Abstract
Appropriate response to others is necessary for social interactions. Yet little is known about how neurotransmitters regulate attractive and repulsive social cues. Using genetic and pharmacological manipulations in Drosophila melanogaster, we show that dopamine is contributing the response to others in a social group, specifically, social spacing, but not the avoidance of odours released by stressed flies (dSO). Interestingly, this dopamine-mediated behaviour is prominent only in the day-time, and its effect varies depending on tissue, sex and type of manipulation. Furthermore, alteration of dopamine levels has no effect on dSO avoidance regardless of sex, which suggests that a different neurotransmitter regulates this response.
Keywords: social spacing, dSO avoidance, dopamine, VMAT, pale, Catsup
1. Introduction
Social space is a measurable characteristic of individuals in groups, and is based on a balance of attractive and repulsive social cues [1]. Abnormal social spacing is observed in individuals with disorders such as autism spectrum or Williams syndrome [2]. However, few studies have investigated the neural mechanisms underlying this basic social response to others.
We used the genetically tractable Drosophila melanogaster model and compared two types of response to another individual: social spacing [3–9], and avoidance of the marking left by flies that have been stressed, i.e. Drosophila stress odorant (dSO), composed partially of CO2 [10,11].
Previous social experience affects social spacing in Drosophila; and vision but not classical odorant perception is necessary to maintain this distancing [3,7]. Expression of genes involved in synaptic function have been reported to be necessary for proper social spacing [6–8], and the neural circuitry underlying CO2 perception has been identified [10]. However, the neurotransmitters involved in these social behaviours have yet to be determined.
The dopamine system is proposed to underlie a conserved network for social decision-making from eusocial insects such as bees [12], through birds and rodents [13], to humans [14]. Therefore, dopaminergic signalling is a strong candidate for the modulation of social interactions. We manipulated tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis [15], present in both neuronal and hypodermal dopamine cells [16]. However, intracellular monoamine homeostasis is controlled by the vesicular monoamine transporter (VMAT) [17], and is thus also an excellent candidate for studies aiming to identify the role of dopamine modulation on behaviour.
2. Material and methods
All lines of Drosophila used are described in the electronic supplementary material. Locomotion was used as an indicator of activity level [18], and is known to be altered in the mutants and conditions tested (in dopamine synthesis [19] and in alteration of VMAT expression [20]). Social space and dSO avoidance were performed as described in [4] and [11]. We fed drugs altering dopamine biosynthesis [15]—30 mM 3-iodotyrosine (a pathway inhibiter) or 1 mM l-DOPA (converted to dopamine)—to 3–4 day old male flies for 24 h. Statistical analyses were performed using GraphPad Prism 7. Detailed descriptions can be found online, in the electronic supplementary material.
3. Results
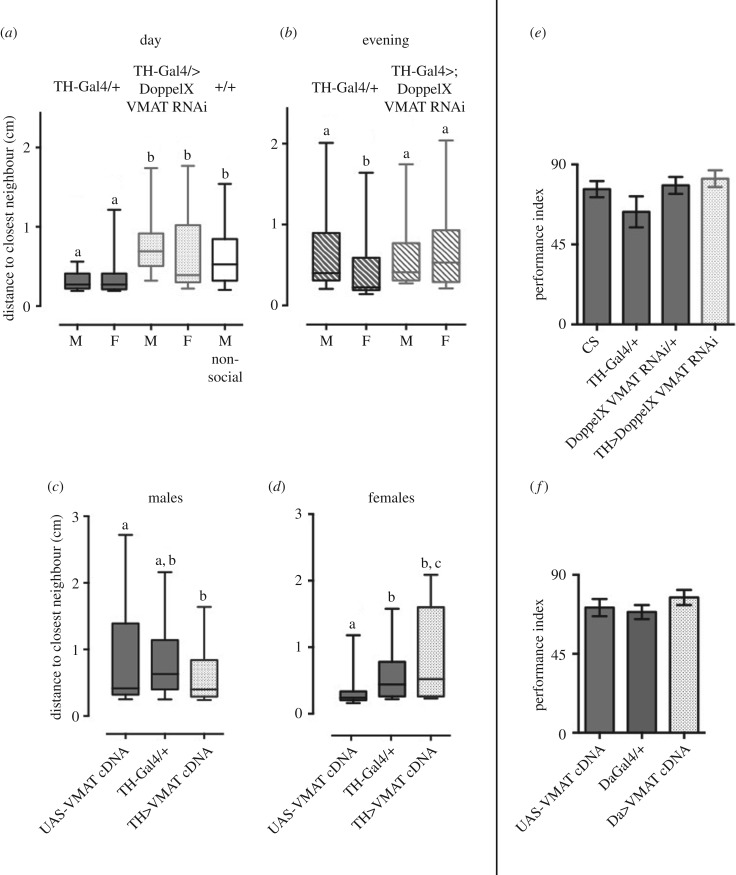
VMAT loss-of-function mutants—which have less available intracellular dopamine [20]—and flies with a reduced expression of VMAT in dopaminergic cells (TH>VMAT RNAi) display an increase in social spacing, similar to non-social flies, regardless of their sex (figure 1a and table 1, and electronic supplementary material, figure S1a–c). However, in the evening, the TH>VMAT RNAi lines are not different from the controls (figure 1b). In contrast, overexpression of the nerve cells variant, VMAT-A, using either TH-Gal4 driver (expressed in most dopaminergic cells [16]) or Da-Gal4 driver (not expressed in serotonin neurons [20]) led to sex-specific altered social spacing. Males are closer (figure 1c and electronic supplementary material, figure S1d), and females are further apart (figure 1d). In both sexes, there is no change in dSO avoidance in any manipulation of VMAT expression performed (figure 1e and electronic supplementary material, figure S2).
Figure 1.
(a,b) Social spacing of males (M) and females (F) overexpressing VMAT RNAi (DoppelX) with a TH-Gal4 driver during (a) the day (number of hours since light turned on (ZT) = 5–7) and (b) the evening (ZT = 11–13). (c,d) Social spacing of (c) males and (d) females overexpressing UAS-VMAT with a TH-Gal4 driver. (e,f) dSO avoidance of mixed sex Canon-S (CS) emitter flies by flies of the indicated genotypes. Letters (a, b, c) indicate groups that are statistically different in multiple comparisons (table 1). The data are represented as typical box and whiskers (box: 50% of the data distribution, middle line: median, whiskers 10%–90%).
Table 1.
Experimental conditions and statistical tests performed.
| experiment | replicates and conditions | statistical test performed |
|---|---|---|
| figure 1a | n = 2 independent repeats of 40 flies in large chambers for each genotype | comparison of medians, Kruskal–Wallis test p < 0.0001; Dunnet post-test multiple comparison a#b p < 0.01 |
| figure 1b | n = 2 independent repeats of 40 flies in large chambers for each genotype | comparison of medians, Kruskal–Wallis test p < 0.0001; Dunnet post-test multiple comparison a#b p < 0.01 |
| figure 1c | n = 6 for VMAT cDNA/+ and TH-Gal4/+, n = 10 for TH>VMAT cDNA independent repeats of 15 individuals in small chambers | Kruskal–Wallis test p < 0.02; Dunnet post-test multiple comparison a#b p < 0.02 |
| figure 1d | n = 6 for UAS-VMAT/+ and TH-Gal4/+, n = 10 for TH>VMAT cDNA independent repeats of 15 individuals in small chambers | Kruskal–Wallis test p < 0.0001; Dunn’ post-test multiple comparison a#b p < 0.0002 |
| figure 1e | Canton-S, n = 6, TH-Gal4 n = 8, DoppelX VMAT RNAi/+, TH>DoppelX VMAT RNAi n = 9; 3 independent trials of approx. 30 flies for 60 s, with 2–4 internal repeats | one-way ANOVA p = 0.1113; t-test p > 0.21 for each comparison with Canton-S |
| figure 1f | UAS-VMAT cDNA/+ n = 6; DaGal4/+, n = 10; Da>VMAT cDNA n = 8 independent repeats of 30 individuals | one-way ANOVA p = 0.3751; t-test p > 0.197 for each comparison with UAS-VMAT/+ |
| experiment | replicates | control used for normalization |
|---|---|---|
|
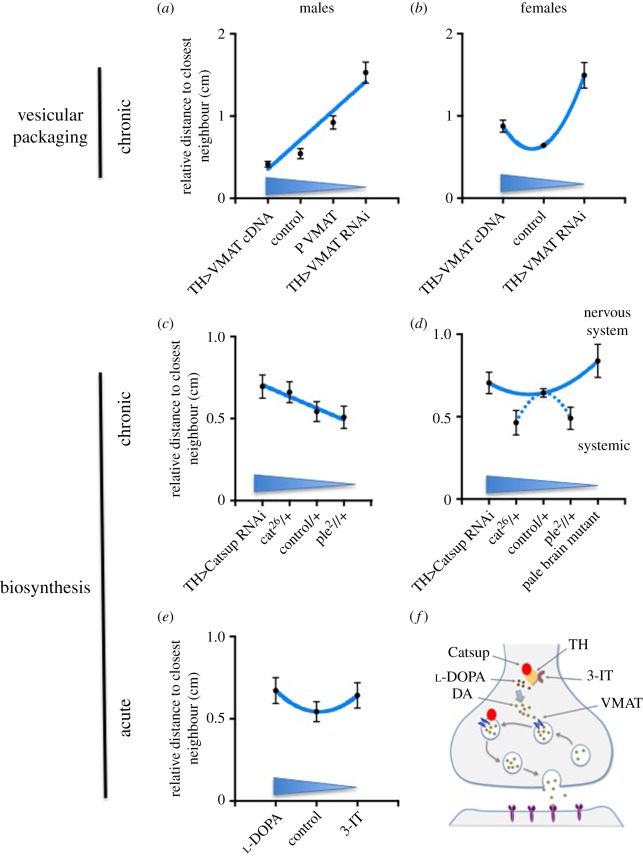
figure 2a, male VMAT |
TH>VMAT cDNA n = 12; control n = 18; P VMAT n = 5; TH>VMAT RNAi (DoppelX) n = 12 | Canton-S |
|
figure 2b, female VMAT |
TH>VMAT cDNA n = 10; control n = 0; TH>VMAT RNAi (DoppelX) n = 2 (in this case, s.e.m. obtained from approx. 80 flies) | Canton-S |
|
figure 2c, male biosynthesis |
TH>Catsup RNAi n = 5, cat26/+ n = 3, control n = 9, ple2/+ n = 3 | for TH>Catsup RNAi control was TH-Gal4/+; for cat26/+ and ple2 control was Canton-S |
|
figure 2d, female biosynthesis |
TH>Catsup RNAi n = 3, cat26/+ n = 3, control n = 14, ple2/+ n = 3, pale brain mutant n = 6 | for TH>Catsup RNAi control was TH-Gal4/+; for cat26/+ and ple2 control was Canton-S |
|
figure 2e, male acute |
3 independent repeats with 2–4 internal replicates of 40, such that control n = 11, 3-IT n = 8 and l-DOPA n = 9 | Canton-S fed vehicle |
Females lacking tyrosine hydroxylase in the nervous system [16] have increased social spacing (electronic supplementary material, figure S3a). The effect is also strongly diminished in the evening (electronic supplementary material, figure S3b). As expected [21], their locomotion is reduced (electronic supplementary material, figure S3c). However, male but not female flies that expressed an RNAi against an inhibitor of tyrosine hydroxylase, catsup, display an increased social spacing (electronic supplementary material, figure S3d). Additional milder mutants, of either catsup (Cat26/+) or tyrosine hydroxylase (ple2/+) lead to no effect in males, whereas females appear closer (electronic supplementary material, figure S3e).
Finally, we fed male Canton-S flies two drugs, l-DOPA and 3-iodotyrosine (3-IT), respectively increasing and decreasing dopamine synthesis in an acute manner [22]. In contrast to the chronic modifications of dopamine synthesis, both treatments led to a similar increase in social spacing, and no effect on dSO avoidance (electronic supplementary material, figure S3f,g), but the previously reported effect on their locomotion [19] (electronic supplementary material, figure S3h).
In order to compare these different treatments, we normalized the data to their respective controls (figure 2—see electronic supplementary material for details). Dopamine affects social spacing in a sex-specific manner that is dependent on whether only the TH-neurons or all tissues are affected and whether the treatment was chronic or acute.
Figure 2.
Linear (a,c) and curvilinear (U-shaped, b,d,e) distribution of social spacing in response to presynaptic modification in dopamine levels, depending on type of alteration (chronic or acute, systemic or nervous system, biosynthesis or vesicular packaging), and sex. The genotypes or treatments are ordered in expected decrease in dopamine (from high to low—blue arrows). Data are represented as relative means (± s.e.m.) of the social space distributions. (f) Diagram of a synapse, indicating the proteins altered by the pharmacological and genetic manipulations in this study. DA, dopamine.
4. Discussion
Abnormal expression of VMAT in dopaminergic neurons alters social spacing, with no effect on dSO avoidance. Furthermore, both chronic and acute modifications of dopamine synthesis also affect social spacing. Although dopamine and VMAT alterations affect locomotion, including in the mutants tested here [3,18,19], we observed no correlation between the social spacing and the locomotion, as reported before [4].
In males, increasing dopamine in the TH-neurons leads to an increase in social space, a result suggested previously, in the context of courtship behaviour [23,24]. However, increasing dopamine in all tissues leads to the opposite effect. In females any manipulation in the TH-neurons leads to increased social space, while manipulation affecting all tissues leads to reduced social space. Although the underlying mechanisms are yet to be understood, these linear (in males) or U-shaped (in females) dose-dependent effects have been proposed by others [25]. It is not surprising to observe different functional consequences for abnormal dopamine concentration in the hypodermal cells versus abnormal dopamine tone at the synapse. Dopamine might affect emission of social cues in hypodermic cells, while affecting the decision process in response to those cues in neuronal cells. Similarly, the sex-specific variations probably reflect differences in how the genetic manipulations performed alter dopamine concentrations in the two sexes. Indeed, adult females have higher dopamine content than males [26]. Furthermore, flies might rely on sexually dimorphic dopaminergic neurons to generate proper social spacing, as shown previously for stress response [27].
Finally, we found no effect on social spacing of loss of function of VMAT in dopaminergic cells in the evening, which supports previous reports of the role of dopamine and VMAT in sleep and arousal, although no change in activity patterns themselves has been reported [28–32].
In summary, we show for the first time to our knowledge that dopamine is a key component of the regulation of social space in Drosophila melanogaster, probably at the level of both emitting and perceiving social signals. Better understanding of the sex- and cell-specificity of dopamine requirements in these social responses might reveal conserved neural correlates.
Supplementary Material
Data accessibility
The datasets are available at Dryad: (http://dx.doi.org/10.5061/dryad.dn5tk) [33]. Supplementary material (results, figures, table, material and methods, and references) is provided in an electronic form online (https://dx.doi.org/10.6084/m9.figshare.c.3838141).
Authors' contributions
Data acquisition: R.W.F., A.A.A., M.N., O.F., M.L. and R.R.A., and with A.F.S. data analysis; writing: R.W.F., M.L. and A.F.S.; editing and critiquing: A.A.A., M.N., O.F., R.R.A. and J.M.O.; supervision: A.F.S. and J.M.O.; conception: A.F.S. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We declare no conflict of interest.
Funding
PSC-CUNY Awards 41, 42 and 43, jointly funded by the Professional Staff Congress and the City University of New York, Western Foundation internal grant and NSERC RGPIN-2015-04275 grant to A.F.S.; training support from the Merck Ciencia Hispanic Scholars Program to R.W.F.; Robert Noyce Mathematics and Science Teachers and NYC Louis Stroke Alliance for Minority Participation scholarships to A.A.A.
References
- 1.Mogilner A, Edelstein-Keshet L, Bent L, Spiros A. 2003. Mutual interactions, potentials, and individual distance in a social aggregation. J. Math. Biol. 47, 353–389. ( 10.1007/s00285-003-0209-7) [DOI] [PubMed] [Google Scholar]
- 2.Lough E, Hanley M, Rodgers J, South M, Kirk H, Kennedy DP, Riby DM. 2015. Violations of personal space in young people with autism spectrum disorders and Williams syndrome: insights from the Social Responsiveness Scale. J. Autism Dev. Disord. 45, 4101–4108. ( 10.1007/s10803-015-2536-0) [DOI] [PubMed] [Google Scholar]
- 3.Simon AF, Chou MT, Salazar ED, Nicholson T, Saini N, Metchev S, Krantz DE. 2012. A simple assay to study social behavior in Drosophila: measurement of social space within a group. Genes Brain Behav. 11, 243–252. ( 10.1111/j.1601-183X.2011.00740.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeil A, Jolley SN, Akinleye AA, Nurilov M, Rouzyi Z, Milunovich A, Chambers MC, Simon AF. 2015. Conditions affecting social space in Drosophila melanogaster. J. Vis. Exp. 105, e53242 ( 10.3791/53242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson BB, Scott A, Dukas R. 2016. Social behavior and activity are decoupled in larval and adult fruit flies. Behav. Ecol. 27, 820–828. ( 10.1093/beheco/arv225) [DOI] [Google Scholar]
- 6.Hahn N, et al. 2013. Monogenic heritable autism gene neuroligin impacts Drosophila social behaviour. Behav. Brain Res. 252, 450–457. ( 10.1016/j.bbr.2013.06.020) [DOI] [PubMed] [Google Scholar]
- 7.Burg ED, Langan ST, Nash HA. 2013. Drosophila social clustering is disrupted by anesthetics and in narrow abdomen ion channel mutants. Genes Brain Behav. 12, 338–347. ( 10.1111/ggb.12025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise A, et al. 2015. Drosophila mutants of the autism candidate gene neurobeachin (rugose) exhibit neuro-developmental disorders, aberrant synaptic properties, altered locomotion, impaired adult social behavior and activity patterns. J. Neurogenet. 29, 135–143. ( 10.3109/01677063.2015.1064916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur K, Simon AF, Chauhan V, Chauhan A. 2015. Effect of bisphenol A on the behavior of Drosophila melanogaster. Behav. Brain Res. 284, 77–84. ( 10.1016/j.bbr.2015.02.001) [DOI] [PubMed] [Google Scholar]
- 10.Suh GSB, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. 2004. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431, 854–859. ( 10.1038/nature02980) [DOI] [PubMed] [Google Scholar]
- 11.Fernandez RW, Akinleye AA, Nurilov M, Feliciano O, McDonald IS, Simon AF. 2014. Straightforward assay for quantification of social avoidance in Drosophila melanogaster. J. Vis. Exp. 94, e52011 ( 10.3791/52011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheiner R, Baumann A, Blenau W. 2006. Aminergic control and modulation of honeybee behaviour. Curr. Neuropharmacol. 4, 259–276. ( 10.2174/157015906778520791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodson JL, Kingsbury MA. 2013. What's in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm. Behav. 64, 103–112. ( 10.1016/j.yhbeh.2013.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. 2010. Genetics of human social behavior. Neuron 65, 831–844. ( 10.1016/j.neuron.2010.02.020) [DOI] [PubMed] [Google Scholar]
- 15.Monastirioti M. 1999. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc. Res. Tech. 45, 106–121. () [DOI] [PubMed] [Google Scholar]
- 16.Friggi-Grelin F, Iche M, Birman S. 2003. Tissue-specific developmental requirements of Drosophila tyrosine hydroxylase isoforms. Genesis 35, 260–269. ( 10.1002/gene.1082) [DOI] [PubMed] [Google Scholar]
- 17.Lawal HO, Krantz DE. 2013. SLC18: vesicular neurotransmitter transporters for monoamines and acetylcholine. Mol. Aspects Med. 34, 360–372. ( 10.1016/j.mam.2012.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang HY, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. 2006. Overexpression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Mol. Psychiatry 11, 99–113. ( 10.1038/sj.mp.4001742) [DOI] [PubMed] [Google Scholar]
- 19.Hanna ME, Bednarova A, Rakshit K, Chaudhuri A, O'Donnell JM, Krishnan N. 2015. Perturbations in dopamine synthesis lead to discrete physiological effects and impact oxidative stress response in Drosophila. J. Insect Physiol. 73, 11–19. ( 10.1016/j.jinsphys.2015.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon AF, et al. 2009. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics 181, 525–541. ( 10.1534/genetics.108.094110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riemensperger T, et al. 2011. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl Acad. Sci. USA 108, 834–839. ( 10.1073/pnas.1010930108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, et al. 2011. Catecholamines up integrates dopamine synthesis and synaptic trafficking. J. Neurochem. 119, 1294–1305. ( 10.1111/j.1471-4159.2011.07517.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu T, Dartevelle L, Yuan C, Wei H, Wang Y, Ferveur JF, Guo A. 2008. Increased dopamine level enhances male–male courtship in Drosophila. J. Neurosci. 28, 5539–5546. ( 10.1523/JNEUROSCI.5290-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Dartevelle L, Yuan C, Wei H, Wang Y, Ferveur JF, Guo A. 2009. Reduction of dopamine level enhances the attractiveness of male Drosophila to other males. PLoS ONE 4, e4574 ( 10.1371/journal.pone.0004574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Swinderen B, Andretic R. 2011. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proc. R. Soc. B 278, 906–913. ( 10.1098/rspb.2010.2564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denno ME, Privman E, Venton BJ. 2014. Analysis of neurotransmitter tissue content of Drosophila melanogaster in different life stages. ACS Chem. Neurosci. 6, 117–123. ( 10.1021/cn500261e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Argue KJ, Neckameyer WS. 2013. Sexually dimorphic recruitment of dopamine neurons into the stress response circuitry. Behav. Neurosci. 127, 734–743. ( 10.1037/a0033807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClung CA. 2013. How might circadian rhythms control mood? Let me count the ways. Biol. Psychiatry 74, 242–249. ( 10.1016/j.biopsych.2013.02.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nall AH, Sehgal A. 2013. Small-molecule screen in adult Drosophila identifies VMAT as a regulator of sleep. J. Neurosci. 33, 8534–8540. ( 10.1523/JNEUROSCI.0253-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno T, Masuda N, Kume S, Kume K. 2012. Dopamine modulates the rest period length without perturbation of its power law distribution in Drosophila melanogaster. PLoS ONE 7, e32007 ( 10.1371/journal.pone.0032007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasture A, El-Kasaby A, Szollosi D, Asjad HM, Grimm A, Stockner T, Hummel T, Freissmuth M, Sucic S. 2016. Functional rescue of a misfolded Drosophila melanogaster dopamine transporter mutant associated with a sleepless phenotype by pharmacological chaperones. J. Biol. Chem. 291, 20876 ( 10.1074/jbc.M116.737551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nall AH, Shakhmantsir I, Cichewicz K, Birman S, Hirsh J, Sehgal A. 2016. Caffeine promotes wakefulness via dopamine signaling in Drosophila. Sci. Rep. 6, 20938 ( 10.1038/srep20938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez RW, Akinleye AA, Nurilov M, Feliciano O, Lollar M, Aijuri RR, O'Donnell JM, Simon AF. 2017. Data from: Modulation of social space by dopamine in Drosophila melanogaster, but no effect on the avoidance of the Drosophila stress odorant. Dryad Digital Repository. ( 10.5061/dryad.dn5tk) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fernandez RW, Akinleye AA, Nurilov M, Feliciano O, Lollar M, Aijuri RR, O'Donnell JM, Simon AF. 2017. Data from: Modulation of social space by dopamine in Drosophila melanogaster, but no effect on the avoidance of the Drosophila stress odorant. Dryad Digital Repository. ( 10.5061/dryad.dn5tk) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets are available at Dryad: (http://dx.doi.org/10.5061/dryad.dn5tk) [33]. Supplementary material (results, figures, table, material and methods, and references) is provided in an electronic form online (https://dx.doi.org/10.6084/m9.figshare.c.3838141).