Abstract
The interplay between range expansion and concomitant diversification is of fundamental interest to evolutionary biologists, particularly when linked to intercontinental dispersal and/or large scale extinctions. The evolutionary history of true frogs has been characterized by circumglobal range expansion. As a lineage that survived the Eocene–Oligocene extinction event (EOEE), the group provides an ideal system to test the prediction that range expansion triggers increased net diversification. We constructed the most densely sampled, time-calibrated phylogeny to date in order to: (i) characterize tempo and patterns of diversification; (ii) assess the impact of the EOEE; and (iii) test the hypothesis that range expansion was followed by increased net diversification. We show that late Eocene colonization of novel biogeographic regions was not affected by the EOEE and surprisingly, global expansion was not followed by increased net diversification. On the contrary, the diversification rate declined or did not shift following geographical expansion. Thus, the diversification history of true frogs contradicts the prevailing expectation that amphibian net diversification accelerated towards the present or increased following range expansion. Rather, our results demonstrate that despite their dynamic biogeographic history, true frogs diversified at a relatively constantly rate, even as they colonized the major land masses of Earth.
Keywords: diversification shift, Ranidae phylogeny, biogeography
1. Introduction
Geographical distribution of species richness is a function of lineage diversification through time and space. Shifts in diversification rate in relation to expansion of geographical ranges can therefore be an important factor underlying geographical patterns in biodiversity. Movement of lineages into new areas has frequently been associated with increased diversification rate in response to various biotic and abiotic factors such as favourable climate, ecological opportunities, lack of competition/predation and the evolution of key innovations [1,2]. Diversification rate increases can also be triggered by extrinsic factors such as mass extinctions, if surviving lineages rapidly diversify into vacant niches [3]. Understanding the deterministic relationship between dispersal and diversification (‘dispersification’; [1]) therefore requires robust estimates of phylogenetic relationships, divergence times, inference of geographical range evolution, and calculation of diversification rates.
Previous studies have shown that net diversification (speciation minus extinction) of amphibians has accelerated toward the present [4] and was positively associated with global range expansion [5]. We used this expectation to test the Dispersification Hypothesis in true frogs, a globally distributed family with 380 species. The evolutionary history of true frogs spans the Eocene–Oligocene mass extinction event (EOEE), which triggered widespread extinctions from marine invertebrates to mammals of Europe and Asia [6]. However, the impact of the EOEE on amphibians has never been explicitly studied.
We synthesized a novel multilocus molecular dataset from a large proportion (77%) of the world's true frogs to estimate colonization patterns, timing of diversification, and global range evolution of this cosmopolitan amphibian family. We then estimated the significance of shifts in diversification rates to test the prediction that range expansion (and/or the EOEE) was followed by increased net diversification. We show that circumglobal range expansion was not coupled to diversification, contradicting classic biogeographic model predictions.
2. Methods
A total of 402 samples representing 292 of the known 380 true frog species was incorporated into phylogenetic analyses. We obtained from GenBank two mitochondrial (16S, cytochrome b) and two nuclear genes (RAG-1, tyrosinase; electronic supplementary material, table S1). A total of 4328 base-pairs were concatenated and partitioned by gene prior to phylogenetic analysis using the Bayesian program BEAST [7]. We used BEAST's bModelTest to explore substitution model space while simultaneously estimating model parameters and the phylogeny [8]. To establish a temporal framework for true frog diversification, we used four fossil calibration points: three within the genus Rana [9] and one to calibrate the most recent common ancestor of the genus Pelophylax [10]. The fossilized birth–death process was used to model speciation times and topology and our sampling used two independent MCMC chains at 500 million generations each.
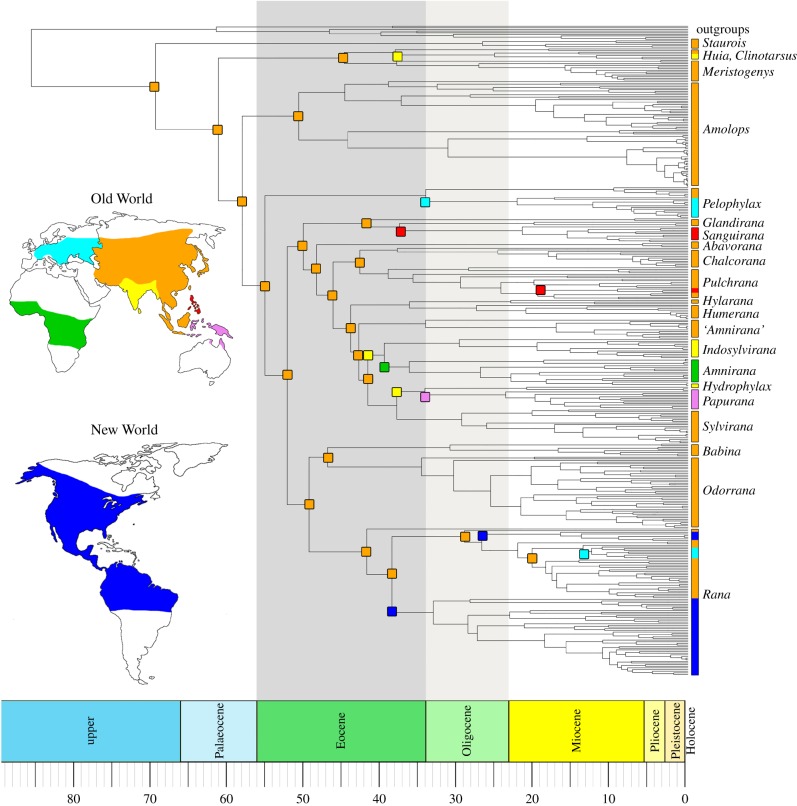
The resulting time-calibrated phylogeny was used to reconstruct the spatio-temporal evolution of geographical ranges in BioGeoBEARS [11]. We defined seven biogeographic regions that are known to be separate land masses during the Cenozoic: America, Europe, Africa, Asia, India, Philippines and Australasia (figure 1), and evaluated the fit of our data to six biogeographic models using likelihood ratios and the Akaike information criterion.
Figure 1.
Time-calibrated ancestral range reconstructions of true frogs.
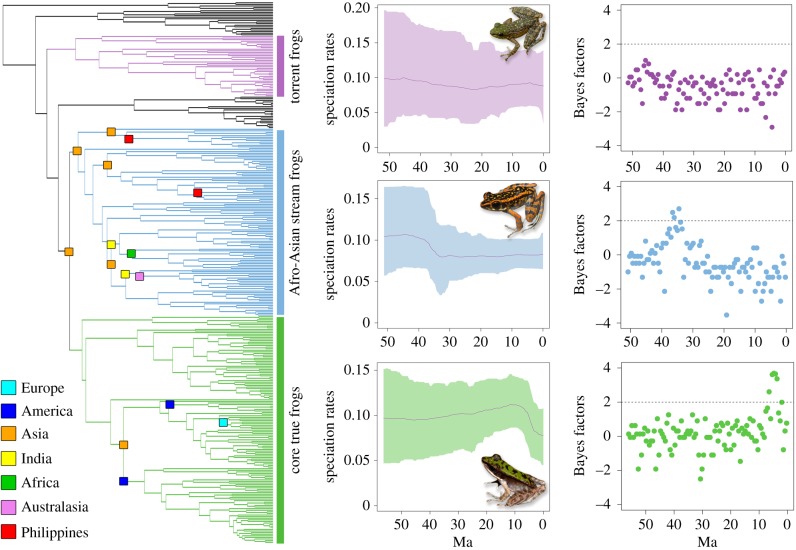
Shifts in net diversification were estimated using the R package ‘TESS’. This method performs reversible-jump MCMC simulation over all possible episodically varying birth–death processes with explicitly modelled mass-extinction events [12]. Our MCMC chain ran until all parameters reached a minimum effective sampling size of 500. Because finer-scale changes may be obscured when larger groups are analysed as a whole [9], we performed analyses at three hierarchical levels to capture diversification shifts at different phylogenetic scales: (i) entire phylogeny; (ii) three major subclades: torrent frogs (Amolops), Afro-Asian stream frogs (Hylarana Complex sensu [13]), core true frogs (Babina, Odorrana, Rana); and (iii) individual subclades that colonized a new region.
3. Results
Our phylogenetic analysis produced high support (posterior probability ≥0.9) for most major nodes (electronic supplementary material, figure S1). Divergence time estimates were generally consistent (± 5 million years) with two previous, more sparsely sampled phylogenetic studies of Ranidae [9,14] but strongly conflicted with another study, which recovered significantly younger ages [13].
Ancestral range reconstructions unambiguously support Asia as the origin of true frogs. Of the six biogeographic models assessed, the BAYAREALIKE + J model was favoured (electronic supplementary material, figure S2 and table S2). A total of 11 major dispersal events were detected, the clear majority of which (n = 8) occurred during the Eocene and Oligocene (figure 1; electronic supplementary material, figure S2). These include three colonizations of the Indian subcontinent by the genera Clinotarsus, Indosylvirana and Hydrophylax; two colonizations of Europe (Pelophylax and Rana); two dispersals into the Philippines (Sanguirana and Pulchrana); two into the Americas (Rana); one to Africa (Amnirana); and one to Australasia (Papurana).
When the TESS analysis was performed on the entire phylogeny, a strong decrease in speciation rate (2lnBF ≈ 5) was detected at approximately 6 mya (electronic supplementary material, figure S3). There was a strong (2 < 2lnBF > 6) decrease in speciation rate in Afro-Asian stream frogs at approximately 35 mya and in core true frogs at approximately 7 mya, but torrent frogs showed no significant shifts (figure 2). No shifts were detected in individual subclades except for the genus Rana which had a similar pattern to core true frogs (electronic supplementary material, figure S4). We detected no shifts in extinction rates, nor signatures of mass extinction (electronic supplementary material, figure S3).
Figure 2.
Speciation rates through time in three major clades of true frogs with significance assessed using Bayes factors.
4. Discussion
Two centuries of global biogeographic and palaeontological treatments have reinforced the expectation that major biotic range expansion into new geographical areas is often followed by an increase in net diversification rates, due to a broad range of phenomena that fall under the general concepts of ecological opportunity and evolutionary innovation [15]. Interestingly, and in striking contrast to expectations, the rapid global range expansion of true frogs was not associated with increased net diversification. On the contrary, diversification rates either decreased or remained unchanged, even as remarkable, circumglobal range expansion via dispersal and colonization of novel regions occurred. The Afro-Asian stream frogs, which underwent the most extensive and rapid range expansion, actually exhibited a decrease in net diversification following range expansions at the Eocene–Oligocene boundary. A similar but stronger shift has been reported in core true frogs in the Miocene [9]. Although studies have shown that an overall decrease in diversification rates in larger groups (e.g. at the family or generic level) may obscure increased diversification rates in smaller subgroups [9], our results showed that diversification rate patterns were consistent across different phylogenetic scales.
Our divergence time estimates are largely congruent with the timing of several well characterized tectonic events. All three independent colonizations of the Indian subcontinent occurred between 35 and 40 mya, after the Indian–Eurasian collision at 40 mya [16]. The separation between eastern and western Palaearctic lineages of Pelophylax was estimated at approximately 35, coinciding with the closure of the Turgai Straits, an event that resulted in a land connection between southern Europe and southwestern Asia, and facilitation of faunal exchange between these two regions [6]. The colonization of the Philippines from East Asia by members of the Glandirana/Sanguirana clade precisely matches the timing, polarity of inferred dispersal, and phylogenetic relationships postulated by the ‘Palawan Ark’ hypothesis [17], reinforcing the interpretation of isolation and palaeotransport of true frog lineages to the Philippines via the Palawan Microcontinent Block [18]. An extensive, archipelago-wide Philippine Sanguirana radiation, most closely related to coastal Eurasian Glandirana (but conspicuously absent from Sunda Shelf land masses), precisely resembles inferred patterns from other Philippine radiations, lending strong statistical support in yet another independent lineage for the Palawan Ark biogeographic mechanism, which has initiated several spectacular Philippine radiations [17,19].
True frogs began to disperse out of Asia at the end of the Eocene and by the beginning of the Miocene, colonized every continent except Antarctica. Yuan and colleagues demonstrated that the New World was colonized via the Beringian land bridge [9]. In contrast, our results showed that the dispersal of Amnirana from India/Asia into Africa at approximately 37–40 mya could not have occurred over land as Africa and Eurasia were separated by the neo-Tethys ocean until approximately 27 mya [20]. This dispersal event coincides with the middle Eocene climatic optimum (MECO), a period of pronounced warming in the middle to late Eocene [21]. Numerous other intercontinental faunal exchanges have been documented during this period [22,23], indicating that the MECO could have been an important facilitator of intercontinental faunal exchange in many unrelated vertebrate groups.
The EOEE was followed by accelerated extinction rates in marine life, mammals and vegetation [6]. However, we found no evidence for an EOEE-associated reduction of diversity in true frogs. Extinction rates remained relatively constant through time, indicating that shifts in net diversification were caused by decreased speciation rates, likely due to global cooling during the Oligocene or the lack of key innovations that prevented ecological generalists from competing with incumbent species [24]. The diversification history of true frogs goes against the current thesis that amphibian net diversification accelerated towards the present [4] or increased following range expansion [5]. Our study demonstrates that, despite their dynamic biogeographic history of pan-global range expansion, true frog diversity did not increase with range expansion. Rather, a relatively constant, linear accumulation of taxonomic diversity through time, coupled with instances of decreased speciation, characterized the evolutionary history of the planet's cosmopolitan true frogs.
Acknowledgements
We thank Carl Hutter for comments on a previous version of the manuscript.
Ethics
Treatment of animals adhered to the University of Kansas IACUC protocol (KU IACUC Authorization 158-04).
Data accessibility
Data supporting this article are available at the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.6d5m2) [25].
Authors' contributions
K.O.C. designed the study, performed analyses and drafted the manuscript; R.M.B. contributed to conceptualization, writing and interpretation. Both authors approved the final publication and agreed to be held accountable for the content therein.
Competing interests
We have no competing interests.
Funding
This project was supported by the University of Kansas Biodiversity Institute Panorama Fund (2013) and The National Geographic Explorer's grant no. 9722-15.
References
- 1.Moore BR, Donoghue MJ. 2007. Correlates of diversification in the plant clade Dipsacales: geographic movement and evolutionary innovations. Am. Nat. 170, S28–S55. ( 10.1086/519460) [DOI] [PubMed] [Google Scholar]
- 2.Drummond CS, Eastwood RJ, Miotto STS, Hughes CE. 2012. Multiple continental radiations and correlates of diversification in Lupinus (Leguminosae): testing for key innovation with incomplete taxon sampling. Syst. Biol. 61, 443–460. ( 10.1093/sysbio/syr126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruta M, Pisani D, Lloyd GT, Benton MJ. 2007. A supertree of Temnospondyli: cladogenetic patterns in the most species-rich group of early tetrapods. Proc. R. Soc. B 274, 3087–3095. ( 10.1098/rspb.2007.1250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roelants K, Gower DJ, Wilkinson M, Loader SP, Biju SD, Guillaume K, Moriau L, Bossuyt F. 2007. Global patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA 104, 887–892. ( 10.1073/pnas.0608378104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pramuk JB, Robertson T, Sites JW, Noonan BP. 2008. Around the world in 10 million years: biogeography of the nearly cosmopolitan true toads (Anura: Bufonidae). Glob. Ecol. Biogeogr. 17, 72–83. [Google Scholar]
- 6.Sun J, Ni X, Bi S, Wu W, Ye J, Meng J, Windley BF. 2014. Synchronous turnover of flora, fauna, and climate at the Eocene–Oligocene Boundary in Asia. Sci. Rep. 4, 7463 ( 10.1038/srep07463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond AJ, Bouckaert RR.. 2015. Bayesian evolutionary analysis with BEAST, p. 260 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Bouckaert R, Drummond A. 2017. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 17, 1–11. ( 10.1186/s12862-017-0890-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan ZY, et al. 2016. Spatiotemporal diversification of the true frogs (genus Rana): a historical framework for a widely studied group of model organisms. Syst. Biol. 65, 824–842. ( 10.1093/sysbio/syw055) [DOI] [PubMed] [Google Scholar]
- 10.Roček Z, Rage J-C. 2003. Evolution of anuran assemblages in the Tertiary and Quaternary of Europe, in the context of palaeoclimate and palaeogeography. Amphib-Reptil. 24, 133–167. ( 10.1163/156853803322390408) [DOI] [Google Scholar]
- 11.Matzke NJ. 2014. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Syst. Biol. 63, 951–970. ( 10.1093/sysbio/syu056) [DOI] [PubMed] [Google Scholar]
- 12.Höhna S, May MR, Moore BR. 2015. TESS: an R package for efficiently simulating phylogenetic trees and performing Bayesian inference of lineage diversification rates. Bioinformatics 32, 789–791. ( 10.1093/bioinformatics/btv651) [DOI] [PubMed] [Google Scholar]
- 13.Oliver LA, Prendini E, Kraus F, Raxworthy CJ. 2015. Systematics and biogeography of the Hylarana frog (Anura: Ranidae) radiation across tropical Australasia, Southeast Asia, and Africa. Mol. Phylogenet. Evol. 90, 176–192. ( 10.1016/j.ympev.2015.05.001) [DOI] [PubMed] [Google Scholar]
- 14.Wiens JJ, Sukumaran J, Pyron RA, Brown RM. 2009. Evolutionary and biogeographic origins of high tropical diversity in Old World frogs (Ranidae). Evolution 63, 1217–1231. ( 10.1111/j.1558-5646.2009.00610.x) [DOI] [PubMed] [Google Scholar]
- 15.Wiens JJ, Donoghue MJ. 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644. ( 10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 16.Bouilhol P, Jagoutz O, Hanchar JM, Dudas FO. 2013. Dating the India–Eurasia collision through arc magmatic records. Earth Planet. Sci. Lett. 366, 163–175. ( 10.1016/j.epsl.2013.01.023) [DOI] [Google Scholar]
- 17.Brown RM. et al 2013. Evolutionary processes of diversification in a model island archipelago. Annu. Rev. Ecol. Evol. Syst. 44, 411–435. ( 10.1146/annurev-ecolsys-110411-160323) [DOI] [Google Scholar]
- 18.Yumul GP, Dimalanta CB, Marquez EJ, Queaño KL. 2009. Onland signatures of the Palawan microcontinental block and Philippine mobile belt collision and crustal growth process: a review. J. Asian Earth Sci. 34, 610–623. ( 10.1016/j.jseaes.2008.10.002) [DOI] [Google Scholar]
- 19.Brown RM, Su YC, Barger B, Siler CD, Sanguila MB, Diesmos AC, Blackburn DC. 2016. Phylogeny of the island archipelago frog genus Sanguirana: another endemic Philippine radiation that diversified ‘Out-of-Palawan’. Mol. Phylogenet. Evol. 94, 531–536. ( 10.1016/j.ympev.2015.10.010) [DOI] [PubMed] [Google Scholar]
- 20.McQuarrie N, Van Hinsbergen DJJ. 2013. Retrodeforming the Arabia–Eurasia collision zone: age of collision versus magnitude of continental subduction. Geology 41, 315–318. ( 10.1130/G33591.1) [DOI] [Google Scholar]
- 21.Bohaty SM, Zachos JC. 2003. Significant southern ocean warming event in the late middle Eocene. Geology 31, 1017–1020. ( 10.1130/G19800.1) [DOI] [Google Scholar]
- 22.Beard KC, Qi T, Dawson MR, Wang B, Chuankuei L. 1994. A diverse new primate fauna from middle Eocene fissure-fillings in southeastern China. Nature 368, 604–609. ( 10.1038/368604a0) [DOI] [PubMed] [Google Scholar]
- 23.Chaimanee Y. et al 2012. Late Middle Eocene primate from Myanmar and the initial anthropoid colonization of Africa. Proc. Natl Acad. Sci. USA 109, 10 293–10 297. ( 10.1073/pnas.1200644109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duellman WE, Trueb L. 1994. Biology of amphibians. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 25.Chan KO, Brown RM. 2017. Data from: Did true frogs ‘dispersify’? Dryad Digital Repository. ( 10.5061/dryad.6d5m2) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Chan KO, Brown RM. 2017. Data from: Did true frogs ‘dispersify’? Dryad Digital Repository. ( 10.5061/dryad.6d5m2) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data supporting this article are available at the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.6d5m2) [25].