Abstract
Measures of increased tundra plant productivity have been associated with the accelerating retreat of the Arctic sea-ice. Emerging studies document opposite effects, advocating for a more complex relationship between the shrinking sea-ice and terrestrial plant productivity. I introduce an autoregressive plant growth model integrating effects of biological and climatic conditions for analysing individual ring-width growth time series. Using 128 specimens of Salix arctica, S. glauca and Betula nana sampled across Greenland to Svalbard, an overall negative effect of the retreating June sea-ice extent was found on the annual growth. The negative effect of the retreating June sea-ice was observed for younger individuals with large annual growth allocations and with little or no trade-off between previous and current year's growth.
Keywords: Arctic, dwarf-shrub, annual growth, autoregression, tree-ring time series, sea-ice
1. Introduction
A comprehensive number of studies support the common notion that the accelerating retreat of the Arctic sea-ice has caused the recently observed increase in plant productivity across the Arctic tundra [1,2]. The positive trend in observed satellite-based normalized difference vegetation indices (NDVI) has most often been used to support the causal relationship between sea-ice extent and plant productivity [1,4]. Specifically, the loss of near-coastal sea-ice has caused the temperature to rise along the Arctic Ocean [1], resulting in the observed increase in plant productivity [3]. Invoking warming as the mechanism for increased growth has been supported by the in situ warming experiments across the western Arctic [4].
Although the relationships between trends of NDVI and sea-ice extent are largely negative, notable variations are found across the Arctic, even with locally significant declines in productivity [2,5] and greenness of above-ground tundra vegetation [5–8]. Specifically, on Svalbard, browning of dwarf shrubs has been reported probably related to increased frequency of winter icing effects [9]. NDVI, especially satellite-based indices, measures congregated vegetation responses to climate without incorporating potential biotic interactions such as individual life-history traits [10] and plant–animal interactions [5,11]. Indeed, herbivory of muskoxen and caribou have been found to repress growth responses of arctic deciduous shrubs to experimental warming with up to 46% [11]. Hence, local environmental conditions may potentially interact with plant growth responses to climate change [10], which calls for an individual-based approach.
Recently, the role of the declining sea-ice extent on plant productivity was addressed specifically by integrating analyses of individual-based time series of tree-ring width measurements [12]. These results demonstrated a consistent growth decline during the late twentieth century warming through enhanced moisture stress amplified by the declining sea-ice concentration. Elaborating, I introduce an autoregressive plant growth model for analysing how current year plant growth is concurrently influenced by intrinsic temporal dependence (i.e. previous year's growth allocations), herbivory and climate variations mediated by the Arctic sea-ice extent. Rather than developing true chronologies for species [13], I have opted for an individual-based plant growth model, which allows for comparing individual growth responses within as well as across sampling locality. Capturing such individual variations would not be possible using across-individual dendroclimatological reconstructions [14]. The individual-based autoregressive model is applied to tundra shrub tree-ring time series from eight locations across Greenland to central Svalbard.
2. Material and methods
(a). Individual plant growth model
It has long been recognized [15,16] that time-series data of tree-ring widths display significant autocorrelation and rarely represent white noise. The within and across species variance in the estimates of the autoregressive coefficients have not been discussed previously in detail for annual plant growth time series. However, it is known that variations in the auto-covariate structure of biological time series provide significant information on intrinsic as well as inter-trophic biotic interactions [17,18].
Assuming concurrent additive effects of lagged temporal dependence and climate, current year's growth (Gt) of a long-lived, iteroparous plant species may be written as a second-order autoregressive model with climate (Ct) as a covariate,
| 2.1 |
where the autoregressive coefficients b1 and b2 quantify the direct effect of last year's growth allocation (Gt−1) and effect of delayed growth allocation (Gt−2), respectively. Following previous model studies [18,19], we may argue that direct temporal dependence (b1) embraces the combined effects related to growth constraints imposed on previous year's growth allocation and/or to density dependence in resource acquisition [19]. Specifically, for b1 ≥ 1 we find no trade-off between current and last year's growth. However, for b1 < 1 this trade-off will increase with decreasing values of b1. Delayed temporal dependence estimated by b2 has been shown to depend on inter-trophic interactions [18]; for annual growth time series of the plant species of Salix and Betula, the former may reflect changes in herbivory [20].
The long-term annual growth equilibrium G* is found from equation (2.1) when Gt = Gt−1 = Gt−2 = G*,
| 2.2 |
Hence, for long-lived, iteroparous plant species, equation (2.2) suggests that G* is related to intrinsic trade-off between previous and current year growth (b1) and extrinsic influences of climate (c1) and herbivory (b2).
(b). Data and analyses
During 2002–2010, a total of 128 individual dwarf-shrubs were sampled across eight arctic locations from southwestern Greenland to Svalbard, of which 27 individuals were B. nana, 43 S. glauca and 58 S. arctica (figure 1a; electronic supplementary material, figure S1). Year of sampling was not included in the tree-ring time series. All species are long-lived, tundra shrub species native to the Arctic. In contrast to the prostrate growth of B. nana and S. arctica, S. glauca grows erect and may be found up to 2–3 m in favourable habitats in Greenland [22]. In all species, growth is seasonal with well-defined, annual growth rings suitable for establishing time series of annual growth-ring chronologies [23].
Figure 1.
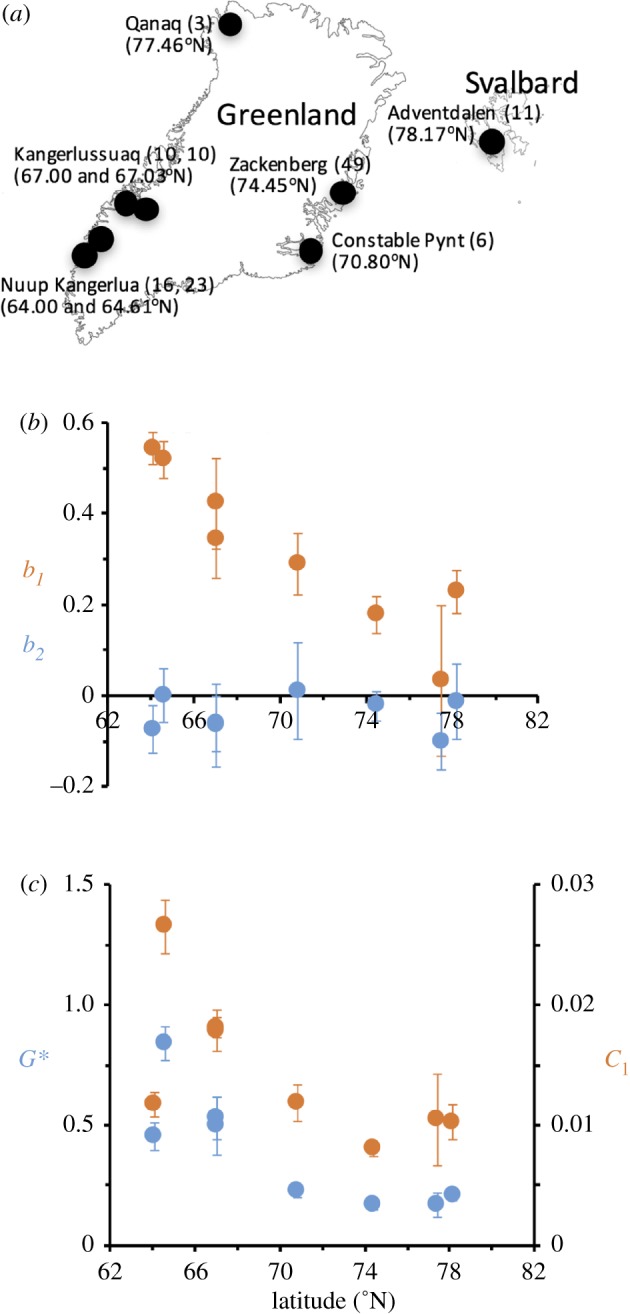
(a) Map with the six locations where 128 individual specimens of S. glauca, S. arctica and B. nana were collected. For each location is given latitude and the number of collected individuals. Average length of growth time series was 34.27 ± 1.62 (±s.e.m.) years with a minimum length of 11 years and a maximum length of 116 years. (b) Variations in the average estimated autoregressive coefficients b1 (direct temporal dependence) and and b2 (delayed temporal dependence) (equation (2.1)) across locations. (c) Latitudinal variations in the estimated average effect of June sea-ice extent (c1) and annual growth (Gt) and long-term annual growth equilibrium (G*). Horizontal bars indicate ± s.e.m.
Following techniques previously applied [24], 20 µm microsections were cut from each stem, stained and then mounted on slide frames. Slides were then digitally scanned for increased photographic contrasting and finally enlarged for the process of measuring tree-ring widths. On each microsection 3–9 radii were measured electronically [13]. To provide optimal cross-dating within each locality, radii were compared to find missing rings following previous applied standard procedures using multiple cross-sections of the sampled stem. For details, see references [13,14].
To estimate the relative importance of intrinsic (autoregressive; b1, b2) and extrinsic (climatic; c1) influences on annual growth across individuals and locations, we confronted each of the sampled 128 growth time series with the autoregressive plant growth model in equation (2.1), where Gt is the width in millimetres of tree-ring year t and Ct the Arctic sea-ice extent (million km2) in June, year t. Time-series models specifically integrating nonlinearity of density-dependence or multiplicative population processes use ln-transformations [18,19]. As the current model does not a priori consider such processes, the ring-width data used here were not ln-transformed. The June sea-ice extent was provided for the period 1979–2015 by the US National Snow and Ice Data Center, University of Colorado, Boulder (http://nsidc.org). In Greenland and on Svalbard, the June sea-ice extent displays a strong inverse relationship with summer temperatures and depth of active layer but only a tendency to correlate positively with summer precipitation (electronic supplementary material, table S1 and figure S3).
All analyses were done in R [21]. The function arima was used with the order = c(2, 0, 0) and the external vector xreg defined by the June sea-ice extent time series. Model fitting was done using maximum likelihood [21]. For intrinsic comparisons across sample locations, for each time series the G* was estimated from a pure first-order autoregressive model with the ar.mle function, that is for b2 = c1 = 0 in equation (2.2).
3. Results
The autoregressive structures of the annual growth time series for B. nana, S. glauca and S. arctica displayed considerable variance across the sampled locations (figure 1a). Specifically, direct temporal dependence (b1) increased (i.e. decreasing b1-values) across locations towards north with the strongest direct temporal dependence found in the specimens from Svalbard (B. nana) and North Greenland (S. arctica; figure 1b). By contrast, delayed temporal dependence (b2) was insignificant in most time series and displayed no trend across latitudes (figure 1b).
Overall, the June sea-ice extent (Ct) was found to have a significant positive effect on current year growth (Gt, figure 1c) in 120 of the 128 time series. However, opposite to direct temporal dependence (b1), the effect of current year sea-ice extent in June on annual growth (c1) decreased towards north (figure 1c) similar to its effect on summer temperatures (electronic supplementary material, figure S3). The long-term annual growth equilibrium (G*) also decreased from south to north (figure 1c).
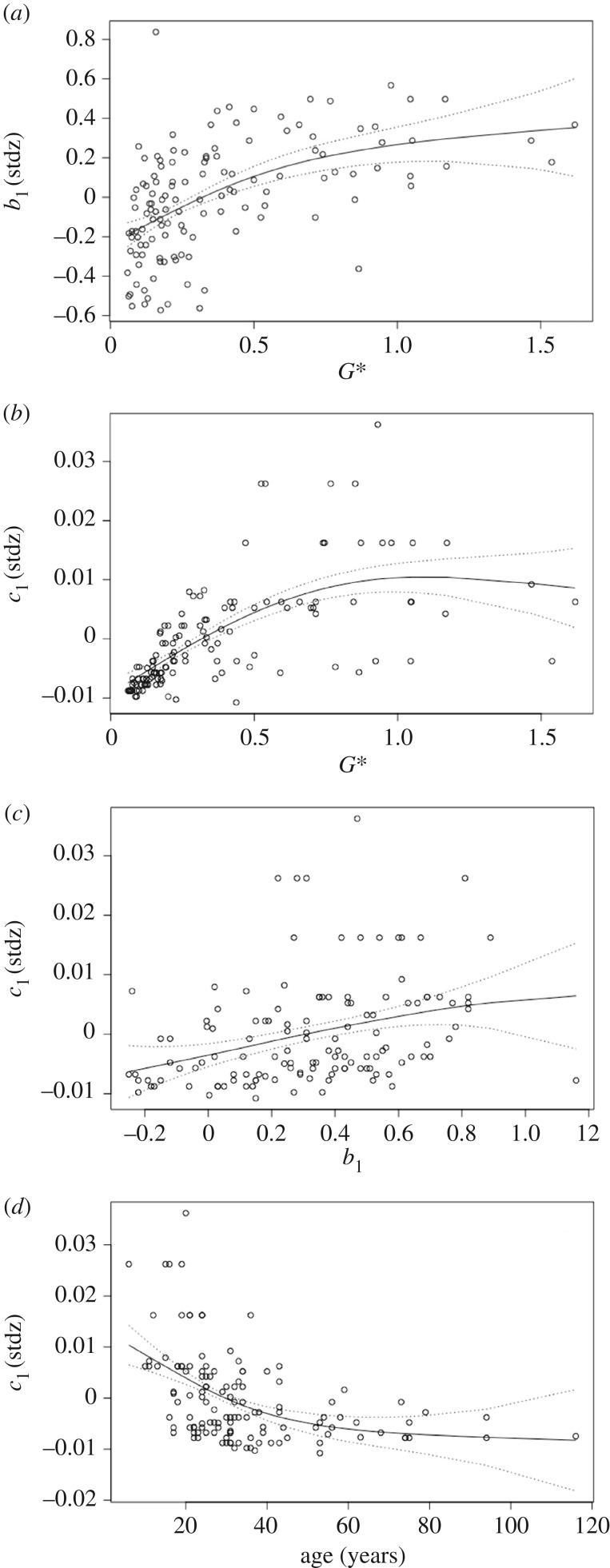
Looking across species and locations, the estimated effects of intrinsic and extrinsic predictors on the annual growth response in Arctic dwarf shrubs covaried significantly. First, as G* increased, direct temporal dependence (b1) decreased (b1 → 1; figure 2a) and the effect of June sea-ice extent (c1) increased (figure 2b). Second, as direct temporal dependence decreased, the effect of June sea-ice extent increased (figure 2c). And, finally, the effect of June sea-ice extent was dependent on the age of the dwarf shrubs: as age increased, c1 decreased, in particular for younger shrubs little or no change in effect for individual shrubs aged 60 years and above (figure 2d).
Figure 2.
(a) Direct temporal dependence (b1) plotted against growth allocation equilibrium (G*) (R2 = 0.30, F1,125 = 48.2, p < 0.001). Effect of current-year June sea-ice extent (c1) on current-year growth (Gt) plotted against (b) G* (R2 = 0.49, F1,125 = 88.6, p < 0.001), (c) b1 (R2 = 0.12, F1,125 = 15.7, p < 0.001) and (d) age (R2 = 0.26, F1,125 = 29.7, p < 0.001). Lines in (a–d) are fitted generalized additive models using non-parametric smoothing splines [21].
4. Discussion
The overall negative influence of the retreating June sea-ice in the Arctic on the long-term annual growth patterns of B. nana, S. arctica and S. glauca across Greenland to Svalbard (figure 1a), reported here, contrasts analyses of satellite data and in situ measurements [1,3]. Indeed, surface warming from increasing loss of sea-ice has been associated with considerable advances in plant phenology and timing of peak primary production together with a longer plant growth season [3]. Also, increases in the abundance and cover of shrubs have been directly associated with warming experiments across the Arctic tundra [4]. So, focusing on the reported changes in timing and extent of plant growth season, the Arctic is greening, arguably as a direct consequence of sea-ice induced summer warming [3].
However, focusing on the long-term changes in individual annual plant growth, as in this study, the coupling to climate may be more complex [25]. In fact, a recent study using long-term variations in tree-ring chronologies demonstrated spatially divergent trends, where, the sensitivity of annual shrub growth to summer temperature varied considerably across the Arctic tundra to local variations in soil moisture, growth forms and the presence of melting permafrost [26]. Specifically, for the species investigated in this study, both significant positive (50%) and negative (50%) responses to increased summer temperatures were found in Greenland. By contrast, the annual growth of S. polaris and Cassiope tetragona on Svalbard demonstrated positive responses to increased summer temperature [26]. As these species are exposed to different snow and moisture regimes compared to B. nana, the divergent results emphasize the importance of integrating species-specific requirements including individual growth forms across landscape gradients to evaluate growth responses to climate changes [26].
The scientific preamble of this study rests upon the reported variability in annual growth responses to changes in climate and offers an explanation to this by introducing a growth model integrating both biotic and abiotic predictors (equation (2.1)). In particular, my analyses suggest that the spatial decrease in the effect of sea-ice extent on annual shrub growth towards north (c1, figure 1c) may be related to an increased dependence on previous year's growth (figures 1c and 2c). Physiological trade-offs embracing energy allocation between two or more functions competing for the same resources within a single individual is at the heart of classic life-history theory [27]. Although variations in the effect of temporal dependence (b1) may indicate some energetic trade-off between current and future growth, trade-off between growth and reproduction among years is likely to be involved implicitly as well, why the correlative interaction between b1 and c1 (figure 2c) needs to be followed up by controlled, experimental studies; in particular, integrating detailed biotic and abiotic data analyses on a landscape-level contemplating the relative influence of local winter and summer warming [28,29] is an important follow-up from large-scale gradient studies.
As suggested previously [25], individual growth form as indicated by the long-term annual growth equilibrium (G*) as well as individual age were found to interact with the effect of June sea-ice extent on annual growth (figure 2b,d), making young, fast-growing individuals more susceptive to changes in sea-ice cover. Nevertheless, although spatially variable, the present analyses suggest an overall annual growth decline in dwarf shrubs across Greenland to Svalbard to retreating Arctic sea-ice (figure 1c). Indeed, as inferred from the strong inverse relationship between summer temperature and sea-ice extent (electronic supplementary material, figure S3 and table S1), the observed surface warming following the retreat of sea-ice may also act as a potent driver of increased regional drought stress reducing growth [12]. Especially in the Arctic with an atmosphere characterized by low humidity, vapour-pressure deficit may be amplified by sea-ice induced surface warming [12]. The autoregressive plant growth model presented suggests that climate-growth dynamics interact with growth form, age and other species-specific traits; interactions central for a fuller understanding of the effects of concurrent large-scale changes in climate, such as the accelerating retreating Arctic sea-ice.
Supplementary Material
Acknowledgements
I extend my sincere thanks to L. Stewart and three anonymous referees for comments on an earlier version of the manuscript.
Data accessibility
Data are available from: http://dx.doi.org/10.5061/dryad.302f1 [30].
Competing interests
We declare we have no competing interests.
Funding
This study was supported initially by the Danish Council for Independent Research, Natural Sciences. The University Centre in Svalbard provided financial support through internal strategic funding.
References
- 1.Bhatt US, et al. 2010. Circumpolar Arctic tundra vegetation is linked to sea-ice decline. Earth Inter. 14, 1–20. ( 10.1175/2010EI315.1) [DOI] [Google Scholar]
- 2.Dutrieux LP, Bartholomeus H, Herold H, Verbesselt J. 2012. Relationships between declining summer sea ice, increasing temperatures and changing vegetation in the Siberian Arctic tundra from Modis time series (2000–11). Environ. Res. Lett. 7, 1–12. ( 10.1088/1748-9326/7/4/044028) [DOI] [Google Scholar]
- 3.Post E, et al. 2013. Ecological consequences of sea-ice decline. Science 341, 519–524. ( 10.1126/science.1235225) [DOI] [PubMed] [Google Scholar]
- 4.Elmendorf SC, et al. 2012. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol. Lett. 15, 164–175. ( 10.1111/j.1461-0248.2011.01716.x) [DOI] [PubMed] [Google Scholar]
- 5.Bjerke JW, Bjerke JW, Karlsen SR, Høgda KA, Malnes E, Jepsen JU, Lovibond S, Vikhamar-Schuler D, Tømmervik H. 2014. Record-low primary productivity and high plant damage in the Nordic Arctic Region in 2012 caused by multiple weather events and pest outbreaks. Environ. Res. Lett. 9, 084006 ( 10.1088/1748-9326/9/8/084006) [DOI] [Google Scholar]
- 6.Bhatt US, Walker DA, Raynolds MK, Bieniek PA, Epstein HE, Comiso JC, Pinzon JE, Tucker CJ, Polyakov IV. 2013. Recent declines in warming and vegetation greening trends over pan-Arctic tundra. Remote Sensing 5, 4229–4254. ( 10.3390/rs5094229) [DOI] [Google Scholar]
- 7.Bieniek PA, et al. 2015. Climate drivers linked to changing seasonality of Alaska coastal tundra vegetation productivity. Earth Interact. 19, 1–29. ( 10.1175/EI-D-15-0013.1) [DOI] [Google Scholar]
- 8.Bjerke JW, Tømmervik H, Zielke M, Jørgensen M. 2015. Impacts of snow season on ground-ice accumulation, soil frost and primary productivity in a grassland of sub-Arctic Norway. Environ. Res. Lett. 10, 095007 ( 10.1088/1748-9326/10/9/095007) [DOI] [Google Scholar]
- 9.Hansen BB, Isaksen K, Benestad RE, Kohler J, Pedersen ÅØ, Loe LE, Coulson SJ, Larsen JO, Varpe Ø.. 2014. Warmer and wetter winters: characteristics and impplications of an extreme weather event in the High Arctic. Environ. Res. Lett. 9, 114021 ( 10.1088/1748-9326/9/11/114021) [DOI] [Google Scholar]
- 10.Reich PB, Sendall KM, Rice K, Stefanski A, Hobbie SE, Montgomery RA. 2015. Geographic range predicts photosynthetic and growth response to warming in co-occuring tree species. Nature CC 5, 148–152. ( 10.1038/nclimate2497) [DOI] [Google Scholar]
- 11.Post E, Pedersen C. 2008. Opposing plant community responses to warming with and without herbivores. Proc. Natl Acad. Sci. USA 105, 12 353–12 358. ( 10.1073/pnas.0802421105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardin MP, Guo XJ, Jong RD, Kinnard C, Bernier P, Raulier D. 2014. Unusual forest growth decline in boral North America covaries with retreat of Arctic sea ice. Glob. Change Biol. 20, 851–866. ( 10.1111/gcb.12400) [DOI] [PubMed] [Google Scholar]
- 13.Schmidt NM, Baittinger C, Forchhammer MC. 2006. Reconstructing century-long snow regimes using estimates of high Arctic Salix arctica radial growth. Arc. Antarc. Alp. Res. 38, 257–262. ( 10.1657/1523-0430(2006)38[257:RCSRUE]2.0.CO;2) [DOI] [Google Scholar]
- 14.Schmidt NM, Baittinger C, Kollman J, Forchhammer MC. 2010. Consistent dendrochronological response of dioecious Salix arctica to variation in local snow precipitation across gender and vegetation types. Arc. Antarc. Alp. Res. 42, 471–475. ( 10.1657/1938-4246-42.4.471) [DOI] [Google Scholar]
- 15.Monserud RA. 1986. Time-series analyses of tree-ring chronologies. Forest Sci. 32, 349–372. [Google Scholar]
- 16.Hallinger M, Manthey M, Wilmking M. 2010. Establishing a missing link: war summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytol. 186, 890–899. ( 10.1111/j.1469-8137.2010.03223.x) [DOI] [PubMed] [Google Scholar]
- 17.Bjørnstad ON, Falck W, Stenseth NC. 1995. A geographic gradient in small rodent density fluctuations: a statistical modeling approach. Proc. R. Soc. Lond. B 262, 127–133. ( 10.1098/rspb.1995.0186) [DOI] [PubMed] [Google Scholar]
- 18.Hendrichsen DK, Topping CJ, Forchhammer MC.. 2009. Predation and fragmentation portrayed in the statistical structure of prey time-series. BMC Ecol. 9, 10 ( 10.1186/1472-6785-9-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Post E, Forchhammer MC, Stenseth NC, Callaghan TV. 2001. The timing of life-history in a changing climate. Proc. R. Soc. Lond. B 208, 15–23. ( 10.1098/rspb.2000.1324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forchhammer MC, Schmidt NM, Høye TT, Berg TB, Hendrichsen DK, Post E. 2008. High-Arctic plant-herbivore interactions under climate change. Adv. Ecol. Res. 40, 391–419.. ( 10.1016/S0065-2504(07)00017-7) [DOI] [Google Scholar]
- 21.Core Team R. 2015. R: a language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing; (https://www.R-project.org/) [Google Scholar]
- 22.Feilberg J, Fredskild B, Holt S. 1984. Flowers of Greenland. Ringsted, Denmark: Forlaget Regnbuen. [Google Scholar]
- 23.Myers-Smith IH, et al. 2015. Methods for measuring arctic and alpine shrub growth: a review. Earth Sci. Rev. 140, 1–13. ( 10.1016/j.earscirev.2014.10.004) [DOI] [Google Scholar]
- 24.Woodcock H, Bradley RS. 1994. Salix arctica: its potential for dendroclimatological studies in the high Arctic. Dendrochronologia 12, 11–22. [Google Scholar]
- 25.Michaletz ST, Cheng D, Kerkhoff AJ, Enquist BJ. 2014. Convergence of terrestrial plant production across global climate gradients. Nature 512, 39–43. ( 10.1038/nature13470) [DOI] [PubMed] [Google Scholar]
- 26.Myers-Smith IH, et al. 2015. Climate sensitivity of shrub growth across the tundra biome. Nature 5, 887–891. ( 10.1038/nclimate2697) [DOI] [Google Scholar]
- 27.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 28.Hollesen J, Buchwal A, Rachlewicz G, Hansen BU, Hansen MO, Stecher O, Elberling B. 2015. Winter warming as an important co-driver for Betula nana growth in western Greenland during past century. Glob. Change Biol. 21, 2410–2423. ( 10.1111/gcb.12913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Wal R, Stien A. 2014. High-arctic plants like it hot: a long-term investigation of between-year variability in plant biomass. Ecology 95, 3414–3427. ( 10.1890/14-0533.1) [DOI] [Google Scholar]
- 30.Forchhammer M. 2017. Data from: Sea-ice induced growth decline in Arctic shrubs. Dryad Digital Repository. ( 10.5061/dryad.302f1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Forchhammer M. 2017. Data from: Sea-ice induced growth decline in Arctic shrubs. Dryad Digital Repository. ( 10.5061/dryad.302f1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from: http://dx.doi.org/10.5061/dryad.302f1 [30].