Study of Severe Cutaneous Adverse Reactions (EuroSCAR) study failed to find such an association.37 Testing for herpes virus reactivation in SCAR syndromes may assist in clarifying the diagnosis in cases where the cutaneous and other clinical findings are non-specific, and may also be of prognostic value.31,21,38
Recent advances in pharmacogenomics in SCAR
Individuals with certain HLA genotypes carry higher risks of developing SCAR syndromes. Over the last decade, clinically significant pharmacogenomics associations have been discovered, leading to specific recommendations regarding HLA genotyping before prescription of drugs to reduce the risks in susceptible populations. However, for common causal medications, in particular, antibiotics, very few clinically meaningful HLA associations exist.39 Medications that are considered to have strong pharmacogenomic associations with severe T-cell mediated ADRs, of which routine genetic screening prior to their prescription have already or in future may soon become the standard of clinical practice are presented herein (Table 2).
Table 2.
Therapeutic recommendations where evidence exists for strong HLA associations for various adverse drug reaction phenotypes†.
| Medications | HLA | Phenotype | Populations studied | Therapeutic recommendation | Selected references |
|---|---|---|---|---|---|
| Abacavir | HLA-B*57:01 | HSS | All | HLA-B*57:01 testing prior to abacavir prescription and avoid abacavir use in HLA-B*57:01 positive individuals | 41,42,45–48 |
| Carbamazepine | HLA-B*15:02§ | SJS/TEN | Han Chinese (China, Hong Kong, Taiwan), Thai, Malaysian, Indian (South Asians) | Avoid carbamazepine in all HLA-B*15:02 positive individuals†† Screening currently recommended for at risk populations (Han Chinese, southeast and south Asians) or unknown ethnicity |
51,58,64,167,168 |
| Carbamazepine | HLA-A*31:01 | DRESS/HSS>SJS/TEN | Han Chinese, Japanese, Korean, Caucasian | If alternative therapeutic agent exists, avoid carbamazepine in all carbamazepine naïve HLA-A*31:01 positive individuals | 51,58,63,52,55,61,62,64 |
| Allopurinol | HLA-B*58:01 | DRESS/HSS and SJS/TEN | Han Chinese (China and Hong Kong), Thai, Korean, Japanese, European | Avoid allopurinol use in the setting of allopurinol naïve HLA-B*58:01 posivite individuals Widespread guidelines for screening prior to use have not been issued¶ |
54,66–74 |
SCAR: severe cutaneous adverse reactions, HLA: human leucocyte antigen, HSS: hypersensitivity syndrome, SJS: Stevens-Johnson syndrome, TEN: toxic epidermal necrolysis, DRESS: drug reaction with eosinophilia and systemic symptoms.
For any individual carrying an HLA risk allele, if they have already tolerated the drug for > 12 continuous weeks currently or in the past, then it is safe for them to continue the drug or for the drug to be reinstituted in the future.
Carbamazepine SJS/TEN is also associated with other B75 serotype HLA alleles such as HLA-B*15:21, B*15:08, B*15:11 and potentially B*15:30 and B*15:31, therefore additional caution should be exerted for carbamazepine use if these HLA types are identified.
Although a much weaker association exists between HLA-B*15:02 and other aromatic amine anticonvulsants, such as oxcarbamazepine, eslicarbamzepine, lamotrigine, phenytoin and fosphenytoin, consideration should be given to choosing an alternative non-aromatic anticonvulsant in the case of identified HLA-B*15:02+.
The American College of Rheumatology Guidelines for Management of Gout (2012) have recommended HLA-B*58:01 testing prior to allopurinol prescription in specific popualtions including 1) those of increased risk (Southeast Asian) and 2) Subpopualtions with increase risk based on advanced chronic renal failure (stage 3).169
Abacavir
Abacavir (ABC), an antiretroviral drug used in combination therapy to treat HIV, is associated with hypersensitivity syndrome (HSS) in 5% (range 0 – 14%) of patients.40 The hypersensitivity syndrome associated with ABC is differentiated from DRESS/DIHS in that the median time to presentation with fever and malaise is 8 days with latency periods as short as 1 day and rash, which does not occur in up to 30%, is often a late feature of the presentation. The skin involvement in ABC HSS is typically a mild to moderate exanathem without evidence of blistering or epidermal detachment. De-challenge after withdrawal of drug occurs rapidly with disappearance of the fever, malaise and even skin rash within 72 hours of abacavir withdrawal. HLA-B*57:01 was found to be a significant risk allele for ABC-HSS by two independent groups.41,42 The lack of specificity of clinical symptoms and signs associated with ABC HSS in HIV positive individuals led to a high clinical false positive rate and an apparent lack of sensitivity of HLA-B*57:01 for ABC HSS. This was particularly apparent in ethnicities with a lower prevalence of HLA-B*57:01 such as African Americans. ABC patch testing was found to be a sensitive and specific means to identify true immunologically-mediated ABC HSS.43,44 A randomised double-blind controlled trial with a co-primary endpoint of clinically and immunologically (patch-test) confirmed ABC HSS demonstrated the clinical utility of HLA-B*57:01 screening to completely eliminate immunologically-mediated cases of ABC-HSS in those of European ancestry.45 A case-control study confirmed the generalizability of this utility to African Americans.46 Several factors favoured successful translation of HLA-B*57:01 screening into routine clinical practice including: 100% negative predictive value, low numbers (n=30) needed to test to prevent one case of true-immunologically mediated ABC HSS, generalisability of the test across all ethnic groups and availability of cost-effective quality-assured laboratory methods with rapid turn-around times.46–48
Carbamazepine
Carbamazepine is an aromatic amine anticonvulsant and is associated with cutaneous adverse reactions in up to 10% of patients.49 Although two digit HLA associations had been previously described between allopurinol SJS/TEN and sulfa antimicrobial SJS/TEN, the association between HLA-B*15:02 and carbamazepine SJS/TEN in a Taiwanese population was the first four digit association for SJS/TEN and the strongest overall for SJS/TEN in the literature to-date.50 A recent meta-analysis showed that HLA-B*15:02 is strongly associated with carbamazepine-induced SJS/TEN in Han Chinese and Southeast Asians who carry high allele frequency (pooled Odds Ratio (OR) 113.4, 95% CI 51.2 – 251.0, p<1×10−5).51 However, such association was lacking in Japanese,52–54 Koreans,55 and Caucasians,56,57 in whom the allele carrier frequency was estimated to be <1%58. HLA-B*15:02 testing provides positive predictive value (PPV) of 1.8% and negative predictive value (NPV) of 100% respectively in susceptible populations, with proven cost-effectiveness for screening.51,59,60
Although HLA-B*15:02 is a risk variant strongly associated with carbamazepine SJS/TEN, there is no evidence to suggest that it is associated with hypersensitivity syndrome (HSS) or maculopapular exathems.51,58
Unlike HLA-B*15:02, HLA-A*31:01 is common with allele carrier frequencies >3% across many ethnic groups58. HLA-A*31:01 was shown to be associated with all SCAR phenotypes across populations including Han Chinese, Japanese, Koreans and Caucasians.52,55,58,61,62 However, HLA-A*31:01 showed a stronger association with DRESS (pooled OR 13.2, 95% CI 8.4 – 20.8, p<0.001) over SJS/TEN (pooled OR 3.94, 95% CI 1.4 – 11.5, p=0.01).58,63 This effect was particularly noted in populations where HLA-B*15:02 carriage is prevalent where it is likely that the strong association between HLA-B*15:02 and carbamazepine SJS/TEN overshadows that of HLA-A*31:01. In contrast, in Europeans, the higher frequency of the HLA-A*31:01 allele appears to overshadow the effect of the uncommon HLA-B*15:02 allele.47,51
Regulatory agencies such as the US Food and Drug Administration (FDA) and the European Medicines Agency have issued recommendations regarding genotyping before initiation of carbamazepine in certain at-risk populations.64 Genetic testing for HLA-B*15:02 is recommended in Han Chinese, Southeast and South Asians or in patients whose ethnic origin is unknown (Level A). HLA-A*31:01 testing may be considered in patients of all ancestries (level B); however, there is no current recommendation for routine screening for HLA-A*31:01 before initiation of carbamazepine therapy. In patients who are positive for HLA-B*15:02, alternatives to carbamazepine should be used, preferably avoiding all aromatic amine anticonvulsants since SJS/TEN has been more weakly associated with HLA-B*15:02 with these drugs in Southeast Asians. In the case of HLA-A*31:01 positivity, ideally, alternative first-line medication to carbamazepine should be used in carbamazepine naïve individuals unless there are no identifiable alternatives, in which case patients should be followed with extremely close monitoring for the first signs of evolving SCAR.58
Allopurinol
Allopurinol accounts for up to 5% of all cases with SCAR.65 An association between allopurinol induced SCAR (SJS/TEN and HSS phenotypes) and HLA-B*58:01 genotype was first described in Taiwanese Han Chinese population.66 Thereafter, studies in other ethnic groups including, Han Chinese from mainland China67,68 and Hong Kong,69 Thai,70 Koreans,71,72 Japanese,54 and Europeans73,74 have replicated similar associations, although the strength of association was much weaker with a lower negative predictive value in Japanese and Europeans, likely owing to different allele frequencies across ethnic groups. The NPV of HLA-B*58:01 screening for allopurinol induced SCAR in Southeast Asian populations is 100%.75 A modelling study from Singapore showed that routine genetic screening to prevent an episode of SCAR, even in high risk populations, did not appear to be cost-effective.76 The extreme short and long-term morbidity and mortality that is in particular associated with SJS/TEN, the lack of comparably inexpensive treatment options to allopurinol, the development of newer and less expensive molecular assays for HLA-B*58:01 and the availability of a prospective screening study suggesting a significantly reduced incidence of allopurinol SCAR with HLA-B*58:01 screening in Taiwan suggest that further attention and implementation of HLA-B*58:01 screening may be warranted.
Causality assessment through clinical, in vivo and ex vivo testing
Assigning drug causality is often difficult in SCAR syndromes, especially when multiple agents are implicated, in particular, antimicrobials.78 Conversely, in situations of a single implicated drug (e.g. carbamazepine, allopurinol), utilisation of appropriate clinical algorithms is often sufficient to assign causality,5,79 especially in histologically confirmed cases.80,81 Drug causality may be clinically established through several different validated methods/algorithms, each with own strengths and limitations (Table 3). Nonetheless, in vivo and ex vivo diagnostics are being increasingly employed to aid causality and management of patients with SCAR.82 Guidelines exist for the recommended concentrations of drugs to be used in in vivo testing for delayed hypersensitivity,83,84 although universal consensus has not been established.
Table 3.
Three major approaches to drug causality assessment in severe cutaneous adverse drug reactions.
| Method | Description | Strengths | Weaknesses | Selected references |
|---|---|---|---|---|
| Global introspection | Inference of causality by expert clinical judgement. | Consensus opinion by a group of experts. Often serves as the gold standard in causality assessment. |
Subjective, influenced by the experience, knowledge and biases of the assessor(s). Poor reproducibility. |
5,170,171 |
| Bayesian approach | Uses clinical and epidemiological data to transform a prior into a posterior probability. | Allows simultaneous assessment of multiple causes. Previous knowledge of the culprit drug profile is not required. |
Time consuming and highly technical. | 172 |
| Drug causality algorithms (see A & B) | Collection of specific data points followed by problem solving operations resulting in an objective assessment of probability. | Structured and standardised method. Reproducible and transparent. |
Clinical utility may be limited in cases where more than one drug is administered. Clinical judgement may be required at various stages. Some algorithms may not be able to identify novel ADRs or first cases of ADRs.* |
5,172 |
| (A).Naranjo Scale | Consists of 10 questions and yields a final assessment of causality as: ‘definite’, ‘probable’, ‘possible’ or ‘doubtful’ that a drug administered in therapeutic doses caused an adverse event. | Well-validated. Widely used and quick/simple tool. |
Classifies >90% of suspected adverse drug reactions as ‘possible.’ Does not take into account drug-drug interactions. |
173 |
| (B) ALDEN | Specific algorithm for assessing drug causality in SJS and TEN. The final assessment of causality is expressed as ‘very probable’. ‘probable’, ‘possible’, ‘unlikely’ or ‘very unlikely.’ |
Developed by experts in SJS/TEN. Validated on cases enrolled in the EuroSCAR study in a case-control analysis. |
Only validated for SJS/TEN. | 5 |
ADR: Adverse drug reaction, ALDEN: Algorithm for drug causality for epidermal necrolysis, SJS: Stevens-Johnson syndrome, TEN: toxic epidermal necrolysis.
Patch testing
Patch testing (PT) involves the application of an implicated and/or potentially cross-reactive drug with a control vehicle (petroleum jelly) to skin for 48 hours82 and subsequently read after 48–96 hours and if possible 7 days. The safety of PT in SCAR has been increasingly demonstrated.85–91 Systemic (but non-life threatening) reactions have been reported infrequently with PT, although mostly for anti-tuberculosis drugs in HIV patients.92–96 The recommendations have been to perform skin testing at least 6 weeks post-resolution of SCAR.97 The sensitivity of patch testing appears highest for ABC HSS (87%)43,44 and DRESS (31.6%–58%) and lowest for SJS/TEN (20%–24%) and AGEP (18%).85,86,90 The sensitivity also appears to be affected by the investigated drug, highest for abacavir, anticonvulsants and beta-lactam antibiotics,87 in particular for abacavir (87%), amoxicillin (up to 44.4%), and lowest for vancomycin (9.1%), trimethoprim-sulfamethoxazole (8.6%), macrolides (4.8%), hepatitis C antivirals98 and cephalosporins (4.4%).85 The use of oral provocation after a negative PT should be used with caution in patients with SCAR, considering the low sensitivity of PT.
Intradermal testing
Intradermal testing (IDT) utilising 0.02–0.05 ml of the highest non-irritant concentration of drug, has been reported in DRESS and other SCAR phenotypes in a number of small series.86,99–101 IDT with delayed readings has been utilised extensively for T-cell mediated hypersensitivity, in particular for non-SCAR phenotypes related to beta-lactams.102,103 IDT avoids the inconvenience of patch testing and reactions will often occur within 6–24 hours. Barbaud et al. demonstrated in a small cohort of predominately beta-lactam SCAR that IDT appeared to have a greater sensitivity than PT when performed following negative PT and was not associated with adverse events.86 Guidelines also support the use of IDT following negative PT in patients with SCAR, outside of SJS/TEN.83 IDT is often limited by the availability of a sterile injectable formulation of the investigated drug. Like PT, oral provocation testing after a negative IDT should be undertaken with caution.
Ex vivo diagnostics
The stimulation of patient peripheral blood mononuclear cells (PBMCs) to measure T-cell responses in the setting of drug-associated SCAR has been increasingly investigated in research and clinical settings. Whilst responses have been detected out to 20 years post-index event, a blood sample from ‘acute bleeds’ or in the early recovery phase is likely to display greater sensitivity.104–106 The lymphocyte transformation test (LTT),99,107 which typically incubates investigated drugs with PBMCs for 5–7 days or longer, measures T-cell responses to a variety of drugs (e.g. antimicrobials, anticonvulsants, analgesics and diuretics) via a stimulation index.104,107–117 Enzyme-linked immunospot assay (ELISpot) has been primarily employed for antiretroviral and antimicrobial hypersensitivity and SCAR syndromes,118–121 especially when in vivo testing has been negative.94,99,113,122,123 Variability in testing methods, incubation periods (1 vs. 2 vs. 5 days), co-stimulation factors (e.g. IL-7/IL-15) and measured outputs (e.g. granulysin, IFN-γ, TNF-α) make comparison within and between testing modalities difficult.8,106,124,125 The known drug epitopes are unknown for most T-cell mediated hypersensitivity syndromes126,127 Currently LTT or ELISpot should not be employed to exclude a suspected drug due to low sensitivity (24–70%125,128 and 60%–80%,125 respectively.) Whilst LTT has demonstrated a higher sensitivity in other types of anticonvulsant hypersensitivity (70–90%),129 lower rates have still been noted in lamotrigine-SJS.130
Indeed, Polak and colleagues’ study compared the lymphocyte proliferation assay (LPA) against combination INF-γ and IL-4 drug ELISpot assays in patients with delayed-type drug hypersensitivity reactions in the acute phase. In their study, the assays demonstrated a test specificity of 95%, 83% and 92% for LPA, INF-γ and IL-4, respectively. During acute drug hypersensitivity reactions, the sensitivity of combined measurement of drug-specific INF-γ and IL-4 cytokines was greater than that of LPA (82% vs. 50%). Thus, these investigators determined that in vitro assays of drug-specific INF-γ and IL-4 production may be more sensitive than LPA for the detection of drug-specific T-cells in the acute setting.131 Further, a recent study by Haw et al. concluded that cytokine assays (INF-γ and IL-4) are superior to LPA in identifying the causative drug in the paediatric population; however, these investigators suggested that when combined, they offer even greater utility in the diagnosis and post-recovery of delayed-type hypersensitivity reactions.132
The sensitivity and hence NPV of ex-vivo testing in the future is likely to be enhanced by co-utilisation of flow cytometry and intracellular cytokine staining methods.118,133–136
The importance of drug cross-reactivity between structurally-related drugs
Structurally-related drugs can cause cross-reactions with SCAR. Although the specific epitopes remain elusive with regards to drug-self peptide responses, it is recognized that the immune system may recognise structural similarities Knowledge regarding the likelihood of cross-reactivity between drugs is important as exposure to structurally similar compounds after an index reaction can precipitate another severe episode. On the contrary, excessive avoidance of medications with low risk of cross-reactivity can lead to unwarranted restriction on therapeutic options that can adversely impact upon clinical care.
Beta-lactams
All beta-lactams (penicillins, cephalosporins, carbapenems and monobactams) share the core beta-lactam structure but with differing side-chains (Fig. 2). Evolving evidence to date suggests that side chain structures are commonly implicated in beta-lactam cross-reactivity for most immediate and delayed reactions. Table 4 further provides a list of commonly prescribed beta-lactams which share similar side chain structures.
Figure 2.
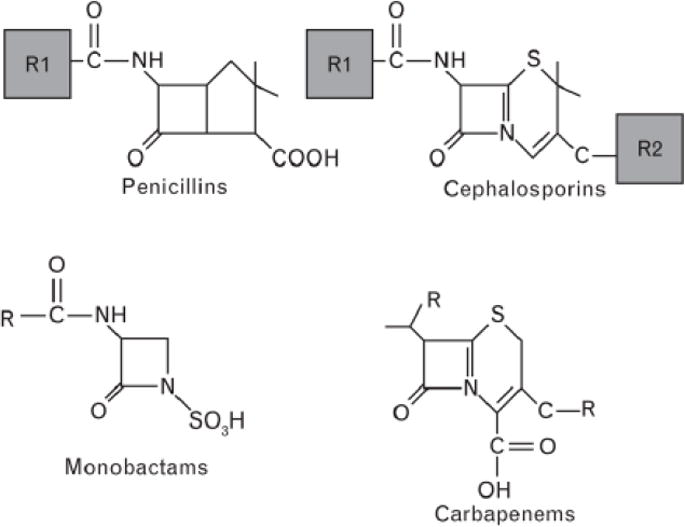
Basic structures of beta-lactams (adapted from Trubiano et al.174). R denotes side chains. Cephalosporins have two side chains, R1 and R2. However, R2 is lost during hydrolysis.
Table 4.
Beta-lactams with similar R1 side-chain structures (adapted from Trubiano et al.174)
| Penicillin G | Amoxicillin | Ampicillin | Ceftriaxone | Cefoxitin | Ceftamandole | Ceftazidime |
|---|---|---|---|---|---|---|
| Cephaloridine | Cefadroxil | Cefaclor | Cefotaxime | Cephaloridine | Cefonicid | Aztreonam |
| Cephalothin | Cefprozil | Cephalexin | Cefpodoxime | Cephalothin | ||
| Cefoxitin | Cefatrizine | Cephadrine | Cefditoren | |||
| Cephalexin | Cephaloglycin | Ceftizoxime | ||||
| Loracarbef | Cefmenoxime | |||||
| Cefepime |
Cephalosporins
R1 side-chains of cephalosporins (Fig. 2) are highly conserved and have been demonstrated to promote cross-reactions with penicillins containing similar structures. This is particularly true between aminopenicillins (amoxicillin, ampicillin and bacampillin) and aminocephalosporins (cephalexin and cefaclor), with recent studies demonstrating that the cross-reactivity rates between the amino compounds may be as high as 18.7%.137,138 On the contrary, patients with delayed aminopenicillin allergy have recently been shown to have complete absence of cross-reactivity and good tolerance to therapeutic challenge to non-amino cephalosporins (cefuroxime and ceftriaxone).137
Overall, low rates of cross-reactivity exist between penicillins and third and fourth generation cephalosporins of dissimilar side chain structures (1.1% vs. 10.9% for first and second generation cephalosporins which share similar side chains).138
Further, an interesting in vitro study by El-Ghaiesh et al. in eight cystic fibrosis patients with delayed hypersensitivity reactions to piperacillin, compared to five tolerant controls, demonstrated the critical role of drug-specific CD4+ and CD8+ T-cell clones in pathogenesis, which did not cross-react to a multitude of penicillins and cephalosporins including those that share similar side chain to piperacillin (e.g. cefoperazone). This study highlights the drug-specific nature of T-cell mediated hypersensitivity reactions as well as the highly complex nature of cross-reactivity to other beta-lactams, with some unknown mechanisms in addition to ‘structural similarities,’ likely further contributing to its pathogenesis.113
Carbapenems and monobactams
Although a cross-reactivity rate of 5.5% to imipenem has been previously reported in penicillin-allergic patients.139 A more recent study involving 204 patients demonstrated that none of the patients with delayed penicillin hypersensitivity cross-reacted to imipenem, meropenem or ertapenem, and all tolerated therapeutic doses of drug challenge.140 In view of the reportedly low (<1%) rates of cross-reactivity to carbapenems in patients with immediate penicillin hypersensitivity reactions,141,142 the true cross-reactivity rates in delayed reactions are likely very low (<1%) and therefore, carbapenems may be judiciously considered in patients who have limited therapeutic options.
In contrast, virtually zero percent cross-reactivity to aztreonam has been consistently demonstrated in patients with delayed penicillin hypersensitivity reactions.137,143 The only caveat is that aztreonam should be avoided in patients with ceftazidime allergy due to side-chain similarities.
It should also be noted that although cross-reactivity rates between penicillins and later generation cephalosporins or carbapenems are low, the vast majority of patients included in these studies had benign skin reactions and few patients with definitive SCAR phenotypes were represented. As such, considerable caution should be taken when prescribing beta-lactam antibiotics to patients with SCAR.
Aromatic anticonvulsants
Commonly prescribed aromatic anticonvulsants include carbamazepine, oxcarbazepine, lamotrigine, phenytoin and phenobarbital.144 Cross-reactivity between these structurally related aromatic anticonvulsants was originally thought to be mediated by arene oxides, toxic metabolites produced through cytochrome P450 pathway.145,146 However, it is now clear that poor metabolisers (e.g. CYP2C9*3) are at higher risk for SCAR associated with some anticonvulsants such as phenytoin.147 Earlier studies suggested that approximately 70% will experience some degree of cross-reactivity between aromatic anticonvulsants.146,148–150 There is also evidence suggesting that HLA-B*15:02 and other B75 serotype HLA alleles confer risk of developing SJS/TEN to other aromatic anticonvulsants, however, to a much lesser degree compared to carbamazepine.151,152 What is currently unclear is the extent to which HLA cross-reactivity occurs since cases of HLA-B*15:02 positive individuals who have reacted to one aromatic amine anticonvulsant but tolerated another (despite the association of HLA-B*15:02 with all aromatic amine anticonvulsant SCAR) have been well-described. Additionally Seitz et al. also noted that 21.7% of patients with carbamazepine hypersensitivity also displayed cross-reactivity to tricyclic antidepressants.150 However, this has not been substantiated as an effect that is seen in-vivo and in the case of HSS to carbamazepine, recommendations would not dictate avoidance of tricyclic antidepressants. In patients with SCAR to aromatic anticonvulsants, valproate, gabapentin, pregabalin and levetiracetam are safe alternatives.153,154
Conclusion and Future Directions
Recent advances in the knowledge of SCAR syndromes have provided us with a better understanding of immunopathogenic mechanisms, including the potential role of pre-existing cross-reactive T cell responses to viral infections, the discovery of important pharmacogenomic associations, which have become the standard of care, the use of clinical and laboratory methods for causality assessment and the knowledge of drug cross-reactivity mechanisms. Further knowledge on how precisely drugs activate T-cells, the pathomechanism for the generally very low positive predictive value of an HLA risk allele for a specific drug toxicity, more specific pharmacogenomic associations and future mechanistic information including cellular and molecular signatures will be key for pre-clinical prediction and prevention of drug toxicity as well as for enabling personalised approaches to prevention, early intervention and treatment of high morbidity and mortality diseases such as SJS/TEN. As highlighted in this review, numerous aspects of SCAR syndromes merit further interdisciplinary research. Finally, given the overall rarity but high morbidity and mortality of SCAR, collaboration through large international, national and multicentre networks to collect prospective data and biobank samples will further enhance a systematised framework for translating discovery into prevention and improved outcomes for patients.
What’s already known about this topic?
Severe cutaneous adverse reactions (SCARs) encompass a heterogeneous group of delayed hypersensitivity reactions, which are most frequently caused by drugs.
The designation SCAR most commonly includes Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), SJS-TEN overlap, drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS or HSS) and acute generalised exanthematous pustulosis (AGEP).
The pathogenesis underlying T-cell mediated delayed hypersensitivity reactions involves interactions between small molecule drugs, HLA Class I molecules and T-cell receptors.
What does this review add?
The rapid evolution of pharmacogenomic discoveries associating severe T-cell mediated drug hypersensitivity syndromes have created the promise of prevention. This has led either to universal HLA screening prior to drug prescription (e.g. HLA-B*57:01 and abacavir) or specific recommendations regarding HLA genotyping before prescription of drugs in susceptible populations (e.g. HLA-B*15:02 and carbamazepine).
Knowledge of the immunopathogenesis of SCAR and key novel and non-mutually exclusive mechanisms by which drugs activate T-cells has evolved.
In-vivo and ex-vivo diagnostics are being increasingly employed to aid causality assessment.
Knowledge of cross-reactivity between structurally-related medications is still rudimentary; however, this knowledge may avoid precipitating subsequent severe episodes and minimise unwarranted restriction of therapeutic options.
Acknowledgments
Funding sources: Professor Phillips and her work are supported through National Institutes of Health R13AR071267-01, 1P50GM115305-1, 1RO1A1103348-01, 1P30AI110527-01A1, the National Health & Medical Research Council (Australia), the Australian Centre for HIV and Hepatitis Virology Research and the Angela Anderson Foundation.
Footnotes
Conflict of interests: None
References
- 1.Thong BY, Tan TC. Epidemiology and risk factors for drug allergy. Br J Clin Pharmacol. 2011;71:684–700. doi: 10.1111/j.1365-2125.2010.03774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roujeau JC. Clinical heterogeneity of drug hypersensitivity. Toxicology. 2005;209:123–9. doi: 10.1016/j.tox.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331:1272–85. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 4.Creamer D, Walsh SA, Dziewulski P, et al. U.K. guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults 2016. Br J Dermatol. 2016;174:1194–227. doi: 10.1111/bjd.14530. [DOI] [PubMed] [Google Scholar]
- 5.Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. 2010;88:60–8. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]
- 6.Pavlos R, Mallal S, Phillips E. HLA and pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2012;13:1285–306. doi: 10.2217/pgs.12.108. [DOI] [PubMed] [Google Scholar]
- 7.Posadas SJ, Padial A, Torres MJ, et al. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol. 2002;109:155–61. doi: 10.1067/mai.2002.120563. [DOI] [PubMed] [Google Scholar]
- 8.Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343–50. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 9.Bellon T, Alvarez L, Mayorga C, et al. Differential gene expression in drug hypersensitivity reactions: induction of alarmins in severe bullous diseases. Br J Dermatol. 2010;162:1014–22. doi: 10.1111/j.1365-2133.2009.09627.x. [DOI] [PubMed] [Google Scholar]
- 10.Morel E, Escamochero S, Cabanas R, et al. CD94/NKG2C is a killer effector molecule in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Allergy Clin Immunol. 2010;125:703–10. doi: 10.1016/j.jaci.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Morel E, Alvarez L, Cabanas R, et al. Expression of alpha-defensin 1–3 in T cells from severe cutaneous drug-induced hypersensitivity reactions. Allergy. 2011;66:360–7. doi: 10.1111/j.1398-9995.2010.02484.x. [DOI] [PubMed] [Google Scholar]
- 12.Bellon T, Blanca M. The innate immune system in delayed cutaneous allergic reactions to medications. Curr Opin Allergy Clin Immunol. 2011;11:292–8. doi: 10.1097/ACI.0b013e3283489c2c. [DOI] [PubMed] [Google Scholar]
- 13.Grossman I. ADME pharmacogenetics: current practices and future outlook. Expert Opin Drug Metab Toxicol. 2009;5:449–62. doi: 10.1517/17425250902902322. [DOI] [PubMed] [Google Scholar]
- 14.Chung WH, Chang WC, Stocker SL, et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis. 2015;74:2157–64. doi: 10.1136/annrheumdis-2014-205577. [DOI] [PubMed] [Google Scholar]
- 15.Pirmohamed M, Alfirevic A, Vilar J, et al. Association analysis of drug metabolizing enzyme gene polymorphisms in HIV-positive patients with co-trimoxazole hypersensitivity. Pharmacogenetics. 2000;10:705–13. doi: 10.1097/00008571-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 16.White KD, Chung WH, Hung SI, et al. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: The role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015;136:219–34. doi: 10.1016/j.jaci.2015.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlos R, Mallal S, Ostrov D, et al. Fever, rash, and systemic symptoms: understanding the role of virus and HLA in severe cutaneous drug allergy. J Allergy Clin Immunol Pract. 2014;2:21–33. doi: 10.1016/j.jaip.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 19.Picard D, Janela B, Descamps V, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010;2:46–62. doi: 10.1126/scitranslmed.3001116. [DOI] [PubMed] [Google Scholar]
- 20.Descamps V, Mahe E, Houhou N, et al. Drug-induced hypersensitivity syndrome associated with Epstein-Barr virus infection. Br J Dermatol. 2003;148:1032–4. doi: 10.1046/j.1365-2133.2003.05330.x. [DOI] [PubMed] [Google Scholar]
- 21.Tohyama M, Hashimoto K, Yasukawa M, et al. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157:934–40. doi: 10.1111/j.1365-2133.2007.08167.x. [DOI] [PubMed] [Google Scholar]
- 22.Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55:1–8. doi: 10.2332/allergolint.55.1. [DOI] [PubMed] [Google Scholar]
- 23.Chen YC, Chiang HH, Cho YT, et al. Human herpes virus reactivations and dynamic cytokine profiles in patients with cutaneous adverse drug reactions - a prospective comparative study. Allergy. 2015;70:568–75. doi: 10.1111/all.12602. [DOI] [PubMed] [Google Scholar]
- 24.Ahluwalia J, Abuabara K, Perman MJ, et al. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172:1090–5. doi: 10.1111/bjd.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiou CC, Chung WH, Hung SI, et al. Fulminant type 1 diabetes mellitus caused by drug hypersensitivity syndrome with human herpesvirus 6 infection. J Am Acad Dermatol. 2006;54(Suppl 2):S14–7. doi: 10.1016/j.jaad.2005.03.057. [DOI] [PubMed] [Google Scholar]
- 26.Aota N, Hirahara K, Kano Y, et al. Systemic lupus erythematosus presenting with Kikuchi-Fujimoto’s disease as a long-term sequela of drug-induced hypersensitivity syndrome. A possible role of Epstein-Barr virus reactivation Dermatology. 2009;218:275–7. doi: 10.1159/000187619. [DOI] [PubMed] [Google Scholar]
- 27.Funck-Brentano E, Duong T, Family D, et al. Auto-immune thyroiditis and drug reaction with eosinophilia and systemic symptoms (DRESS) associated with HHV-6 viral reactivation. Ann Dermatol Venereol. 2011;138:580–5. doi: 10.1016/j.annder.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 28.Seishima M, Yamanaka S, Fujisawa T, et al. Reactivation of human herpesvirus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2006;155:344–9. doi: 10.1111/j.1365-2133.2006.07332.x. [DOI] [PubMed] [Google Scholar]
- 29.Kano Y, Hiraharas K, Sakuma K, et al. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol. 2006;155:301–6. doi: 10.1111/j.1365-2133.2006.07238.x. [DOI] [PubMed] [Google Scholar]
- 30.Harding DJ, Subramaniam K, MacQuillan G, et al. Severe drug-induced hypersensitivity syndrome with a shared HLA-B allele. Med J Aust. 2012;197:411–3. doi: 10.5694/mja12.10477. [DOI] [PubMed] [Google Scholar]
- 31.Shiohara T, Iijima M, Ikezawa Z, et al. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007;156:1083–4. doi: 10.1111/j.1365-2133.2007.07807.x. [DOI] [PubMed] [Google Scholar]
- 32.Almudimeegh A, Rioux C, Ferrand H, et al. Drug reaction with eosinophilia and systemic symptoms, or virus reactivation with eosinophilia and systemic symptoms as a manifestation of immune reconstitution inflammatory syndrome in a patient with HIV? Br J Dermatol. 2014;171:895–8. doi: 10.1111/bjd.13079. [DOI] [PubMed] [Google Scholar]
- 33.Kano Y, Ushigome Y, Horie C, et al. Immune reconstitution inflammatory syndrome observed in the setting of drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS) Clin Transl Allergy. 2014;4:148. [Google Scholar]
- 34.Peppercorn AF, Miller MB, Fitzgerald D, et al. High-level human herpesvirus-6 viremia associated with onset of Stevens-Johnson syndrome: report of two cases. J Burn Care Res. 2010;31:365–8. doi: 10.1097/BCR.0b013e3181d0f48b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teraki Y, Murota H, Izaki S. Toxic epidermal necrolysis due to zonisamide associated with reactivation of human herpesvirus 6. Arch Dermatol. 2008;144:232–5. doi: 10.1001/archdermatol.2007.48. [DOI] [PubMed] [Google Scholar]
- 36.Haro-Gabaldon V, Sanchez-Sanchez-Vizcaino J, Ruiz-Avila P, et al. Acute generalized exanthematous pustulosis with cytomegalovirus infection. Int J Dermatol. 1996;35:735–7. doi: 10.1111/j.1365-4362.1996.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 37.Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) Br J Dermatol. 2007;157:989–96. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 38.Eshki M, Allanore L, Musette P, et al. Twelve-year analysis of severe cases of drug reaction with eosinophilia and systemic symptoms: a cause of unpredictable multiorgan failure. Arch Dermatol. 2009;145:67–72. doi: 10.1001/archderm.145.1.67. [DOI] [PubMed] [Google Scholar]
- 39.Aung AK, Haas DW, Hulgan T, et al. Pharmacogenomics of antimicrobial agents. Pharmacogenomics. 2014;15:1903–30. doi: 10.2217/pgs.14.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Symonds W, Cutrell A, Edwards M, et al. Risk factor analysis of hypersensitivity reactions to abacavir. Clin Ther. 2002;24:565–73. doi: 10.1016/s0149-2918(02)85132-3. [DOI] [PubMed] [Google Scholar]
- 41.Mallal S, Nolan D, Witt C, et al. Association between presence of HLA-B* 5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–32. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 42.Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–2. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 43.Shear NH, Milpied B, Bruynzeel DP, et al. A review of drug patch testing and implications for HIV clinicians. AIDS. 2008;22:999–1007. doi: 10.1097/QAD.0b013e3282f7cb60. [DOI] [PubMed] [Google Scholar]
- 44.Phillips EJ, Wong GA, Kaul R, et al. Clinical and immunogenetic correlates of abacavir hypersensitivity. AIDS. 2005;19:979–81. doi: 10.1097/01.aids.0000171414.99409.fb. [DOI] [PubMed] [Google Scholar]
- 45.Mallal S, Phillips E, Carosi G, et al. HLA-B* 5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–79. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 46.Saag M, Balu R, Phillips E, et al. High sensitivity of human leukocyte antigen-b* 5701 as a marker for immunologically confirmed abacavir hypersensitivity in white and black patients. Clin Infect Dis. 2008;46:1111–8. doi: 10.1086/529382. [DOI] [PubMed] [Google Scholar]
- 47.Phillips E, Mallal S. Successful translation of pharmacogenetics into the clinic. Mol Diagn Ther. 2009;13:1–9. doi: 10.1007/BF03256308. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz-Iruela C, Padullés-Zamora N, Podzamczer-Palter D, et al. HLA-B* 57: 01 genotyping in the prevention of hypersensitivity to abacavir: 5 years of experience. Pharmacogenet Genomics. 2016;26:390–6. doi: 10.1097/FPC.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 49.Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–15. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 51.Yip V, Marson A, Jorgensen A, et al. HLA genotype and carbamazepine-induced cutaneous adverse drug reactions: a systematic reveiw. Clin Pharmacol Ther. 2012;92:757–65. doi: 10.1038/clpt.2012.189. [DOI] [PubMed] [Google Scholar]
- 52.Ozeki T, Mushiroda T, Yowang A, et al. Genome-wide association study identifies HLA-A* 3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Gen. 2011;20:1034–41. doi: 10.1093/hmg/ddq537. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda H, Takahashi Y, Yamazaki E, et al. HLA Class I markers in Japanese patients with carbamazepine-induced cutaneous adverse reactions. Epilepsia. 2010;51:297–300. doi: 10.1111/j.1528-1167.2009.02269.x. [DOI] [PubMed] [Google Scholar]
- 54.Kaniwa N, Saito Y, Aihara M, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9:1617–22. doi: 10.2217/14622416.9.11.1617. [DOI] [PubMed] [Google Scholar]
- 55.Kim SH, Lee KW, Song WJ, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011;97:190–7. doi: 10.1016/j.eplepsyres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Lonjou C, Thomas L, Borot N, et al. A marker for Stevens-Johnson syndrome: ethnicity matters. Pharmacogenomics J. 2006;6:265–8. doi: 10.1038/sj.tpj.6500356. [DOI] [PubMed] [Google Scholar]
- 57.Alfirevic A, Jorgensen AL, Williamson PR, et al. HLA-B locus in Caucasian patients with carbamazepine hypersensitivity. Pharmacogenomics. 2006;7:813–8. doi: 10.2217/14622416.7.6.813. [DOI] [PubMed] [Google Scholar]
- 58.Amstutz U, Shear NH, Rieder MJ, et al. Recommendations for HLA-B* 15:02 and HLA-A* 31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014;55:496–506. doi: 10.1111/epi.12564. [DOI] [PubMed] [Google Scholar]
- 59.Chen P, Lin JJ, Lu CS, et al. Carbamazepine-induced toxic effects and HLA-B* 1502 screening in Taiwan. N Engl J Med. 2011;364:1126–33. doi: 10.1056/NEJMoa1009717. [DOI] [PubMed] [Google Scholar]
- 60.Locharernkul C, Shotelersuk V, Hirankarn N. HLA-B* 1502 screening: time to clinical practice. Epilepsia. 2010;51:936–8. doi: 10.1111/j.1528-1167.2010.02549.x. [DOI] [PubMed] [Google Scholar]
- 61.McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A* 3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364:1134–43. doi: 10.1056/NEJMoa1013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16:297–306. doi: 10.1097/01.fpc.0000199500.46842.4a. [DOI] [PubMed] [Google Scholar]
- 63.Genin E, Chen D, Hung S, et al. HLA-A* 31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: an international study and meta-analysis. Pharmacogenomics J. 2014;14:281–8. doi: 10.1038/tpj.2013.40. [DOI] [PubMed] [Google Scholar]
- 64.Ferrell PB, McLeod HL. Carbamazepine, HLA-B* 1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008;9:1543–6. doi: 10.2217/14622416.9.10.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens–Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333:1600–8. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- 66.Hung SI, Chung WH, Liou LB, et al. HLA-B* 5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA. 2005;102:4134–9. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng L, Xiong Y, Qin C, et al. HLA-B* 58:01 is strongly associated with allopurinol-induced severe cutaneous adverse reactions in Han Chinese patients: a multicentre retrospective case–control clinical study. Br J Dermatol. 2015;173:555–8. doi: 10.1111/bjd.13688. [DOI] [PubMed] [Google Scholar]
- 68.Cao ZH, Wei ZY, Zhu QY, et al. HLA-B* 58:01 allele is associated with augmented risk for both mild and severe cutaneous adverse reactions induced by allopurinol in Han Chinese. Pharmacogenomics. 2012;13:1193–201. doi: 10.2217/pgs.12.89. [DOI] [PubMed] [Google Scholar]
- 69.Chiu M, Hu M, Ng M, et al. Association between HLA-B* 58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br J Dermatol. 2012;167:44–9. doi: 10.1111/j.1365-2133.2012.10894.x. [DOI] [PubMed] [Google Scholar]
- 70.Tassaneeyakul W, Jantararoungtong T, Chen P, et al. Strong association between HLA-B* 5801 and allopurinol-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenetic Genomics. 2009;19:704–9. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- 71.Jung JW, Song WJ, Kim YS, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. 2011;26:3567–72. doi: 10.1093/ndt/gfr060. [DOI] [PubMed] [Google Scholar]
- 72.Kang HR, Jee YK, Kim YS, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011;21:303–7. doi: 10.1097/FPC.0b013e32834282b8. [DOI] [PubMed] [Google Scholar]
- 73.Lonjou C, Borot N, Sekula P, et al. A European study of HLA-B in Stevens–Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18:99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 74.Gonçalo M, Coutinho I, Teixeira V, et al. HLA-B* 58:01 is a risk factor for allopurinol-induced DRESS and Stevens–Johnson syndrome/toxic epidermal necrolysis in a Portuguese population. Br J Dermatol. 2013;169:660–5. doi: 10.1111/bjd.12389. [DOI] [PubMed] [Google Scholar]
- 75.Ko TM, Tsai CY, Chen SY, et al. Use of HLA-B* 58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. Br Med J. 2015;351:4848. doi: 10.1136/bmj.h4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong D, Tan-Koi WC, Teng GG, et al. Cost–effectiveness analysis of genotyping for HLA-B* 5801 and an enhanced safety program in gout patients starting allopurinol in Singapore. Pharmacogenomics. 2015;16:1781–93. doi: 10.2217/pgs.15.125. [DOI] [PubMed] [Google Scholar]
- 77.Cheng L, Zhang L, Gao L, et al. Genotyping HLA-B* 5801 for allopurinol-induced severe cutaneous adverse reactions: an accurate and prompt method. Clin Transl Sci. 2015;8:834–6. doi: 10.1111/cts.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trubiano JA, Aung AK, Nguyen M, et al. A comparative analysis between antibiotic- and nonantibiotic-associated delayed cutaneous adverse drug reactions. J Allergy Clin Immunol Pract. 2016;4:1187–93. doi: 10.1016/j.jaip.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 79.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 80.Cho YT, Liau JY, Chang CY, et al. Co-existence of histopathological features is characteristic in drug reaction with eosinophilia and systemic symptoms and correlates with high grades of cutaneous abnormalities. J Eur Acad Dermatol Venereol. 2016;30:2077–84. doi: 10.1111/jdv.13728. [DOI] [PubMed] [Google Scholar]
- 81.Yawalkar N, Pichler WJ. Immunohistology of drug-induced exanthema: clues to pathogenesis. Curr Opin Allergy Clin Immunol. 2001;1:299–303. doi: 10.1097/01.all.0000011030.52248.1b. [DOI] [PubMed] [Google Scholar]
- 82.Rive CM, Bourke J, Phillips EJ. Testing for drug hypersensitivity syndromes. Clin Biochem Rev. 2013;34:15–38. [PMC free article] [PubMed] [Google Scholar]
- 83.Brockow K, Garvey LH, Aberer W, et al. Skin test concentrations for systemically administered drugs - an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68:702–12. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 84.Brockow K, Romano A, Blanca M, et al. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. 2002;57:45–51. [PubMed] [Google Scholar]
- 85.Pinho A, Coutinho I, Gameiro A, et al. Patch testing - a valuable tool for investigating non-immediate cutaneous adverse drug reactions to antibiotics. J Eur Acad Dermatol Venereol. 2016;31:280–7. doi: 10.1111/jdv.13796. [DOI] [PubMed] [Google Scholar]
- 86.Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555–62. doi: 10.1111/bjd.12125. [DOI] [PubMed] [Google Scholar]
- 87.Hassoun-Kheir N, Bergman R, Weltfriend S. The use of patch tests in the diagnosis of delayed hypersensitivity drug eruptions. Int J Dermatol. 2016;55:1219–24. doi: 10.1111/ijd.13306. [DOI] [PubMed] [Google Scholar]
- 88.Charfi O, Lakhoua G, Sahnoun R, et al. DRESS syndrome following levofloxacin exposure with positive patch-test. Therapie. 2015;70:547–9. doi: 10.2515/therapie/2015046. [DOI] [PubMed] [Google Scholar]
- 89.Fathallah N, Slim R, Rached S, et al. Carbamazepine-induced DRESS with severe eosinophilia confirmed by positive patch test. Dermatitis. 2014;25:282–4. doi: 10.1097/DER.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 90.Santiago F, Goncalo M, Vieira R, et al. Epicutaneous patch testing in drug hypersensitivity syndrome (DRESS) Contact Dermatitis. 2010;62:47–53. doi: 10.1111/j.1600-0536.2009.01659.x. [DOI] [PubMed] [Google Scholar]
- 91.Tchen T, Reguiai Z, Vitry F, et al. Usefulness of skin testing in cutaneous drug eruptions in routine practice. Contact Dermatitis. 2009;61:138–44. doi: 10.1111/j.1600-0536.2009.01578.x. [DOI] [PubMed] [Google Scholar]
- 92.Lehloenya RJ, Todd G, Wallace J, et al. Diagnostic patch testing following tuberculosis-associated cutaneous adverse drug reactions induces systemic reactions in HIV-infected persons. Br J Dermatol. 2016;175:150–6. doi: 10.1111/bjd.14492. [DOI] [PubMed] [Google Scholar]
- 93.Shebe K, Ngwanya MR, Gantsho N, et al. Severe recurrence of drug rash with eosinophilia and systemic symptoms syndrome secondary to rifampicin patch testing in a human immunodeficiency virus-infected man. Contact Dermatitis. 2014;70:125–7. doi: 10.1111/cod.12155. [DOI] [PubMed] [Google Scholar]
- 94.Bensaid B, Rozieres A, Nosbaum A, et al. Amikacin-induced drug reaction with eosinophilia and systemic symptoms syndrome: delayed skin test and ELISPOT assay results allow the identification of the culprit drug. J Allergy Clin Immunol. 2012;130:1413–4. doi: 10.1016/j.jaci.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 95.Giorgini S, Martinelli C, Tognetti L, et al. Use of patch testing for the diagnosis of abacavir-related hypersensitivity reaction in HIV patients. Dermatol Ther. 2011;24:591–4. doi: 10.1111/j.1529-8019.2012.01409.x. [DOI] [PubMed] [Google Scholar]
- 96.Lin YT, Chang YC, Hui RC, et al. A patch testing and cross-sensitivity study of carbamazepine-induced severe cutaneous adverse drug reactions. J Eur Acad Dermatol Venereol. 2013;27:356–64. doi: 10.1111/j.1468-3083.2011.04418.x. [DOI] [PubMed] [Google Scholar]
- 97.Demoly P, Adkinson NF, Brockow K, et al. International Consensus on drug allergy. Allergy. 2014;69:420–37. doi: 10.1111/all.12350. [DOI] [PubMed] [Google Scholar]
- 98.Federico A, Aitella E, Sgambato D, et al. Telaprevir may induce adverse cutaneous reactions by a T cell immune-mediated mechanism. Ann Hepatol. 2015;14:420–4. [PubMed] [Google Scholar]
- 99.Cabanas R, Calderon O, Ramirez E, et al. Piperacillin-induced DRESS: distinguishing features observed in a clinical and allergy study of 8 patients. J Investig Allergol Clin Immunol. 2014;24:425–30. [PubMed] [Google Scholar]
- 100.Arruti N, Villarreal O, Bernedo N, et al. Positive allergy study (intradermal, patch, and lymphocyte transformation tests) in a case of isoniazid-induced DRESS. J Investig Allergol Clin Immunol. 2016;26:119–20. doi: 10.18176/jiaci.0025. [DOI] [PubMed] [Google Scholar]
- 101.Perrin-Lamarre A, Petitpain N, Trechot P, et al. Glycopeptide-induced cutaneous adverse reaction: results of an immunoallergic investigation in eight patients. Ann Dermatol Venereol. 2010;137:101–5. doi: 10.1016/j.annder.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Torres MJ, Sanchez-Sabate E, Alvarez J, et al. Skin test evaluation in nonimmediate allergic reactions to penicillins. Allergy. 2004;59:219–24. doi: 10.1046/j.1398-9995.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 103.Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy Asthma and Immunology et al. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–73. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 104.Kano Y, Hirahara K, Mitsuyama Y, et al. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy. 2007;62:1439–44. doi: 10.1111/j.1398-9995.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 105.Nagao-Dias AT, Teixeira FM, Coelho HL. Diagnosing immune-mediated reactions to drugs. Allergol Immunopathol (Madr) 2009;37:98–104. doi: 10.1016/s0301-0546(09)71112-7. [DOI] [PubMed] [Google Scholar]
- 106.Fu M, Gao Y, Pan Y, et al. Recovered patients with Stevens-Johson syndrome and toxic epidermal necrolysis maintain long-lived IFN-gamma and sFasL memory response. PLoS One. 2012;7:e45516. doi: 10.1371/journal.pone.0045516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jurado-Palomo J, Cabanas R, Prior N, et al. Use of the lymphocyte transformation test in the diagnosis of DRESS syndrome induced by ceftriaxone and piperacillin-tazobactam: two case reports. J Investig Allergol Clin Immunol. 2010;20:433–6. [PubMed] [Google Scholar]
- 108.Gomez E, Torres MJ, Mayorga C, et al. Immunologic evaluation of drug allergy. Allergy Asthma Immunol Res. 2012;4:251–63. doi: 10.4168/aair.2012.4.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic reactions induced by drugs: pathogenesis and diagnostic tests. J Investig Allergol Clin Immunol. 2009;19:80–90. [PubMed] [Google Scholar]
- 110.Beeler A, Zaccaria L, Kawabata T, et al. CD69 upregulation on T cells as an in vitro marker for delayed-type drug hypersensitivity. Allergy. 2008;63:181–8. doi: 10.1111/j.1398-9995.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 111.Britschgi M, Pichler WJ. Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr Opin Allergy Clin Immunol. 2002;2:325–31. doi: 10.1097/00130832-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 112.Dias de Castro E, Leblanc A, Sarmento A, et al. An unusual case of delayed-type hypersensitivity to ceftriaxone and meropenem. Eur Ann Allergy Clin Immunol. 2015;47:225–7. [PubMed] [Google Scholar]
- 113.El-Ghaiesh S, Monshi MM, Whitaker P, et al. Characterization of the antigen specificity of T-cell clones from piperacillin-hypersensitive patients with cystic fibrosis. J Pharmacol Exp Ther. 2012;341:597–610. doi: 10.1124/jpet.111.190900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kardaun SH, de Monchy JG. Acute generalized exanthematous pustulosis caused by morphine, confirmed by positive patch test and lymphocyte transformation test. J Am Acad Dermatol. 2006;55(Suppl. 2):S21–3. doi: 10.1016/j.jaad.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 115.Ogasawara K, Tomitsuka N, Kobayashi M, et al. Stevens-Johnson syndrome associated with intravenous acetazolamide administration for evaluation of cerebrovascular reactivity. Case report. Neurol Med Chir (Tokyo) 2006;46:161–3. doi: 10.2176/nmc.46.161. [DOI] [PubMed] [Google Scholar]
- 116.Kanny G, Pichler W, Morisset M, et al. T cell-mediated reactions to iodinated contrast media: evaluation by skin and lymphocyte activation tests. J Allergy Clin Immunol. 2005;115:179–85. doi: 10.1016/j.jaci.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 117.Romano A, Torres MJ, Di Fonso M, et al. Delayed hypersensitivity to cefazolin: report on a case involving lymphocyte transformation studies with different cephalosporins. Ann Allergy Asthma Immunol. 2001;87:238–42. doi: 10.1016/S1081-1206(10)62233-8. [DOI] [PubMed] [Google Scholar]
- 118.Keane NM, Pavlos RK, McKinnon E, et al. HLA Class I restricted CD8+ and Class II restricted CD4+ T cells are implicated in the pathogenesis of nevirapine hypersensitivity. AIDS. 2014;28:1891–901. doi: 10.1097/QAD.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 119.Keane NM, Roberts SG, Almeida CA, et al. High-avidity, high-IFNgamma-producing CD8 T-cell responses following immune selection during HIV-1 infection. Immunol Cell Biol. 2012;90:224–34. doi: 10.1038/icb.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Esser S, Jablonka R, Heinemann FM, et al. Detection of abacavir hypersensitivity by ELISpot method. Inflamm Allergy Drug Targets. 2012;11:227–34. doi: 10.2174/187152812800392751. [DOI] [PubMed] [Google Scholar]
- 121.Lucas A, Lucas M, Strhyn A, et al. Abacavir-reactive memory T cells are present in drug naive individuals. PLoS One. 2015;10:e0117160. doi: 10.1371/journal.pone.0117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rozieres A, Hennino A, Rodet K, et al. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy. 2009;64:534–42. doi: 10.1111/j.1398-9995.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- 123.Khalil G, El-Sabban M, Al-Ghadban S, et al. Cytokine expression profile of sensitized human T lymphocytes following in vitro stimulation with amoxicillin. Eur Cytokine Netw. 2008;19:131–41. doi: 10.1684/ecn.2008.0132. [DOI] [PubMed] [Google Scholar]
- 124.Scheibenbogen C, Romero P, Rivoltini L, et al. Quantitation of antigen-reactive T cells in peripheral blood by IFNgamma-ELISPOT assay and chromium-release assay: a four-centre comparative trial. J Immunol Methods. 2000;244:81–9. doi: 10.1016/s0022-1759(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 125.Porebski G, Pecaric-Petkovic T, Groux-Keller M, et al. In vitro drug causality assessment in Stevens-Johnson syndrome - alternatives for lymphocyte transformation test. Clin Exp Allergy. 2013;43:1027–37. doi: 10.1111/cea.12145. [DOI] [PubMed] [Google Scholar]
- 126.Yun J, Marcaida MJ, Eriksson KK, et al. Oxypurinol directly and immediately activates the drug-specific T cells via the preferential use of HLA-B*58:01. J Immunol. 2014;192:2984–93. doi: 10.4049/jimmunol.1302306. [DOI] [PubMed] [Google Scholar]
- 127.Yun J, Mattsson J, Schnyder K, et al. Allopurinol hypersensitivity is primarily mediated by dose-dependent oxypurinol-specific T cell response. Clin Exp Allergy. 2013;43:1246–55. doi: 10.1111/cea.12184. [DOI] [PubMed] [Google Scholar]
- 128.Schrijvers R, Gilissen L, Chiriac AM, et al. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Transl Allergy. 2015;5:31. doi: 10.1186/s13601-015-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elzagallaai AA, Knowles SR, Rieder MJ, et al. In vitro testing for the diagnosis of anticonvulsant hypersensitivity syndrome: a systematic review. Mol Diagn Ther. 2009;13:313–30. doi: 10.1007/BF03256336. [DOI] [PubMed] [Google Scholar]
- 130.Tang YH, Mockenhaupt M, Henry A, et al. Poor relevance of a lymphocyte proliferation assay in lamotrigine-induced Stevens-Johnson syndrome or toxic epidermal necrolysis. Clin Exp Allergy. 2012;42:248–54. doi: 10.1111/j.1365-2222.2011.03875.x. [DOI] [PubMed] [Google Scholar]
- 131.Polak ME, Belgi G, McGuire C, et al. In vitro diagnostic assays are effective during the acute phase of delayed-type drug hypersensitivity reactions. Br J Dermatol. 2013;168:539–49. doi: 10.1111/bjd.12109. [DOI] [PubMed] [Google Scholar]
- 132.Haw WY, Polak ME, McGuire C, et al. In vitro rapid diagnostic tests for severe drug hypersensitivity reactions in children. Ann Allergy Asthma Immunol. 2016;117:61–6. doi: 10.1016/j.anai.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 133.Mizukawa Y, Yamazaki Y, Teraki Y, et al. Direct evidence for interferon-gamma production by effector-memory-type intraepidermal T cells residing at an effector site of immunopathology in fixed drug eruption. Am J Pathol. 2002;161:1337–47. doi: 10.1016/s0002-9440(10)64410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mayorga C, Sanz ML, Gamboa P, et al. In vitro methods for diagnosing nonimmediate hypersensitivity reactions to drugs. J Investig Allergol Clin Immunol. 2013;23:213–25. [PubMed] [Google Scholar]
- 135.Almeida CA, Martin AM, Nolan D, et al. Cytokine profiling in abacavir hypersensitivity patients. Antivir Ther. 2008;13:281–8. [PubMed] [Google Scholar]
- 136.Nishio D, Izu K, Kabashima K, et al. T cell populations propagating in the peripheral blood of patients with drug eruptions. J Dermatol Sci. 2007;48:25–33. doi: 10.1016/j.jdermsci.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 137.Romano A, Gaeta F, Valluzzi RL, et al. Cross-reactivity and tolerability of aztreonam and cephalosporins in subjects with a T cell–mediated hypersensitivity to penicillins. J Allergy Clin Immunol. 2016;138:179–86. doi: 10.1016/j.jaci.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 138.Buonomo A, Nucera E, Pecora V, et al. Cross-reactivity and tolerability of cephalosporins in patients with cell-mediated allergy to penicillins. J Investig Allergol Clin Immunol. 2014;24:331–7. [PubMed] [Google Scholar]
- 139.Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to β-lactams. Allergy. 2009;64:1644–8. doi: 10.1111/j.1398-9995.2009.02058.x. [DOI] [PubMed] [Google Scholar]
- 140.Romano A, Gaeta F, Valluzzi R, et al. Absence of cross-reactivity to carbapenems in patients with delayed hypersensitivity to penicillins. Allergy. 2013;68:1618–21. doi: 10.1111/all.12299. [DOI] [PubMed] [Google Scholar]
- 141.Romano A, Viola M, Guéant-Rodriguez RM, et al. Imipenem in patients with immediate hypersensitivity to penicillins. N Engl J Med. 2006;354:2835–7. doi: 10.1056/NEJMc053529. [DOI] [PubMed] [Google Scholar]
- 142.Atanasković-Marković M, Gaeta F, Medjo B, et al. Tolerability of meropenem in children with IgE-mediated hypersensitivity to penicillins. Allergy. 2008;63:237–40. doi: 10.1111/j.1398-9995.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 143.Buonomo A, Nucera E, De Pasquale T, et al. Tolerability of aztreonam in patients with cell-mediated allergy to β-lactams. Int Arch Allergy Immunol. 2010;155:155–9. doi: 10.1159/000318844. [DOI] [PubMed] [Google Scholar]
- 144.Aihara M. Pharmacogenetics of cutaneous adverse drug reactions. J Dermatol. 2011;38:246–54. doi: 10.1111/j.1346-8138.2010.01196.x. [DOI] [PubMed] [Google Scholar]
- 145.Romano A, Pettinato R, Andriolo M, et al. Hypersensitivity to aromatic anticonvulsants: in vivo and in vitro cross-reactivity studies. Curr Pharm Des. 2006;12:3373–81. doi: 10.2174/138161206778193962. [DOI] [PubMed] [Google Scholar]
- 146.Krivoy N, Taer M, Neuman MG. Antiepileptic drug-induced hypersensitivity syndrome reactions. Curr Drug Saf. 2006;1:289–99. doi: 10.2174/157488606777934459. [DOI] [PubMed] [Google Scholar]
- 147.Chung WH, Chang WC, Lee YS, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. 2014;312:525–34. doi: 10.1001/jama.2014.7859. [DOI] [PubMed] [Google Scholar]
- 148.Sierra NM, Garcia B, Marco J, et al. Cross hypersensitivity syndrome between phenytoin and carbamazepine. Pharm World Sci. 2005;27:170–4. doi: 10.1007/s11096-004-1736-z. [DOI] [PubMed] [Google Scholar]
- 149.Wang X, Lang S, Shi X, et al. Cross-reactivity of skin rashes with current antiepileptic drugs in Chinese population. Seizure. 2010;19:562–6. doi: 10.1016/j.seizure.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 150.Seitz CS, Pfeuffer P, Raith P, et al. Anticonvulsant hypersensitivity syndrome: cross-reactivity with tricyclic antidepressant agents. Ann Allergy Asthma Immunol. 2006;97:698–702. doi: 10.1016/S1081-1206(10)61103-9. [DOI] [PubMed] [Google Scholar]
- 151.Man CB, Kwan P, Baum L, et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia. 2007;48:1015–8. doi: 10.1111/j.1528-1167.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 152.He N, Min FL, Shi YW, et al. Cutaneous reactions induced by oxcarbazepine in Southern Han Chinese: incidence, features, risk factors and relation to HLA-B alleles. Seizure. 2012;21:614–8. doi: 10.1016/j.seizure.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 153.Yang CY, Dao RL, Lee TJ, et al. Severe cutaneous adverse reactions to antiepileptic drugs in Asians. Neurology. 2011;77:2025–33. doi: 10.1212/WNL.0b013e31823b478c. [DOI] [PubMed] [Google Scholar]
- 154.Alvestad S, Lydersen S, Brodtkorb E. Cross-reactivity pattern of rash from current aromatic antiepileptic drugs. Epilepsy Res. 2008;80:194–200. doi: 10.1016/j.eplepsyres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 155.Viard I, Wehrli P, Bullani R, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–3. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- 156.Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Chem Immunol Allergy. 2012;97:1–17. doi: 10.1159/000335612. [DOI] [PubMed] [Google Scholar]
- 157.Roujeau JC. Stevens-Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol. 1997;24:726–9. doi: 10.1111/j.1346-8138.1997.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 158.Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69:173.e1–13. doi: 10.1016/j.jaad.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 159.Mockenhaupt M. The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Expert Rev Clin Immunol. 2011;7:803–13. doi: 10.1586/eci.11.66. [DOI] [PubMed] [Google Scholar]
- 160.Walsh S, Diaz-Cano S, Higgins E, et al. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391–401. doi: 10.1111/bjd.12081. [DOI] [PubMed] [Google Scholar]
- 161.Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50–8. doi: 10.1111/bjd.13683. [DOI] [PubMed] [Google Scholar]
- 162.Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): A review and update. J Am Acad Dermatol. 2015;73:843–8. doi: 10.1016/j.jaad.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 163.Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87–92. doi: 10.1111/j.1440-0960.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 164.Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;2015:260928. doi: 10.1155/2015/260928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Speeckaert MM, Speeckaert R, Lambert J, et al. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts. Eur J Dermatol. 2010;20:425–33. doi: 10.1684/ejd.2010.0932. [DOI] [PubMed] [Google Scholar]
- 166.Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322–8. doi: 10.1097/ACI.0b013e32832cf64e. [DOI] [PubMed] [Google Scholar]
- 167.Amstutz U, Ross CJ, Castro-Pastrana LI, et al. HLA-A* 31:01 and HLA-B* 15:02 as Genetic Markers for Carbamazepine Hypersensitivity in Children. Clin Pharmacol Ther. 2013;94:142–9. doi: 10.1038/clpt.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cheung YK, Cheng SH, Chan EJ, et al. HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia. 2013;54:1307–14. doi: 10.1111/epi.12217. [DOI] [PubMed] [Google Scholar]
- 169.Khanna D, Khanna PP, FitzGerald JD, et al. 2012 American College of Rheumatology Guidelines for Management of Gout Part II: Therapy and Anti-inflammatory Prophylaxis of Acute Gouty Arthritis. Arthritis Care Res. 2012;64:1447–61. doi: 10.1002/acr.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Arimone Y, Begaud B, Miremont-Salame G, et al. Agreement of expert judgment in causality assessment of adverse drug reactions. Eur J Clin Pharmacol. 2005;61:169–73. doi: 10.1007/s00228-004-0869-2. [DOI] [PubMed] [Google Scholar]
- 171.Hayashi PH. Drug-Induced Liver Injury Network Causality Assessment: Criteria and Experience in the United States. Int J Mol Sci. 2016;17:201. doi: 10.3390/ijms17020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Agbabiaka TB, Savovic J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf. 2008;31:21–37. doi: 10.2165/00002018-200831010-00003. [DOI] [PubMed] [Google Scholar]
- 173.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 174.Trubiano J, Phillips E. Antimicrobial stewardship’s new weapon? A review of antibiotic allergy and pathways to ‘de-labeling’. Curr Opin Infect Dis. 2013;26:526–37. doi: 10.1097/QCO.0000000000000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pavlos R, Mallal S, Ostrov D, et al. T cell-mediated hypersensitivity reactions to drugs. Annu Rev Med. 2015;66:439–54. doi: 10.1146/annurev-med-050913-022745. [DOI] [PMC free article] [PubMed] [Google Scholar]


