Abstract
Background
The use of selective internal radiation therapy with yttrium 90 resin microspheres (SIR-Spheres®) in chemotherapy-resistant colorectal cancer liver metastases has been associated with favorable progression-free survival (PFS) and overall survival when given alone or concurrently with chemotherapy. We conducted a single institute retrospective trial to explore the potential impact of SIR-Spheres® with concurrent chemotherapy vs. SIR-Spheres® alone on liver PFS in patients with colorectal liver metastases (CRLM).
Methods
Patients with 5-fluorouracil-refractory CRLM treated with SIR-Spheres® between 2009 and 2014 were identified. Patients were excluded if they received any chemotherapy/targeted regimen following radioembolization on which they did not previously progress. This strategy was adopted to minimize the impact of post-SIR-Spheres® systemic therapy bias on PFS.
Results
Twenty-seven patients satisfied inclusion criteria and were included in this analysis. Patients’ demographics were similar between the two treatment arms, except for the median number of prior therapies. No associated ≥ grade 3 toxicities were noted. Liver disease control rates were 84% and 14% on the SIR-Spheres® plus chemotherapy arms and SIR-Spheres® alone arms, respectively (P=0.001). Median PFS in the liver was 176 days in the SIR-Spheres® plus chemotherapy group vs. 91 days in the SIR-Sphere® alone group (P=0.0009). Median overall survival was 212 days in the SIR-Spheres® plus chemotherapy group vs. 154 days in the SIR-Spheres® alone group (P=0.1023).
Conclusions
In patients with 5-fluorouracil-refractory disease, SIR-Spheres® plus chemotherapy is associated with an increased liver disease control rate and a prolonged liver PFS in comparison with SIR-Spheres® alone.
Keywords: SIR-Spheres®, concurrent chemotherapy, colorectal liver metastases
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related mortality in the United States. It is estimated that 20% of patients with CRC present with metastatic disease while another 30% develop metastatic disease after an initial presentation with local or regional disease (1). The liver is the most common CRC metastatic site and is the only site of disease for 30% of patients (2). Moreover, two-thirds of CRC deaths are due to liver metastases (2). Liver resection significantly prolongs overall survival in patients with colorectal liver metastases (CRLM), however, only 20% of CRLM patients are eligible for liver resection (3-5) and more than 50% experience hepatic recurrence (2). Patients with unresectable CRLM are candidates for a variety of liver directed therapies to improve hepatic disease control, and ultimately delay hepatic failure (6).
Hepatic radioembolization, or selective internal radiation therapy (SIRT), delivers yttrium-90 (Y-90) microspheres into liver metastases. Y-90 beads are injected into the main hepatic artery circulation and then lodge at the tumor arterioles where they emit high energy beta-radiation to the surrounding tumor. SIR-Spheres® have been FDA approved for the treatment of unresectable liver metastases from primary CRC with adjuvant intra-hepatic artery chemotherapy.
SIR-Spheres® have been investigated in CRC patients with liver only or liver-predominant disease in the first-line, second-line, and refractory settings (7-12). While the addition of SIR-Spheres® to FOLFOX (± bevacizumab) in the first-line setting was associated with an improvement in hepatic response rate and prolongation in hepatic progression-free survival, its contributions to improvement in overall survival remain largely unknown (13). In contrast, several single-arm and randomized clinical trials have shown favorable survival results for SIR-Spheres® alone or with concurrent chemotherapy in refractory colorectal cancer (7,12,14,15). However, the value of adding concurrent chemotherapy to SIR-Spheres® over SIR-Spheres® alone has not been adequately investigated.
In this study, we retrospectively explored the potential impact of adding concurrent chemotherapy to SIR-Spheres® by comparing liver progression-free survival in refractory CRC patients who either received SIR-Spheres® alone (SIRT) or concurrent chemotherapy with SIR-Spheres® (SIRT-C).
Methods
Patient selection
With institutional review board approval, we retrospectively reviewed medical records of patients with refractory CRLM treated with SIR-Spheres® at our institution between 2009 and 2014. CRLM patients who received SIR-Spheres® as a first-line treatment were excluded. CRLM patients treated with SIRT or with SIRT-C were excluded if they received any chemotherapy/targeted regimen following radioembolization on which they did not previously progress. This strategy was adopted specifically to minimize the impact of post-SIR-Spheres® systemic therapy bias on liver progression-free survival outcome. The patients who received chemotherapy at outside institutions were also excluded. We compared the two treatment groups on patient characteristics including demographics, liver involvement pattern, KRAS status, and volume of extrahepatic disease. Types of concurrent chemotherapies, and post-SIR-Spheres® therapies were collected on both arms. The volume of extrahepatic disease was defined as low if it was: (I) fewer than five nodules which were less than 1 cm in size or a single nodule of less than 2 cm in lung; and/or (II) lymph node involvement as single anatomic size less than 2 cm in diameter. All patients had documented liver metastases progression prior to SIR-Spheres® treatment.
Treatment
Initial mapping angiogram was performed two weeks before the radioembolization procedure. Branch coil embolization was performed prophylactically where it was indicated. All patients were evaluated with a macroaggregated albumin scan to rule out hepatic-lung shunting. SIR-Spheres® were administered in the outpatient setting to either one or both sides of the liver. For some patients with bilobar disease or with borderline liver impairment, lobar treatments were performed in a sequential fashion, 4–6 weeks apart. Concurrent chemotherapy in the SIRT-C group included infusional 5-FU, capecitabine, irinotecan, or FOLFOX, as per treating physicians’ discretion. SIRT-C patients were eligible for analysis only if they were maintained on the same pre-SIR-Spheres® chemotherapy regimen following their radioembolization.
Response assessment
For each study arm best response was determined by applying RECIST 1.1 criteria to pre- and post-treatment PET/CT imaging. CT or PET scans were typically done 6 to 12 weeks after the procedure unless patients presented with significant symptoms or liver function test abnormalities. Liver progression-free survival (PFS) was defined as the time from first SIR-Spheres® treatment to the first progression in the treated lobe of liver and without any switch in pre-treatment systemic therapy for the SIRT-C group. Toxicities were collected from the medical record. Kaplan-Meier estimation with the log-rank test was used to compare groups on PFS and overall survival (OS) analysis. All statistics were performed using Stata/MP 13.1 (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics and treatment
Out of 54 metastatic colorectal cancer patients who received radioembolization at our institution between 2009 and 2014, 27 patients were included in this retrospective analysis. Twenty-seven patients were excluded for the following reasons: (I) 2 patients received SIR-Spheres® as their first-line therapy on a clinical trial; (II) 10 patients were from an outside hospital and follow-up images were unavailable; (III) 15 patients received chemotherapy/targeted regimens following radioembolization on which they did not previously progress. Out of the 27 eligible patients, 14 patients received SIRT alone; 13 patients received SIRT-C. Concurrent chemotherapy included infusional 5-FU, capecitabine, FOLFOX, or irinotecan. Median age was 62 and 63 for the SIRT and SIRT-C group, respectively. There were more RAS wild-type tumors in the SIRT group and more RAS mutant tumors in the SIRT-C group. As a result, patients in the SIRT arm had undergone a median of three lines of systemic therapy before SIR-Sphere® treatment vs. a median of two lines of systemic therapy in the SIRT-C arm (Table 1).
Table 1. Baseline characteristics of metastatic colorectal cancer patients.
| Patients’ characteristics | SIRT | SIRT-C |
|---|---|---|
| Number of patients | 14 | 13 |
| Mean age | 62 | 63 |
| Sex | ||
| Female | 4 | 5 |
| Male | 10 | 8 |
| Primary lesion | ||
| Rectal | 3 | 1 |
| Colon | 11 | 12 |
| Extrahepatic disease | ||
| None | 3 | 3 |
| Low | 4 | 3 |
| High | 7 | 7 |
| RAS status | ||
| Mutated | 4 | 10 |
| Wild Type | 10 | 3 |
| Median line of prior therapies | 3 | 2 |
| Concurrent chemotherapy with SIR-Spheres® | 0 | |
| 5-FU | 5 | |
| Capecitabine | 3 | |
| FOLFOX | 4 | |
| Irinotecan | 1 |
SIRT, SIR-Spheres® alone; SIRT-C, SIR-Spheres® with concurrent chemotherapy; 5-FU, 5-fluorouracil; FOLFOX, 5-FU, leucovorin, and oxaliplatin; FOLFIRI, 5-FU, leucovorin, and irinotecan.
Toxicity
The toxicity profile was similar for SIRT and SIRT-Cwith the most common toxicities grade 1–2 fatigue (5 vs. 6 patients, respectively), and grade 1–2 abdominal discomfort including epigastric pain (4 vs. 3 patients, respectively). One patient in the SIRT-C group developed upper gastrointestinal bleeding from a peptic ulcer one year after radioembolization. Elevated AST liver function tests (Grade 2 AST and ALT) were observed in one patient in the SIRT group immediately after radioembolization. In the SIRT-C group two patients developed cirrhosis on long-term follow-up, and one patient developed liver failure immediately after radioembolization. Grade 1–2 nausea and vomiting were observed in both SIRT and SIRT-C (1 vs. 3 patients, respectively). One patient in the SIRT group developed acalculous cholecystitis requiring cholecystectomy with final pathology showing resin beads in the inflamed gallbladder. One patient in the SIRT-C group also developed acalculous cholecystitis and recovered with conservative antibiotics alone. In general, most patients tolerated treatment well and toxicities were addressed with conservative management such as antiemetics and proton pump inhibitors (Table 2).
Table 2. Treatment-related adverse events (grades 1–2).
| Adverse events | SIRT | SIRT-C |
|---|---|---|
| Fatigue | 5 | 6 |
| Abdominal pain | 4 | 3 |
| Peptic ulcer disease | 0 | 1 |
| Nausea and vomiting | 1 | 3 |
| Liver function abnormality | 1 | 3 |
| Acalculous cholecystitis | 1 | 1 |
| Pleural effusion | 0 | 1 |
SIRT, SIR-Spheres® alone; SIRT-C, SIR-Spheres® with concurrent chemotherapy.
Response
Treatment response was evaluated based on available imaging studies 6–12 weeks after radioembolization. Best response was reported per RECIST 1.1 criteria. Disease control rates were 84% (2/13 partial response and 9/13 stable disease) on the SIRT-C arm and 14% (2/14 stable disease) on the SIRT arm (P=0.001, Table 3). Median PFS in the liver was 176 days in the SIRT-C group and 91 days in the SIRT group (P=0.0009, Figure 1). Median OS was 212 vs. 154 days in the SIRT-C and SIRT groups, respectively (P=0.1023, Figure 2).
Table 3. Efficacy of SIRT or SIRT-C.
| Cohorts | PD (%) | SD (%) | PR (%) | DCR* (%) | PFS+ (days) | OS# (days) |
|---|---|---|---|---|---|---|
| SIRT | 12/14 (85.7) | 2/14 (14.3) | 0/14 (0) | 2/14 (14.3) | 91 | 154 |
| SIRT-C | 2/13 (15.4) | 9/13 (69.2) | 2/13 (15.4) | 11/13 (84.6) | 176 | 212 |
PD, progression of disease; SD, stable disease; PR, partial response; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; SIRT, SIR-Spheres® alone; SIRT-C, SIR-Spheres® with concurrent chemotherapy. *, P=0.001; +, P=0.0009; #, P=0.1023.
Figure 1.
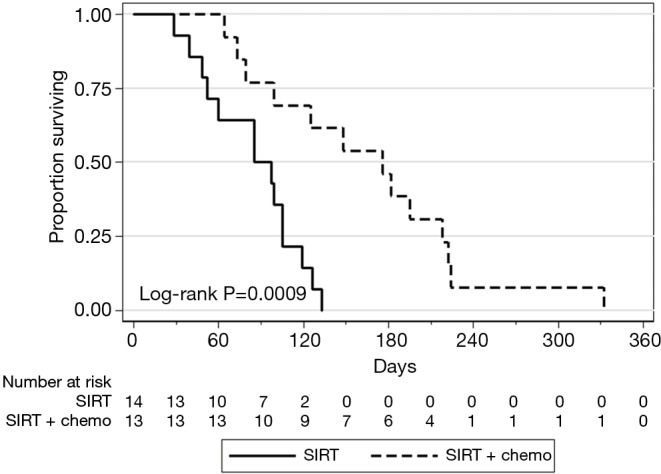
Progression-free survival in liver after SIRT and SIRT-C therapy. SIRT, SIR-Spheres® alone; SIRT-C, SIR-Spheres® with concurrent chemotherapy.
Figure 2.
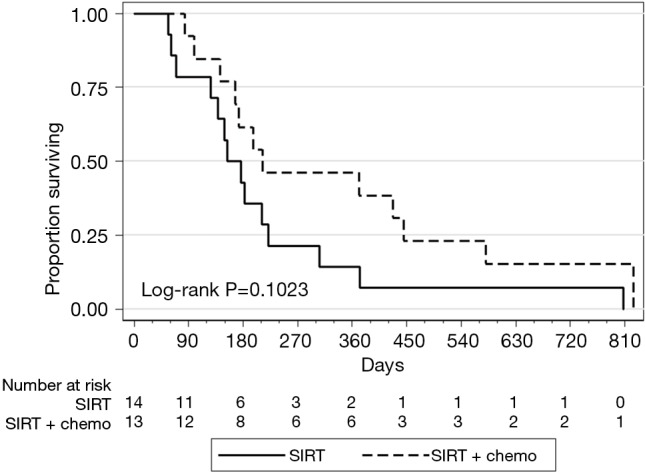
Overall survival in liver after SIRT and SIRT-C therapy. SIRT, SIR-Spheres® alone; SIRT-C, SIR-Spheres® with concurrent chemotherapy.
Discussion
SIR-Spheres® capitalize on the differential arterial blood supply to tumors and normal parenchyma in the liver. SIR-Spheres® were originally FDA approved following a phase III trial of intrahepatic arterial infusion of floxuridine (HAI-FUDR) with or without SIR-Spheres® microspheres in the first-line therapy of patients with CRLM (7). In this phase III study, response rate (RR) improved from 17.6% to 44% and liver PFS increased from 9.9 to 15.9 months with the addition of SIR-Spheres®. SIR-Spheres® have been subsequently incorporated with or without concurrent chemotherapy in the treatment of patients with refractory CRLM (7,12,14,15). Chemotherapy such as infusional 5-FU, FOLFOX, irinotecan, or oxaliplatin have been given concurrently with SIR-Spheres® in several prospective clinical trials in earlier lines of treatment. A randomized first-line phase II clinical trial in patients with CRLM demonstrated an improved response rate, PFS, and OS with concurrent 5-FU/LV and SIR-Spheres® vs. 5FU/LV (12). A phase I study of SIR-Spheres® and FOLFOX4 in the first-line treatment of patients with CRLM showed a remarkable response of 90%, prompting the investigation of this regimen in the randomized phase III SIRFLOX trial (16). The SIRFLOX trial recently reported that FOLFOX plus SIR-Spheres® had a higher median liver PFS (20.5 vs. 12.6 months; P=0.002) than FOLFOX alone; however, OS results from this study are still pending (13). While these studies suggest an advantage for concurrent chemotherapy and SIR-Spheres® in controlling liver disease over chemotherapy alone, they do not reflect the impact of chemo radioembolization vs. radioembolization alone.
To our knowledge, only one prior retrospective study compared the outcome of SIRT-C vs. SIRT in the salvage setting (8). In this study, the response rate and overall survival was more favorable with SIRT-C than with SIRT alone. Six percent of the patients did not have prior chemotherapy, 50% of patients had one prior line of chemotherapy, and 43% of patients had two or three lines. Overall survival was higher with concurrent chemotherapy and SIR-Spheres® versus SIR-Spheres® alone (13 vs. 7 months; P=0.017). However, this study did not adjust for the type of chemotherapy used during chemo radioembolization or for post- chemo radioembolization chemotherapy. Therefore, the favorable OS noted in the SIRT-C on this study could be attributed to post-SIRT chemotherapy. In our study, most patients were heavily treated prior to SIR-Sphere® therapy with a median of 3 lines in the SIRT arm and a median of 2 lines in SIRT-C arm. Importantly, we excluded all patients who received non-cross-resistant post-SIRT chemotherapy. This strategy allowed us to explore a true synergistic interaction between chemotherapy and SIRT. We found a significantly higher liver disease control rate in the SIRT-C arm (84%) vs. the SIRT alone arm (14%), despite the relatively higher rate of RAS-mutations with the SIRT-C arm. When limiting analysis to the RAS mutant patients, SIRT-C was associated with a disease control rate of 61% and a PFS of 150 days. These findings are in contrast to a retrospective study by Lahti et al. which suggested a lack of significant benefit from SIRT in RAS mutated CRLM patients (median OS =4.8 m) (17). We postulate that the lack of benefit for RAS mutant patients in the study by Lahti and colleagues may be due to chance associated with a small sample size (a type II error).
The median OS demonstrated in the SIRT-C arm was 212 days compared with 154 days in the SIRT arm though significance was not reached (P=0.1023). The lack in significance in OS between arms in our study may be in part due to progression of extrahepatic sites of disease, and to an underpowered patient population size, similar to what we suggested about Lahti’s study. Additionally, our SIRT-C cohort had a greater proportion of RAS-mutant tumors (10/13 patients) than the SIRT cohort; this association may account for more aggressive phenotypes at all disease sites and therefore mitigate survival benefit. The association between RAS mutations and poor prognosis in the metastatic CRC has been reviewed elsewhere (18). The lack of OS benefit may also be related to post-progression salvage therapy that could not be controlled for in our study design.
We acknowledge that our study has limitations. Given the retrospective nature of the study, we encountered imbalances in patient characteristics such as RAS mutation status, extent of extrahepatic disease, and the type of chemotherapies received before and after SIR-Sphere® treatment. Any of these factors may have influenced progression-free survival in the liver, or overall survival. We aimed to minimize the impact of post-SIR-Sphere® systemic therapy bias on SIRT and SIRT-C liver-PFS by excluding patients who received chemotherapy/targeted regimens following SIRT on which they did not previously progress. However, we were unable to control post-progression treatments, which may influence OS. Future prospective studies that take these factors into account are warranted.
In summary, our research is consistent with previous studies that investigated SIR-Spheres® with concurrent chemotherapy in refractory CRLM patients. SIRT-C was shown to be well tolerated and to provide promising disease control in a heavily pretreated patient population. Our study suggests that concurrent chemotherapy may improve tumor response to SIR-Spheres® therapy in refractory CRLM patients. Larger prospective studies are needed to determine predictive biomarkers of response to SIR-Spheres® treatment, optimal timing of SIR-Spheres®, and the optimal concurrent regimen in patients with CRLM.
Acknowledgements
None.
Ethical Statement: The study was approved by City of Hope Institutional review board (No 15003). Informed consents were not required since this is a retrospective study and informed consent waiver was obtained from the IRB.
Footnotes
Conflicts of Interest: Dr. Park has received honoraria for proctoring services provided to SIRTEX Medical; Dr. Fakih has received honoraria for consulting services provided to SIRTEX Medical. Other authors have no conflicts of interest to declare. Material has not been presented at an SIR Annual Scientific Meeting.
References
- 1.Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol 1986;150:195-203. 10.1002/path.1711500308 [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997;15:938-46. 10.1200/JCO.1997.15.3.938 [DOI] [PubMed] [Google Scholar]
- 3.Beard SM, Holmes M, Price C, et al. Hepatic resection for colorectal liver metastases: A cost-effectiveness analysis. Ann Surg 2000;232:763-76. 10.1097/00000658-200012000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Ooijen B, Wiggers T, Meijer S, et al. Hepatic resections for colorectal metastases in The Netherlands. A multiinstitutional 10-year study. Cancer 1992;70:28-34. [DOI] [PubMed] [Google Scholar]
- 5.Robertson DJ, Stukel TA, Gottlieb DJ, et al. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer 2009;115:752-9. 10.1002/cncr.24081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho M, Gong J, Fakih M. The state of regional therapy in the management of metastatic colorectal cancer to the liver. Expert Rev Anticancer Ther 2016;16:229-45. 10.1586/14737140.2016.1129277 [DOI] [PubMed] [Google Scholar]
- 7.Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12:1711-20. 10.1023/A:1013569329846 [DOI] [PubMed] [Google Scholar]
- 8.Chua TC, Bester L, Saxena A, et al. Radioembolization and systemic chemotherapy improves response and survival for unresectable colorectal liver metastases. J Cancer Res Clin Oncol 2011;137:865-73. 10.1007/s00432-010-0948-y [DOI] [PubMed] [Google Scholar]
- 9.Gibbs P, Heinemann V, Sharma NK, et al. SIRFLOX: Randomized phase III trial comparing first-line mFOLFOX6 ± bevacizumab (bev) versus mFOLFOX6 + selective internal radiation therapy (SIRT) ± bev in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2015;33(suppl; abstr 3502). [DOI] [PubMed]
- 10.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 11.Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for 90Y resin microspheres. J Gastrointest Oncol 2015;6:134-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78-85. 10.1002/jso.20141 [DOI] [PubMed] [Google Scholar]
- 13.van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients With Metastatic Colorectal Cancer. J Clin Oncol 2016;34:1723-31. 10.1200/JCO.2015.66.1181 [DOI] [PubMed] [Google Scholar]
- 14.Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687-94. 10.1200/JCO.2010.28.5643 [DOI] [PubMed] [Google Scholar]
- 15.van Hazel GA, Pavlakis N, Goldstein D, et al. Treatment of fluorouracil-refractory patients with liver metastases from colorectal cancer by using yttrium-90 resin microspheres plus concomitant systemic irinotecan chemotherapy. J Clin Oncol 2009;27:4089-95. 10.1200/JCO.2008.20.8116 [DOI] [PubMed] [Google Scholar]
- 16.Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol 2007;25:1099-106. 10.1200/JCO.2006.08.7916 [DOI] [PubMed] [Google Scholar]
- 17.Lahti SJ, Xing M, Zhang D, et al. KRAS Status as an Independent Prognostic Factor for Survival after Yttrium-90 Radioembolization Therapy for Unresectable Colorectal Cancer Liver Metastases. J Vasc Interv Radiol 2015;26:1102-11. 10.1016/j.jvir.2015.05.032 [DOI] [PubMed] [Google Scholar]
- 18.Gong J, Cho M, Fakih M. RAS and BRAF in metastatic colorectal cancer management. J Gastrointest Oncol 2016;7:687-704. 10.21037/jgo.2016.06.12 [DOI] [PMC free article] [PubMed] [Google Scholar]


