Abstract
Early environmental enrichment improves postnatal cognition in animals and humans. Here, we examined the effects of the prenatal acoustic environment (parental song rate) on prenatal attention in superb fairy-wren (Malurus cyaneus) embryos, the only songbird species with evidence of prenatal discrimination of maternal calls and in ovo call learning. Because both adults also sing throughout the incubation phase, we broadcast songs to embryos and measured their heart rate response in relation to parental song rate and tutor identity (familiarity, sex). Embryos from acoustically active families (high parental song rate) had the strongest response to songs. Embryos responded (i) strongest to male songs irrespective of familiarity with the singer, and (ii) strongest if their father had a high song rate during incubation. This is the first evidence for a prenatal physiological response to particular songs (potential tutors) in the egg, in relation to the prenatal acoustic environment, and before the sensitive period for song learning.
Keywords: prenatal responsiveness, song learning, female song, tutor selection
1. Introduction
Early life experiences affect perceptual preference and cognitive development. In particular, prenatal auditory exposure influences the development of the auditory system and postnatal perceptual preferences in humans [1,2] and birds [3–5]. The fitness benefits of early environmental enrichment include improved postnatal learning [6,7] and the capacity to show an adaptive response to novelty [8], which could increase survival.
New research is shedding light on when a vocal learning organism may start to learn sound. For decades, the sensitive phase for song acquisition in songbirds was believed to start after hatching. Vocal learning relies on the identification and selection of appropriate tutors [9] and is generally guided by genetic predisposition [10,11] or acoustic and social criteria [11–13]. Recent findings have shown that the auditory brain regions involved in song discrimination (and thus song learning) differentiate before hatching [14].
In superb fairy-wrens (Malurus cyaneus), offspring respond to and learn vocal cues from maternal calls in ovo [15–18]. Females also produce songs inside the nest whereas males sing near the nest [19], and fledglings acquire their song elements via social transmission from both parents [20–21]. Embryos could thus exhibit preference for particular tutors based on familiarity. If embryos respond to song, prenatal song exposure could minimize mistakes during song acquisition, increase transmission of particular song characteristics, and reduce the risk of hybridization as parental singing close to the nest can increase familiarity-based preference for a vocal tutor [22]. Thus natural selection could favour costly parental singing [18,20] if high song rate during early development promotes offspring quality or adaptive preference for vocal tutors.
Here, we examined the effects of the prenatal acoustic environment (parental song rate) and singer identity (familiarity, sex) on the magnitude of prenatal response to conspecific songs. Presentation of relevant stimuli usually produces an orientation reflex [23] including both behavioural (e.g. head turn) and physiological (e.g. heart rate change) response [24]. Change in a physiological response, such as heart rate, thus provides insights into cognitive mechanisms, whereby a decrease in heart rate is associated with increased attention [16,25,26].
2. Material and methods
We recorded adult songs and embryo heart rates (HR) at Cleland Wildlife Sanctuary, South Australia (34°58′ S, 138°41′ E) between September and December 2015. We measured the correlation between parental song rate during incubation and change in prenatal HR to experimental broadcast of (i) mother's songs (n = 7), (ii) social father's songs (n = 7), (iii) unfamiliar female's songs (n = 7), or (iv) unfamiliar male's songs (n = 7).
We prepared 14 stimulus tracks (seven females, seven males), each consisting of 1 min of pre-playback silence (pre), 1 min of playback (trial; consisting of six evenly spaced songs) and 1 min of silence post-playback (post). For each track, we used three adult chatter songs (repeated twice; one individual per track) without overlapping sound. We recorded songs several days prior to playback using a Telinga parabolic microphone (Telinga Microphones, Sweden) connected to a portable Sound Devices 722 digital audio recorder (Sound Devices LLC, USA) at 44.1 kHz sampling rate, 16-bit depth. We removed sounds less than 1.5 kHz with a high pass filter, normalized playback (−15 db) and saved all tracks as uncompressed 16 bit wave files in Amadeus Pro 1.5 (Hairersoft Inc., Switzerland). We transferred all tracks onto an Apple iPod (Apple Inc., USA) connected to a Moshi Bass burger speaker (Moshi Corporation, USA; sensitivity: more than 80 dB; frequency response: 280 Hz to 16 kHz).
We collected 28 eggs from 28 clutches from 20 families (one egg per nest; incubation day 11 or 12). All tested nests had a clutch size of three eggs and were found in small native bushes in open grassland and hence had similar habitats. HR was measured in the field using a digital egg monitor (Buddy™, Vetronic Services, UK) [16,27], which was placed on a small portable heat pack to control for temperature. Once the egg was in the monitor, we broadcast a selected track (at approx. 83 db at 1 m) via iPod/speaker placed 5–10 cm from the monitor. Each egg was tested once with one stimulus track; HR was scored every 10 s. Eggs were returned to their nest for hatching. Parental song rate was recorded as the number of songs during two 1-h incubation protocols [18,19]. Parental age was 1 to 3+ years.
Data were analysed with SPSS 22 for Windows (SPSS Inc., USA). η2 and R2 are presented as measures of effect size. All data were normally distributed (Shapiro–Wilk: all p > 0.16). We applied ANOVA to the pre and post HR values with playback type as a fixed effect. We tested the impact of the family of origin, age of the eggs and breeding attempt (1–3) on the pre, trial and post HR values with MANOVA. We used a paired t-test to investigate difference in male and female song rate, and MANOVA to test for differences in song characteristics (frequencies, length, number of elements, element versatility). We used ANOVA to test for a relationship between parental age categories and singing rate. We defined HR response as the change between pre-trial and trial when exposed to conspecific song. We used linear regression to compare HR response with parental song rate, and ANOVA to test the effects of familiarity (familiar, unfamiliar) and sex of the singer (male, female) on HR response.
3. Results
HR values were approximately 249 ± 12 bpm (range 83–419 bpm) in pre-trial and approximately 216 ± 11 bpm (range 80–410 bpm) in post-trial. We found no difference in HR values in relation to playback type in the pre- (ANOVA: F3,27 = 1.61; p = 0.21; η2 = 0.17) and post-trial (F3,27 = 0.59; p = 0.63; η2 = 0.07), and no impact of the family of origin, age of the eggs or breeding attempt on the HR values (all p > 0.13).
Male songs only differed from female songs in peak frequencies, with higher frequencies for males (MANOVA: F1,13 = 5.49, p = 0.04; η2 = 0.31; all other p > 0. 12). Male song rate during incubation (12.6 ± 3.0 songs h−1) was higher (×2) than female song rate (6.5 ± 1.3), but the difference was not statistically significant (paired t-test: t25 = 1.83, p = 0.08). We did not find any relationship between parental age and song rate (ANOVA—males: F4,24 = 0.69, p = 0.61; females: F5,24 = 0.28, p = 0.92).
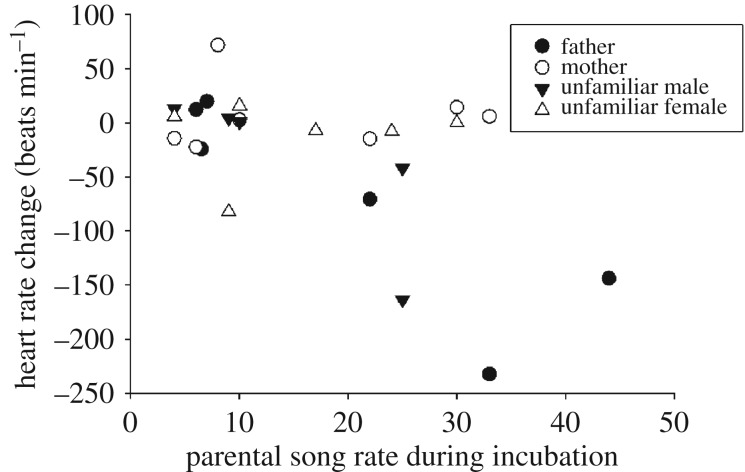
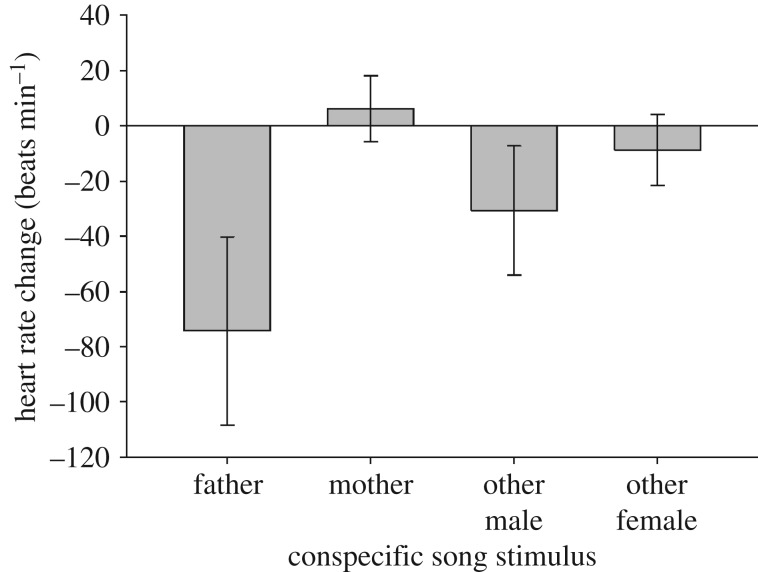
Embryos exposed to higher parental song rate during incubation had a stronger prenatal response to playback of conspecific song (linear regression: r = −0.47, t25 = −2.59, p = 0.02; R2 = 0.19; figure 1). Embryos had a stronger prenatal response to their father's song when fathers had high song rate (r = −0.40, t = −2.03, p = 0.05, R2 = 0.15), but prenatal response to mother's song was not predicted by maternal song rate (r = −0.32, t = −1.61, p = 0.12, R2 = 0.10). Embryos decreased their HR more after hearing male songs than after hearing female songs (ANOVA: F1,27 = 5.21, p = 0.03; η2 = 0.18; figure 2), irrespective of their familiarity with the singer (F1,27 = 0.40, p = 0.53; η2 = 0.02).
Figure 1.
Change in prenatal heart rate (beats min−1) following exposure to conspecific song (father, mother, unfamiliar male, unfamiliar female) shown in relation to parental song rate (songs h−1) during incubation. Embryos with larger negative response had lowered HR, which signals a more attentive response.
Figure 2.
Change in prenatal heart rate (beats per minute; mean ± s.e.) to broadcast of chatter song from the embryo's social father (n = 7), mother (n = 7), unfamiliar male (n = 7), or unfamiliar female (n = 7). Lower HR is indicative of greater attention towards the stimulus.
4. Discussion
Prenatal enrichment has been shown to facilitate postnatal auditory responsiveness and perceptual preferences [3]. Here, we show that prenatal enrichment not only affects postnatal but also prenatal preferences. Eggs exposed to higher parental song rate had stronger in ovo response to song compared to those exposed to lower song rate. Embryos also demonstrated a greater response to male songs, perhaps because male songs had higher peak frequencies than female songs. Within families, fathers sang more frequently than mothers during incubation and paternal song rate significantly correlated with the magnitude of prenatal response to fathers' song.
To our knowledge, this is the first evidence that discrimination towards particular songs (and hence potential tutors) is exhibited in the egg, well before the sensitive period (believed to start only after hatching). Song learning is supposedly guided by both learned and genetically inherited templates [28]. Determining whether early song discrimination is a fully innate behaviour or whether birds are influenced by templates learnt early in their development is challenging [29]. Most studies that have identified early song discrimination have argued for innate predisposition for vocal templates or tutors [30]. However, birds were often collected from their original nests as nestlings and therefore individuals may have been influenced by early experiences, either as younger nestlings or during incubation. The findings of this study suggest that cues perceived during embryonic development could influence selectivity for song tutors and that some behaviour that has previously been considered innate could be the result of embryonic experience, with implications for song dialects and divergence [31].
Acknowledgements
Thanks to Petra Hanke and Christine Evans for field assistance and song recordings.
Ethics
Permission to carry out fieldwork was received from the Department of Environment Water and Natural Resources (Z24699). This study was approved by the Animal Welfare Committee of Flinders University (E325-404).
Data accessibility
Data are deposited in the Flinders University data repository at doi:10.4226/86/59278bd972839.
Authors' contributions
D.C.-N. and S.K. conceived and designed the project, acquired and analysed the data, and wrote the manuscript. All authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
The authors declare no funding.
References
- 1.Moon C, Lagercrantz H, Kuhl PK. 2013. Language experienced in utero affects vowel perception after birth: a two-country study. Acta Paediatr. 102, 156–160. ( 10.1111/apa.12098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partanen E, Kujala T, Tervaniemi M, Huotilainen M. 2013. Prenatal music exposure induces long-term neural effects. PLoS ONE 8, e78946 ( 10.1371/journal.pone.0078946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain SU, Sharma RA, Wadhwa S. 2004. Effect of prenatal species-specific and music stimulation on the postnatal auditory preference of domestic chick. Indian J. Physiol. Pharmacol. 48, 174–183. [PubMed] [Google Scholar]
- 4.Chaudhury S, Jain S, Wadhwa S. 2010. Expression of synaptic proteins in the hippocampus and spatial learning in chicks following prenatal auditory stimulation. Dev. Neurosci. 32, 114–124. ( 10.1159/000279758) [DOI] [PubMed] [Google Scholar]
- 5.Harshaw C, Lickliter R. 2011. Biased embryos: prenatal experience alters the postnatal malleability of auditory preferences in bobwhite quail. Dev. Psychobiol. 53, 291–302. ( 10.1002/dev.20521) [DOI] [PubMed] [Google Scholar]
- 6.Kim H, et al. 2006. Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain Dev. 28, 109–114. ( 10.1016/j.braindev.2005.05.008) [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Yu L, Cai R, Zhang J, Sun X. 2009. Early auditory enrichment with music enhances auditory discrimination learning and alters NR2B protein expression in rat auditory cortex. Behav. Brain Res. 196, 49–54. ( 10.1016/j.bbr.2008.07.018) [DOI] [PubMed] [Google Scholar]
- 8.Jones RB. 1982. Effects of early environmental enrichment upon open-field behavior and timidity in the domestic chick. Dev. Psychobiol. 15, 105–111. ( 10.1002/dev.420150203) [DOI] [PubMed] [Google Scholar]
- 9.Laland KN. 2004. Social learning strategies. Anim. Learn. Behav. 32, 4–14. ( 10.3758/BF03196002) [DOI] [PubMed] [Google Scholar]
- 10.Wheatcroft D, Qvarnström A. 2015. A blueprint for vocal learning: auditory predispositions from brains to genomes. Biol. Lett. 11, 20150155 ( 10.1098/rsbl.2015.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marler P, Peters S. 1977. Selective vocal learning in a sparrow. Science 198, 519–521. ( 10.1126/science.198.4316.519) [DOI] [PubMed] [Google Scholar]
- 12.Greig EI, Taft BN, Pruett-Jones S. 2012. Sons learn songs from their social fathers in a cooperatively breeding bird. Proc. R. Soc. B 279, 3154–3160. ( 10.1098/rspb.2011.2582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheelwright NT, Swett MB, Levin II, Kroodsma DE, Freeman-Gallant CR, Williams H. 2008. The influence of different tutor types on song learning in a natural bird population. Anim. Behav. 75, 1479–1493. ( 10.1016/j.anbehav.2007.08.030) [DOI] [Google Scholar]
- 14.Kirn JR. 2010. The relationship of neurogenesis and growth of brain regions to song learning. Brain. Lang. 115, 29–44. ( 10.1016/j.bandl.2009.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colombelli-Négrel D, Hauber ME, Robertson J, Sulloway FJ, Hoi H, Griggio M, Kleindorfer S. 2012. Embryonic learning of vocal passwords in superb fairy-wrens reveals intruder cuckoo nestlings. Curr. Biol. 22, 2155–2160. ( 10.1016/j.cub.2012.09.025) [DOI] [PubMed] [Google Scholar]
- 16.Colombelli-Négrel D, Hauber ME, Kleindorfer S. 2014. Prenatal learning in an Australian songbird: habituation and individual discrimination in superb fairy-wren embryos. Proc. R. Soc. B 281, 20141154 ( 10.1098/rspb.2014.1154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleindorfer S, Evans C, Colombelli-Négrel D. 2014. Females that experience threat are better teachers. Biol. Lett. 10, 20140046 ( 10.1098/rsbl.2014.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleindorfer S, Hoi H, Evans C, Mahr K, Robertson J, Hauber ME, Colombelli-Négrel D. 2014. The cost of teaching embryos in superb fairy-wrens. Behav. Ecol. 25, 1131–1135. ( 10.1093/beheco/aru097) [DOI] [Google Scholar]
- 19.Kleindorfer S, Evans C, Mahr K. 2016. Female in-nest chatter song increases predation. Biol. Lett. 12, 20150513 ( 10.1098/rsbl.2015.0513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans C. 2016. Female song in the superb fairy-wren (Malurus cyaneus): perspectives informed by function and ontogeny. PhD Thesis, Flinders Univeristy, Adelaide. [Google Scholar]
- 21.Evans C, Kleindorfer S. 2016. Superb fairy-wren (Malurus cyaneus) sons and daughters acquire song elements from adult males and females. Front. Ecol. Evol. 4, 9 ( 10.3389/fevo.2016.00009) [DOI] [Google Scholar]
- 22.Dowling JL, Colombelli-Négrel D, Webster MS. 2016. Kin signatures learned in the egg? Red-backed fairy-wren songs are similar to their mother's in-nest calls and songs. Front. Ecol. Evol. 4, 48 ( 10.3389/fevo.2016.00048) [DOI] [Google Scholar]
- 23.Pavlov IP. 1927. Conditioned reflexes. An investigation of the physiological activity of the cerebral cortex. Oxford, UK: Oxford Univeristy Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein AS. 1969. To what does the orienting response respond? Psychophysiology 6, 338–350. ( 10.1111/j.1469-8986.1969.tb02911.x) [DOI] [PubMed] [Google Scholar]
- 25.Hauber ME, Pearson HE, Reh A, Merges A. 2002. Discrimination between host songs by brood parasitic brown-headed cowbirds (Molothrus ater). Anim. Cogn. 5, 129–137. ( 10.1007/s10071-002-0143-x) [DOI] [PubMed] [Google Scholar]
- 26.Soha JA, Marler P. 2001. Cues for early discrimination of conspecific song in the white-crowned sparrow (Zonotrichia leucophrys). Ethology 107, 813–826. ( 10.1046/j.1439-0310.2001.00713.x) [DOI] [Google Scholar]
- 27.Lierz M, Gooss O, Hafez HM. 2006. Noninvasive heart rate measurement using a digital egg monitor in chicken and turkey embryos. J. Avian Med. Surg. 20, 141–146. ( 10.1647/2005-017R.1) [DOI] [Google Scholar]
- 28.Marler P. 1984. Song learning: innate species differences in the learning process. In The biology of learning, vol 29 (eds Marler P, Terrace HS), pp. 289–309. Berlin, Germany: Springer. [Google Scholar]
- 29.Shizuka D. 2014. Early song discrimination by nestling sparrows in the wild. Anim. Behav. 92, 19–24. ( 10.1016/j.anbehav.2014.03.021) [DOI] [Google Scholar]
- 30.Marler P. 1990. Innate learning preferences: signals for communication. Dev. Psychobiol. 23, 557–568. ( 10.1002/dev.420230703) [DOI] [PubMed] [Google Scholar]
- 31.Wilkins MR, Seddon N, Safran RJ. 2013. Evolutionary divergence in acoustic signals: causes and consequences. Trends Ecol. Evol. 28, 156–166. ( 10.1016/j.tree.2012.10.002) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are deposited in the Flinders University data repository at doi:10.4226/86/59278bd972839.