Abstract
Previously, it has been shown that long-distance migrants, garden warblers (Sylvia borin), were disoriented in the presence of narrow-band oscillating magnetic field (1.403 MHz OMF, 190 nT) during autumn migration. This agrees with the data of previous experiments with European robins (Erithacus rubecula). In this study, we report the results of experiments with garden warblers tested under a 1.403 MHz OMF with various amplitudes (∼0.4, 1, ∼2.4, 7 and 20 nT). We found that the ability of garden warblers to orient in round arenas using the magnetic compass could be disrupted by a very weak oscillating field, such as an approximate 2.4, 7 and 20 nT OMF, but not by an OMF with an approximate 0.4 nT amplitude. The results of the present study indicate that the sensitivity threshold of the magnetic compass to the OMF lies around 2–3 nT, while in experiments with European robins the birds were disoriented in a 15 nT OMF but could choose the appropriate migratory direction when a 5 nT OMF was added to the stationary magnetic field. The radical-pair model, one of the mainstream theories of avian magnetoreception, cannot explain the sensitivity to such a low-intensity OMF, and therefore, it needs further refinement.
Keywords: magnetic compass, radical-pair model, radiofrequency field, orientation, bird migration, garden warbler
1. Introduction
Migratory birds are known to obtain information for orientation and navigation tasks from the geomagnetic field [1–3]. Even though the physiological mechanisms of the magnetic sense are not yet definitely established, it is widely believed that birds use two magnetoreception systems: chemical magnetoreceptor in their eyes [1,2] and magnetite-based magnetoreceptor probably located in the upper beak [3]. The chemical magnetoreception system is presumably used for orientation tasks (magnetic compass) [4] and the iron-based magnetoreceptor may be a part of the magnetic map [5–7].
The magnetic compass in birds was discovered in the 1960s and 1970s [8]. Since that time, many features of this compass system have been revealed. It has an inclination nature, in contrast to the human technical compass and magnetic compass in mammals [9]: migratory birds use the angle between the gravity vector and the lines of the geomagnetic field [8]; therefore, they cannot distinguish between the magnetic North and South, but only between ‘poleward’ and ‘equatorward’ directions in both hemispheres. The magnetic compass of birds is light-dependent and does not work in total darkness [10]; in many orientation experiments songbird migrants chose the appropriate migratory direction under short-wavelength light (UV, blue and green) [11–15] but were disoriented under yellow and red light [11,12,16,17]. Furthermore, we know that information from the magnetoreceptor in their eyes is analysed in the forebrain area called Cluster N. It is a part of the visual Wulst, linked with the eyes via a thalamofugal visual pathway and is highly active during migratory restlessness in nocturnal songbird migrants in contrast to non-migrants [18–21]. With this area lesioned, the birds lose their ability to orient using information from the geomagnetic field, but still can use celestial cues [4].
Despite intensive investigation of the magnetic compass and of avian magnetoreception in general during recent decades, the physical principles of this compass system are not known with certainty. The most well-developed and broadly discussed hypothesis is that it is based on spin-dependent photochemical reactions. The idea that such reactions may play a crucial role in the magnetic compass sense was initially proposed [22] and further developed into the radical-pair model (RPM) by Schulten and co-workers [23]. According to this theory, migratory birds can use cryptochromes, blue-light-sensitive flavoprotein molecules [24], as a magnetoreceptor, because cryptochromes are the only class of organic photoreceptors in vertebrates that can form a long-lived radical pair upon absorption of a photon. Typically, the radical pair forms in a singlet state (with antiparallel spins of the two uncoupled electrons). Owing to hyperfine interaction with magnetic moments of atomic nuclei, the radical pair can transform from this state to a triplet state (with parallel electron spins) and back; this process is known as singlet–triplet interconversion. While in the singlet state, the radicals have a chance to recombine. If they do not recombine, the reaction may go further. For this reason, the reaction yield depends on the efficiency of the singlet–triplet interconversion which, in turn, is affected by the external magnetic field. If the effect of external field varies with the molecule orientation with respect to the magnetic field lines (which could be made possible by anisotropic hyperfine interactions [23]), a chemical compass may be, in principle, realized. The ways of further transduction of the chemical stimulus are debatable; the general idea is that the directions of cryptochromes' molecular axes are correlated with their positions in the retina, giving rise to magnetic field-induced visual patterns [23,25].
This theory is supported by several lines of evidence: (i) four types of cryptochromes (Cry 1a, Cry 1b, Cry 2 and Cry 4) have been found in avian retina [2,26–28]; (ii) expression levels of Cry 1a and Cry 1b, the two putative magnetoreception molecules, depend on the migratory status of birds [29] and expression of Cry 1b may vary with season [30] (but see [31]); (iii) this model can explain the inclination nature of the magnetic compass [23]; (iv) the absorption spectrum of cryptochromes (blue) corresponds to the light conditions (from UV to green) under which migratory birds showed magnetic orientation in behavioural experiments; and (v) lifetime of cryptochrome radicals is sufficient to allow for the Earth-strength magnetic field to modulate radical-pair reaction [26] (for details, see [2,5]). According to the authors of this theory, the disruptive effect of oscillating magnetic fields (OMFs) is one of its crucial proofs [23]. Several studies performed with migratory and non-migratory birds showed that the OMF can destroy the ability to orient using the magnetic compass [32–38] and, therefore, the disruptive effect of the OMF is regarded as a diagnostic tool testing for the radical-pair reaction as the basis of the magnetic compass in other animals [39,40]. In many of these experiments, a medium-distance songbird migrant, namely the European robin (Erithacus rubecula), was used as a model species. Experimental birds were disoriented both in a narrow-band radiofrequency (RF) field [34–37] (but see [38]) and a broadband RF field [35,38]. Very low intensities of the RF field, e.g. 15 and 48 nT, were sufficient to prevent European robins from using their magnetic compass, whereas at 5 nT their orientation was not affected by the time-dependent electromagnetic field [35]. Thus, the sensitivity threshold of the European robin to the OMF is believed to lie between 5 and 15 nT.
The current physical model cannot explain this very strong sensitivity of songbirds to the weak OMF [1,41–43]. In this study, we analysed the magnetic orientation of garden warblers (Sylvia borin) with an even weaker narrow-band 1.403 MHz OMF (with amplitudes of ∼0.4, 1, ∼2.4, 7 and 20 nT) added to the static geomagnetic field of 50 100 nT. Our aim was to find the sensitivity threshold to the disruptive effect of the RF field (in intensity) in this species.
2. Material and methods
2.1. Study site, model species and bird keeping
We performed orientation tests with first-year garden warblers mist-netted during autumn migration on the Courish Spit (Kaliningrad region, Russia; 55°09′ N, 20°52′ E). After capture, we kept the birds outdoors in individual cages (60 × 40 × 40 cm) for at least 3–5 days before the first test to give them an opportunity to acclimate to cage conditions. The birds experienced natural photoperiod, local geomagnetic field conditions and had access to all astronomic orientation cues (Sun, sunset polarization patterns and stars) during their time in captivity. Garden warblers were provided ad libitum with mealworms (Tenebrio molitor), a homemade mix of mashed boiled eggs with grated carrots, black elderberry (Sambucus nigra) and a vitamin supplement in fresh water.
2.2. Behavioural experiments and parameters of oscillating magnetic field
2.2.1. Experiments in autumn 2014–2015
In 2014–2015, the experiments included two phases. During the first phase (mid-August to early September), the migratory orientation of garden warblers was studied in the natural magnetic field (NMF) in Emlen funnels [44] under a simulated overcast sky. We used modified Emlen funnels made of aluminium (top diameter 300 mm, bottom diameter 100 mm, slope 45° with the top opening covered by netting). On top of the Emlen funnels, we put lids made of frosted glass which completely obscured the stars and any other patterns but let enough light in. Thus, the only orientation cue available to the experimental birds during the test was the geomagnetic field. After testing the birds for at least three and up to five times and receiving at least two and up to five results when the birds were sufficiently active, the birds were transferred to the second phase.
In the second phase of tests (early to late September), the same birds were tested in the OMF in similar Emlen funnels but made of cardboard (a dielectric material). The OMF was created by coils 0.75 m in diameter, fed from a commercial, stabilized, high-frequency generator and a custom-made broadband power amplifier, which served to match the generator output with a 50 Ω coaxial cable 40 m in length. Each coil comprised three wraps of isolated copper wire 1 mm in cross section, fixed to a wooden frame. The cable transmitted the RF current to coils and allowed us to place them at a distance from the RF source assembly (generator, amplifier and power supply). The source assembly was put into a wooden case, placed in a clearing in reeds approximately 25 m along the straight line from the centre of another clearing, where six wooden tables for experimental cages were set up. The sound of working devices was not discernible already 2 m apart from the case and all the more near experimental tables; it was far below the natural environmental noise. On the tables, up to 12 coils could be installed, two on each (the distance between RF coils was approx. 0.5 m, between tables with various OMF conditions—at least 2 m). Coils were connected in parallel by small lengths of coaxial cable to the main RF cable and mounted so that one side of the coil was raised above the tabletop to increase the angle between the OMF and the NMF, which as a result was approximately 30°. Emlen funnels were placed on the tables, one in the centre of each coil.
We used narrow-band RF fields with a frequency of 1.403 MHz, matching the Larmor frequency of a free-standing electron spin in the local geomagnetic field of 50 100 nT, and with amplitudes of 20, 7 and 1 nT (20, 7 and 1 nT subsamples, respectively). In addition, the same garden warblers also were tested in the NMF again (0 nT subsample, RF coils were turned off) simultaneously with experiments in the OMF. We did that to control whether birds did not lose their motivation to migrate after being kept in the aviary for a long time or changing their direction of activity in the Emlen funnel during the second part of the season according to the clock-and-compass concept [45]. The parameters of the OMF were controlled before and after each test. To this end, we used a single wire loop 25 cm in diameter, connected to a digital oscilloscope SDS 200A (SoftDSP, Republic of Korea). The loop was placed consequently in the centre of each coil. The periodic in time induction EMF was visualized using the computer software SoftScope v. 2.5 (SoftDSP, Republic of Korea), and its frequency and amplitude were measured, and the latter recalculated into the amplitude of the RF magnetic field.
Each bird was tested in the OMF randomly at least three times but approximately the same number of times for each OMF amplitude (20, 7, 1 and 0 nT). All tests in the OMF were performed under frosted glass, so that the birds had no access to astronomic orientation cues. Orientation tests during both phases (in the NMF and the OMF) started at the beginning of astronomical twilight and lasted for 40 min.
2.2.2. Experiments in autumn 2016
In 2016, we changed the design of our experiments to avoid the effect of progress of season on orientation found in autumn 2015: after catching and keeping them in an outdoor aviary for several days, garden warblers were tested in three experimental conditions at the same time (in the NMF and a 1.403 MHz RF field with ∼0.4 and ∼2.4 nT amplitudes). Oscillating currents to feed OMF coils were produced by a signal generator Rigol DG4162 (Rigol Technologies, Inc., Beaverton, USA). Parameters of the narrow-band oscillating field were controlled with the same 0.25 m loop antenna as in previous years, connected to a digital storage oscilloscope Tektronix 2012B (Tektronix, Inc., USA) before and after each test.
All orientation tests were performed only in cardboard Emlen funnels and lasted 40 min after the beginning of astronomical twilight. We used the same size and position of coils for the OMF as in previous experiments; the birds were tested randomly at least three times in every experimental condition. All tests were performed under frosted glass as in previous years.
2.3. Measurements of frequency spectra of the ambient electromagnetic noise and of the applied oscillating magnetic field
In 2014–2015, the level of ambient magnetic noise was controlled using the same equipment as for measuring the OMF amplitude, and was found to be marginally detectable. In 2016, we attempted a more accurate measurement of the magnetic noise spectrum. To this end, we used a custom-made selective amplifier/detector with a bandwidth of 100 kHz and sensitivity threshold of 30 nV/Hz1/2 and two magnetic antennas with different frequency responses. The first one was a single-loop 1.5 × 1.5 m antenna. In the measured frequency range of 0–10 MHz, its sensitivity was well fitted by the linear equation for electromagnetic induction of a wire loop
| 2.1 |
where U is the detected voltage amplitude, B is the magnetic field amplitude, f is its frequency and S is the loop area. The resulting sensitivity threshold with this antenna was 10 pT at 1 MHz and 1 pT at 10 MHz. The second antenna consisted of two loops of wire of the same size. The frequency response of this antenna was affected by a broad resonance at f = 6 MHz that required careful calibration of this antenna, but at frequencies below this resonance it had two times higher sensitivity (5 pT at 1 MHz). The results of noise measurements are presented in figure 1. As one can see from the graph, the main observed noise power is concentrated near the two bands of AM broadcast radio, 31 and 41 m, and is probably emitted by radio transmitters. The measured noise near the Larmor frequency is lower than 10 pT in the frequency range 1.35–1.45 MHz.
Figure 1.
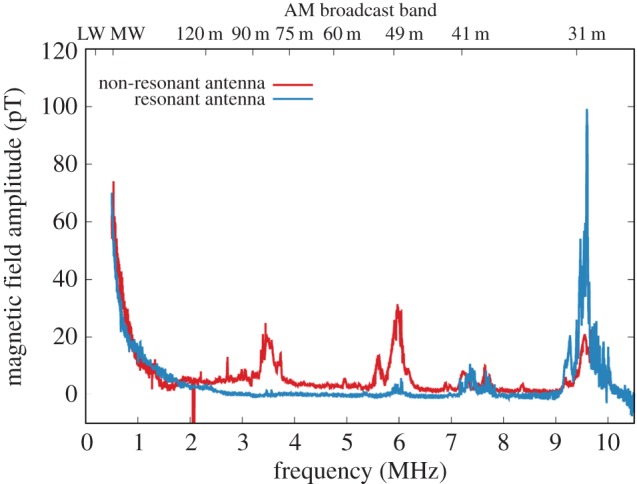
Average amplitude of the magnetic field noise measured at the experimental site as a function of the central position of the 100 kHz detection window. Red curve is the signal measured using a single-loop non-resonant antenna. Blue curve is the signal measured using a two-turn loop resonant antenna. Top x-axis shows a low-frequency border of AM broadcast bands (LW, long-wave; MW, middle-wave, 120 m–31 m; SW, short-wave bands).
The frequency spectrum of the AC voltage produced by our generator was measured with a fast-Fourier-transform spectrum analyser with a resolution bandwidth of 10 kHz, in laboratory conditions. To facilitate comparison with the field measurements of environmental noise and applied RF fields, the data were recalculated into magnetic fields using the parameters of our coil system. As seen from figure 2, the background noise level of the generator is below the ambient noise at our experimental site. The amplitude of the strongest of the higher harmonics is approximately 300 times less than the main signal.
Figure 2.

An example of the frequency spectrum of the oscillating magnetic field, measured at an OMF amplitude of 0.6 nT with a resolution bandwidth of 10 kHz.
We performed a series of tests to evaluate a possible influence of screening on the OMF amplitude inside the Emlen funnel in the case of using aluminium funnels for RF experiments (as in [38]). As expected from the electromagnetic theory, we observed a strong screening of the vertical component of the OMF, inducing eddy currents along the circumference of the aluminium funnel, which diminish the applied OMF according to the Lenz rule. Horizontal components of the OMF were not considerably screened, at least in the central part of the funnel. To quantify the screening of the vertical component, we measured the OMF amplitude in the centre of one of the 0.75 m inductor coils that we used for generating RF fields. The coil was placed horizontally on a wooden table, while an 11 cm loop antenna was placed in its centre 8 cm above the table surface. An aluminium Emlen funnel used routinely for our static-field experiments could be put on the table so that the loop antenna was inside the funnel in its centre.
2.4. Data analysis and statistics
The directionality of the birds' activity was recorded as scratches left by their claws as they hopped in the funnels on a print film covered with a dried mixture of whiting and glue. Two researchers independently determined each bird's mean direction from the distribution of the scratches. In most cases, the mean direction could be very precisely identified using the simple visual estimation method [46]. If a pattern of scratches was not clear, both persons independently counted scratches in each of 36, 10° sectors (some birds leave so many scratches that an exact number of scratches cannot be counted, but must be estimated) and used circular statistics software to assess the directionality based on the numbers of scratches. The mean of the two observers' determined directions was recorded as the orientation result. If both observers considered the scratches to be randomly distributed or if the two mean directions deviated by more than 30°, the bird was considered to be not oriented in the given test. Inactive (fewer than 40 scratches) tests and not sufficiently concentrated trials were excluded from analysis. We used the double-blind control protocol: researchers who carried out behavioural experiments and quantified their results were not aware of the OMF conditions in each table until the end of all experiments.
The mean group directions were calculated based on individual mean directions. The significance of the mean group vectors in each experimental condition was tested using the Rayleigh test. Differences in the mean direction between experimental groups were analysed using the non-parametric Mardia–Watson–Wheeler test (MWW) [47]. We used the bootstrap technique [48] to test whether the significantly oriented group shows more directed orientation behaviour than the non-significantly oriented group (for details, see [49]). Circular statistics and bootstrap analysis were performed in R 3.0.0 (www.r-project.org). Confidence intervals for the magnitude of the orientation vector r shown in figure 3 were calculated using the procedure described in the electronic supplementary material.
Figure 3.

The dependence of the magnitude of the orientation vector r on the amplitude of the oscillating magnetic field (from all our tests).
3. Results
Garden warblers tested in the NMF during the first phase were oriented in the seasonally appropriate migratory direction in 2014 (α = 184°, n = 22, r = 0.39, p = 0.033; 95% CI = 143°–226°; figure 4a) and 2015 (α = 205°, n = 16, r = 0.45, p = 0.036; 95% CI = 164°–247°; figure 4b). These two distributions were not significantly different from each other (MWW: W = 0.202, p = 0.9; 99% CIs overlap: 99% CIs for birds in 2014 and 2015 are 130°–231° and 151°–260°, respectively). Therefore, we pooled the data from the two years; the overall direction of orientation was 194° (n = 38, r = 0.41, p = 0.001; 95% CI = 164°–224°; figure 4c). This direction fits well the mean autumn migratory direction of the same species on the Courish Spit according to recoveries of birds ringed on the Courish Spit (212°, 95% CI = 204°–221°; [50] and unpublished data of the Biological Station Rybachy) and the data obtained in previous experiments in Emlen funnels in garden warblers (α = 247°, 95% CI = 231°–264°; α = 256°, 95% CI = 224°–288° [43,51]).
Figure 4.
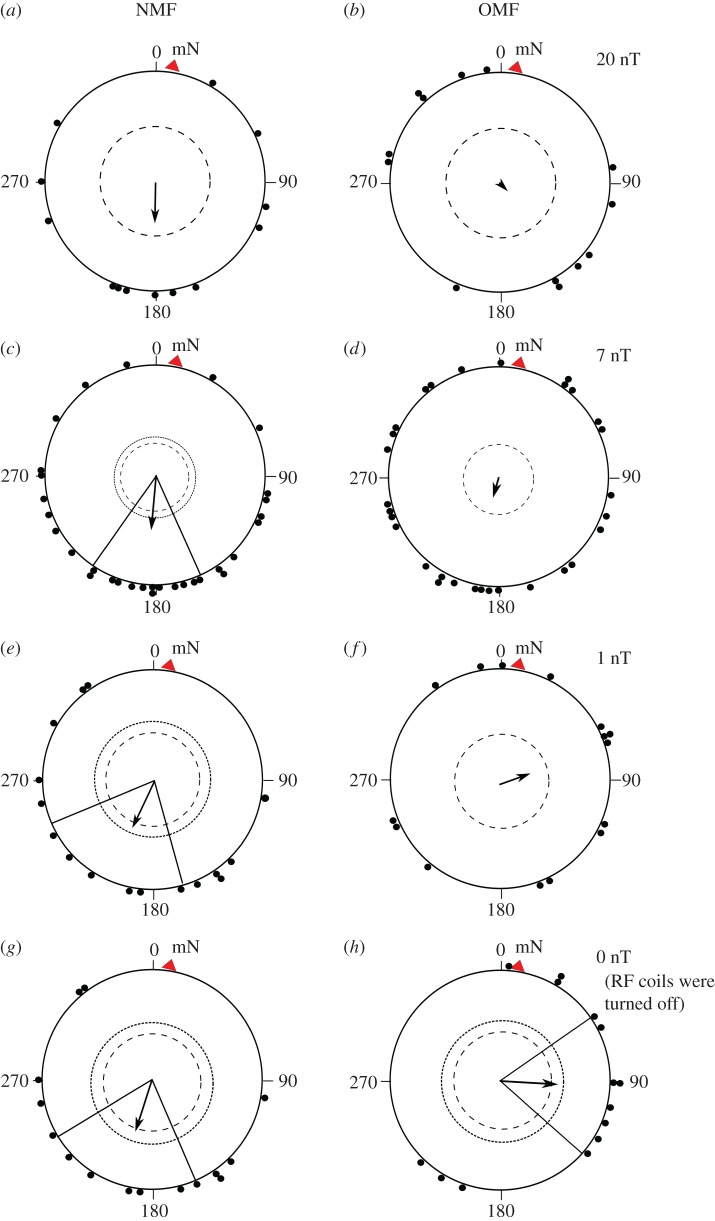
Orientation of garden warblers in the local geomagnetic field (NMF) during the first phase of experiments, autumn 2014–2015: (a) autumn 2014 (α = 184°, n = 22, r = 0.39, p = 0.033; 95% CI = 143°–226°), (b) autumn 2015 (α = 205°, n = 16, r = 0.45, p = 0.036; 95% CI = 164°–247°) and (c) 2 years pooled (α = 194°, n = 38, r = 0.41, p = 0.001; 95% CI 164°–224°). Here α is the direction of the mean vector, r is the length of the mean vector (r varies from 0 to 1), n is the number of birds, p is the significance level and 95% CI is 95% confidence intervals. Dots denote individual directions of each bird; arrow denotes the mean group vector; inner and outer dashed circles are 1 and 5% significance levels of the Rayleigh test, respectively. Radial lines indicate 95% CI. The red triangle and letters mN are the position of the magnetic north.
When we tested the experimental birds in a 20 nT RF field (20 nT subsample), they could not choose any significant direction in circular arenas (α = 129°, n = 13, r = 0.03, p = 0.99; figure 5b). The same subsample was disoriented in the NMF too, but showed a non-significant tendency to orient in the southerly direction (α = 180°, n = 13, r = 0.38, p = 0.16; figure 5a). When we tried to test the birds in a time-dependent electromagnetic field with lower intensities, namely 1 and 7 nT, our expectation was that such weak oscillating fields would not affect the orientation of the birds, because in a previous study European robins did not lose their ability to orient using their magnetic compass under a 5 nT OMF [35]. However, garden warblers that had been significantly oriented in the NMF towards the mean direction of 185° (n = 31, r = 0.45, p = 0.001, 95% CI = 156°–215°; figure 5c) were disoriented in the RF field with a 7 nT amplitude (α = 201°, n = 31, r = 0.16, p = 0.46; figure 5d). Concentration of their individual directions was significantly lower than in the control condition (p < 0.05; 95% CI for r-value in the NMF is 0.26 < r < 0.66 according to bootstrap analysis). Similar results were shown by the birds tested in a 1 nT OMF (α = 71°, n = 16, r = 0.29, p = 0.27; figure 5f); the same subsample showed significant orientation in the autumn migratory direction in the NMF (α = 206°, n = 16, r = 0.45, p = 0.036, 95% CI = 164°–247°; figure 5e). Concentration of directions shown by garden warblers in the 1 nT RF field differed from the behaviour of the same birds in the NMF (p < 0.12, 88% confidence interval for r-value is 0.30 < r < 0.67 calculated by bootstrapping).
Figure 5.
Orientation of garden warblers when 1.403 MHz OMFs with various amplitudes were added to the local geomagnetic field, autumn 2014–2015. (b,d,f,h) Orientation of the experimental birds (subsample) in the OMF during the second phase. (a,c,e,g) Orientation of the same birds (the same subsample) in the NMF during the first phase of experiments. Row (a,b): 20 nT RF field subsample in the NMF ((a) α = 180°, n= 13, r = 0.38, p = 0.16) and OMF ((b) α = 129°, n = 13, r = 0.03, p = 0.99). Row (c,d): 7 nT RF field subsample in the NMF ((c) α = 185°, n = 31, r = 0.45, p = 0.001, 95% CI = 156°–215°) and OMF ((d) α = 201°, n = 31, r = 0.16, p = 0.46). Row (e,f): 1 nT RF field subsample in the NMF ((e) α = 206°, n = 16, r = 0.45, p = 0.036, 95% CI = 164°–247°) and OMF ((f) α = 71°, n = 16, r = 0.29, p = 0.27). Row (g,h): 0 nT RF field subsample in the NMF during the first phase of experiments ((g) α = 198°, n = 15, r = 0.49, p = 0.024, 95% CI = 157°–239°) and the NMF during the second phase when RF coils were turned off ((h) α = 94°, n = 15, r = 0.52, p = 0.016, 95% CI = 55°–132°). For a description of the circular diagrams, see the legend to figure 1.
We also carried out additional orientation experiments in the NMF again during the second phase (0 nT RF field subsample) to control that our experimental birds did not lose the ability to orient in the appropriate migratory direction due to progress of season. In these experiments, garden warblers chose an atypical easterly direction during the second phase (α = 94°, n = 15, r = 0.52, p = 0.016, 95% CI = 55°–132°; figure 5h), whereas the same birds had been significantly oriented in the southwesterly migratory direction in the NMF during the first phase (α = 198°, n = 15, r = 0.49, p = 0.024, 95% CI = 157°–239°; figure 5g). The mean orientation of the birds tested in the NMF for the second time was significantly different from the results obtained in the same condition for the first time (MWW: W = 0.832, p = 0.016 and 99% CIs do not overlap: 99% CIs for birds in the NMF and the OMF are 43°–144° and 144°–252°, respectively).
To tackle this problem, we carried out orientation tests in the NMF and OMF at the same time in autumn 2016. Unfortunately, our experimental birds were not significantly oriented in the NMF: α = 241°, n = 25, r = 0.125, p = 0.68 (figure 6a). When we tested the same birds in the OMF with an amplitude of approximately 2.4 nT, they did not show a directional preference (α = 139°, n = 21, r = 0.201, p = 0.44; figure 6b). However, a very weak narrow-band electromagnetic field (intensity approx. 0.4 nT) did not disrupt the magnetic compass orientation of garden warblers that showed a significant seasonally appropriate orientation (α = 183°, n = 26, r = 0.349, p < 0.05, 95% CI = 140°–226°; figure 6c).
Figure 6.
Orientation of garden warblers in three experimental conditions during 2016 autumn experiments: in the NMF ((a) α = 241°, n = 25, r = 0.125, p = 0.68); in an approximate 2.4 nT RF field ((b) α = 139°, n = 21, r = 0.201, p = 0.44); in an approximate 0.4 nT RF field ((c) α = 183°, n = 26, r = 0.349, p < 0.05, 95% CI = 140°–226°). For a description of the circular diagrams, see the legend to figure 1.
The effect of the OMF on orientation ability of garden warblers is summarized in figure 3, which shows the magnitude of the vector r calculated from all the data for all three experimental seasons, as a function of the OMF amplitude. The results of measurements of the OMF amplitude for several frequencies with and without the aluminium funnel are given in figure 7. In the frequency range from 0.2 to 5 MHz, the amplitude attenuation factor for the vertical OMF due to the electromagnetic screening is not less than 100 (−20 dB).
Figure 7.

The amplitude attenuation factor for the vertical OMF as a function of oscillation frequency.
4. Discussion
It has recently been shown that garden warblers orient in the seasonally appropriate migratory direction in the NMF in the absence of all orientation cues except the geomagnetic field, but lose this ability in the OMF with an amplitude of 190 nT and a frequency of 1.403 MHz [44]. Similar results were shown in behavioural experiments with another songbird migrant, European robin, where the authors used broadband (1–10 MHz) and narrow-band RF fields [34–36]. However, it does not agree with the recent report that European robins were disoriented in circular arenas in a weak broadband OMF (2 kHz–9 MHz) but not in a narrow-band OMF with a 1.363 MHz Larmor frequency [38].
In this study, we found that very weak narrow-band oscillating fields, approximately 2.4 and 7 nT RF fields, could disrupt the ability of garden warblers to choose the correct migratory direction. This sensitivity is higher than that reported for European robins that showed directional behavioural activity in a 5 nT RF field but were disoriented in a weak narrow-band OMF with a 15 nT amplitude [37]. Surprisingly, garden warblers tested in the NMF again (0 nT RF condition, figure 5h) during the second phase preferred the easterly direction. It is not the typical migratory direction for this species during autumn migration within Europe according to ringing recoveries [50,52], and it differed from the orientation of the same birds tested in the NMF for the first time (figure 5g). We tested our birds in the NMF from the middle of August to the beginning of September during the first phase and from the middle of September to the beginning of October during the second phase. We suggest that our data reflect the directional shift (Zugknick) of young inexperienced birds during their first migration. European garden warblers are known to move towards the southwest in the first part of their autumn migration [45,53] and to change towards much more easterly directions late in the season after they have (nearly) crossed the Sahara [54,55].
The results of our experiments indicate that the sensitivity threshold of garden warbler magnetic compass to the disruptive effect of a time-dependent electromagnetic field lies below 5 nT. In the very weak approximately 2.4 nT RF field, our birds were completely disoriented (figure 6b), but showed a non-significant tendency to orient in the easterly direction in a 1 nT RF field (figure 5f). This is not the correct migratory direction in the Baltic area, as discussed above, but the same birds in the NMF showed similar directions at the same time (figure 5h). We tested garden warblers in autumn 2016 in differently designed experiments, in which we started to test birds in the NMF condition and 2.4 and 0.4 nT oscillating fields at the same time, thereby avoiding the calendar effect. Unfortunately, we did not obtain a significantly oriented response from the birds in the NMF (figure 6a), but the same garden warblers were oriented in the southerly direction in the approximately 0.4 nT oscillating field. These results suggest that this very weak RF magnetic field does not disrupt the ability of garden warblers to orient using the magnetic field and the sensitivity threshold to such an influence lies around 2–3 nT. In a study with another songbird migrant, European robin, this threshold was found between 15 and 5 nT [35]. A possible explanation of this difference is that different songbird species have a somewhat varying ability to obtain directional information from the magnetic patterns blurred by the OMF.
The dependence of the magnitude of the orientation vector r, calculated from all data for 3 years (figure 3), illustrates the principal result of our work: the disruption of magnetic orientation in garden warblers by the OMF becomes evident when its amplitude exceeds 2–3 nT. Indeed, orientation is significant at OMF amplitudes less than or equal to 1 nT. At 2.4 nT, there is an insignificant tendency to orientate in the correct direction, and above this amplitude no traces of orientation remain.
It has recently been reported that European robins showed oriented behaviour in the seasonally appropriate migratory direction when tested in a narrow-band OMF but were disoriented in a weak broadband RF field [38]. The authors assumed that their results contradicted earlier reports [34–37] because they carried out experiments in a laboratory shielded from human-induced ambient electromagnetic noise. They propose that the experiments performed in Frankfurt [36–38] may have been influenced by the urban electromagnetic noise and therefore the birds could have experienced unpredictable RF fields during the experiments. This explanation can hardly be applied to our earlier data [43]: in our experiments, we did not use electromagnetic shielding and tested our birds in a rural location where the level of electromagnetic noise was low (figure 1). The spectral power density of the magnetic noise in our location was similar to that inside the shielded and grounded huts used in Oldenburg and much lower than the ambient noise even in rural locations near Oldenburg [56]. All our tests in the OMF were performed using Emlen funnels made from dielectric materials because often-used aluminium Emlen funnels strongly screen oscillating fields. The screening is especially strong when the OMF is applied vertically (figure 7), as was the case in the experiments described in [38] (but not in the previous experiments with electromagnetic noise by the same group [56]).
We independently confirmed the data of previous experiments in European robins and garden warblers [34–36,43] and showed that, at least in garden warblers, the disruptive influence of a narrow-band OMF on their ability to orient using the magnetic compass starts when the field amplitude exceeds 2–3 nT. Orientation is significant at OMF amplitudes less than or equal to 1 nT; at 2.4 nT, garden warblers show an insignificant tendency to orientate in the correct direction, and above this amplitude no traces of orientation remain. The results of this and earlier studies agree with the qualitative predictions of the RPM and cannot be explained by any of other existing theories, to the best of our understanding. However, on the quantitative level, the fact that the OMF with the amplitude of several nT may obstruct the magnetic orientation cannot be explained by the RPM in its current form [41,43,57]. Probably, a new hybrid chemical–ferromagnetic model of the avian magnetic compass [58] will be able to explain the disruptive effect of very weak RF magnetic fields, but it needs to be verified by further behavioural experiments and histological studies.
Supplementary Material
Acknowledgements
We are grateful to Anna Anashina and Julia Loshchagina for their assistance during the experiments.
Ethics
The experiments were conducted in accordance with the national animal welfare legislation of Russia and were approved by the Ethical Committee of the Zoological Institute of Russian Academy of Sciences (permit no. 2015-01-13) and by Kaliningrad Regional Agency for Protection, Reproduction and Use of Animal World and Forests (permit no. 2015-05).
Data accessibility
This article has no additional data.
Authors' contributions
N. Chernetsov, A. Pakhomov, K. Kavokin and J. Bojarinova designed the research. N. Chernetsov, A. Pakhomov, D. Kobylkov, R. Chetverikova and R. Lubkovskaja performed orientation tests and analysed the data. K. Kavokin, P. S. Grigoryev and R. Cherbunin provided technical support for experiments, and analysed the electromagnetic noise in the places of experiments and the influence of the aluminium funnel on the parameters of the OMF. A. Pakhomov, N. Chernetsov, K. Kavokin and R. Cherbunin wrote the paper. All authors commented on the manuscript and gave their final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Financial support for this study in 2014–2015 was made available by the Russian Foundation for Basic Research (grant no. 15-04-05386 to N.C.) and by the Zoological Institute RAS (research project no. AAAA-A16-116123010004-1). We also acknowledge support from St Petersburg State University through projects 1.37.149.2014 (to N.C., D.K., R.Chetverikova, J.B. and R.L.) and 11.37.159.2014 (to R.Cherbunin, P.S.G. and K.K.). Experiments in 2016 were supported by Russian Science Foundation (grant no. 16-14-10159 to N.C.).
References
- 1.Hore P, Mouritsen H. 2016. The radical-pair mechanism of magnetoreception . Annu. Rev. Biophys. 45, 299–344. ( 10.1146/annurev-biophys-032116-094545) [DOI] [PubMed] [Google Scholar]
- 2.Liedvogel M, Mouritsen H. 2010. Cryptochromes—a potential magnetoreceptor: what do we know and what do we want to know? J. R. Soc. Interface 7, S147–S162. ( 10.1098/rsif.2009.0411.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cadiou H, McNaughton PA. 2010. Avian magnetite-based magnetoreception: a physiologist's perspective. J. R. Soc. Interface 7, S193–S205. ( 10.1098/rsif.2009.0423.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zapka M, et al. 2009. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461, 1274–1277. ( 10.1038/nature08528) [DOI] [PubMed] [Google Scholar]
- 5.Kishkinev D, Chernetsov N. 2015. Magnetoreception systems in birds: a review of current research . Biol. Bull. Rev. 5, 46–62. ( 10.1134/S2079086415010041) [DOI] [PubMed] [Google Scholar]
- 6.Kishkinev D, Chernetsov N, Heyers D, Mouritsen H, Kubke MF. 2013. Migratory reed warblers need intact trigeminal nerves to correct for a 1,000 km eastward displacement. PLoS ONE 8, e65847 ( 10.1371/journal.pone.0065847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiltschko W, Wiltschko R. 2007. Magnetoreception in birds: two receptors for two different tasks. J. Ornithol. 148, 61–76. ( 10.1007/s10336-007-0233-2) [DOI] [Google Scholar]
- 8.Wiltschko W, Wiltschko R. 1972. Magnetic compass of European robins. Science 176, 62–64. ( 10.1126/science.176.4030.62) [DOI] [PubMed] [Google Scholar]
- 9.Marhold S, Wiltschko W, Burda H. 1997. A magnetic polarity compass for direction finding in a subterranean mammal. Naturwissenschaften 84, 421–423. ( 10.1007/s001140050422) [DOI] [Google Scholar]
- 10.Stapput K, Thalau P, Wiltschko R, Wiltschko W. 2008. Orientation of birds in total darkness. Curr. Biol. 18, 602–606. ( 10.1016/j.cub.2008.03.046) [DOI] [PubMed] [Google Scholar]
- 11.Muheim R, Bäckman J, Åkesson S. 2002. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 205, 3845–3856. [DOI] [PubMed] [Google Scholar]
- 12.Rappl R, Wiltschko R, Weindler P, Berthold P, Wiltschko W. 2000. Orientation behavior of garden warblers (Sylvia borin) under monochromatic light of various wavelengths. Auk 117, 256–260. ( 10.1642/0004-8038(2000)117%5B0256:OBOGWS%5D2.0.CO;2) [DOI] [Google Scholar]
- 13.Wiltschko R, Gehring D, Denzau S, Nießner C, Wiltschko W. 2014. Magnetoreception in birds: II. Behavioural experiments concerning the cryptochrome cycle. J. Exp. Biol. 217, 4225–4228. ( 10.1242/jeb.110981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiltschko R, Munro U, Ford H, Stapput K, Thalau P, Wiltschko W. 2014. Orientation of migratory birds under ultraviolet light. J. Comp. Phys. A 200, 399–407. ( 10.1007/s00359-014-0898-y) [DOI] [PubMed] [Google Scholar]
- 15.Wiltschko W, Wiltschko R. 1995. Migratory orientation of European robins is affected by the wavelength of light as well as by a magnetic pulse. J. Comp. Phys. A 177, 363–369. ( 10.1007/BF00192425) [DOI] [Google Scholar]
- 16.Wiltschko W, Munro U, Ford H, Wiltschko R. 1993. Red light disrupts magnetic orientation of migratory birds. Nature 364, 525–527. ( 10.1038/364525a0) [DOI] [Google Scholar]
- 17.Wiltschko W, Wiltschko R. 1999. The effect of yellow and blue light on magnetic compass orientation in European robins, Erithacus rubecula. J. Comp. Phys. A 184, 295–299. ( 10.1007/s003590050327) [DOI] [Google Scholar]
- 18.Heyers D, Manns M, Luksch H, Güntürkün O, Mouritsen H, Iwaniuk A. 2007. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS ONE 2, e937 ( 10.1371/journal.pone.0000937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liedvogel M, Feenders G, Wada K, Troje NF, Jarvis ED, Mouritsen H. 2007. Lateralized activation of Cluster N in the brains of migratory songbirds. Eur. J. Neurosci. 25, 1166–1173. ( 10.1111/j.1460-9568.2007.05350.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouritsen H, Feenders G, Liedvogel M, Wada K, Jarvis ED. 2005. Night-vision brain area in migratory songbirds. Proc. Natl Acad. Sci. USA 102, 8339–8344. ( 10.1073/pnas.0409575102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zapka M, Heyers D, Liedvogel M, Jarvis ED, Mouritsen H. 2010. Night-time neuronal activation of Cluster N in a day- and night-migrating songbird. Eur. J. Neurosci. 32, 619–624. ( 10.1111/j.1460-9568.2010.07311.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulten K, Swenberg CE, Weller A. 1978. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z. Phys. Chem. 111, 1–5. ( 10.1524/zpch.1978.111.1.001) [DOI] [Google Scholar]
- 23.Ritz T, Adem S, Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. ( 10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaves I, et al. 2011. The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62, 335–364. ( 10.1146/annurev-arplant-042110-103759) [DOI] [PubMed] [Google Scholar]
- 25.Solov'yov IA, Greiner W. 2013. Light-activated magnetic compass in birds. In Exciting interdisciplinary physics (ed. W Greiner), pp. 481–492. Berlin, Germany: Springer; ( 10.1007/978-3-319-00047-3_38) [DOI] [Google Scholar]
- 26.Liedvogel M, Maeda K, Henbest K, Schleicher E, Simon T, Timmel CR, Hore PJ, Mouritsen H, El-Shemy H. 2007. Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE 2, e1106 ( 10.1371/journal.pone.0001106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Möller A, Sagasser S, Wiltschko W, Schierwater B. 2004. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften 91, 585–588. ( 10.1007/s00114-004-0578-9) [DOI] [PubMed] [Google Scholar]
- 28.Nießner C, Denzau S, Gross JC, Peichl L, Bischof HJ, Fleissner G, Wiltschko W, Wiltschko R, Warrant EJ. 2011. Avian ultraviolet/violet cones identified as probable magnetoreceptors. PLoS ONE 6, e20091 ( 10.1371/journal.pone.0020091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fusani L, Bertolucci C, Frigato E, Foà A. 2014. Cryptochrome expression in the eye of migratory birds depends on their migratory status. J. Exp. Biol. 217, 918–923. ( 10.1242/jeb.096479) [DOI] [PubMed] [Google Scholar]
- 30.Nießner C, Gross JC, Denzau S, Peichl L, Fleissner G, Wiltschko W, Wiltschko R, Warrant EJ.. 2016. Seasonally changing cryptochrome 1b expression in the retinal ganglion cells of a migrating passerine bird. PLoS ONE 11, e0150377 ( 10.1371/journal.pone.0150377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolte P, et al. 2016. Localisation of the putative magnetoreceptive protein cryptochrome 1b in the retinae of migratory birds and homing pigeons. PLoS ONE 11, e0147819 ( 10.1371/journal.pone.0147819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keary N, Ruploh T, Voss J, Thalau P, Wiltschko R, Wiltschko W, Bischof HJ.. 2009. Oscillating magnetic field disrupts magnetic orientation in zebra finches, Taeniopygia guttata. Front. Zool. 6, 25 ( 10.1186/1742-9994-6-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinzon-Rodriguez A, Muheim R. 2017. Zebra finches have a light-dependent magnetic compass similar to migratory birds. J. Exp. Biol. 220, 1202–1209. ( 10.1242/jeb.148098) [DOI] [PubMed] [Google Scholar]
- 34.Ritz T, Thalau P, Phillips JB, Wiltschko R, Wiltschko W.. 2004. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429, 177–180. ( 10.1038/nature02534) [DOI] [PubMed] [Google Scholar]
- 35.Ritz T, Wiltschko R, Hore PJ, Rodgers CT, Stapput K, Thalau P, Timmel CR, Wiltschko W. 2009. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451–3457. ( 10.1016/j.bpj.2008.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thalau P, Ritz T, Stapput K, Wiltschko R, Wiltschko W. 2005. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften 92, 86–90. ( 10.1007/s00114-004-0595-8) [DOI] [PubMed] [Google Scholar]
- 37.Wiltschko R, Thalau P, Gehring D, Nießner C, Ritz T, Wiltschko W. 2015. Magnetoreception in birds: the effect of radio-frequency fields. J. R. Soc. Interface 12, 20141103 ( 10.1098/rsif.2014.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarze S, Schneider NL, Reichl T, Dreyer D, Lefeldt N, Engels S, Baker N, Hore PJ, Mouritsen H.. 2016. Weak broadband electromagnetic fields are more disruptive to magnetic compass orientation in a night-migratory songbird (Erithacus rubecula) than strong narrow-band fields. Front. Behav. Neurosci. 10, 55 ( 10.3389/fnbeh.2016.00055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thalau P, Ritz T, Burda H, Wegner RE, Wiltschko R. 2006. The magnetic compass mechanisms of birds and rodents are based on different physical principles. J. R. Soc. Interface 3, 583–587. ( 10.1098/rsif.2006.0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vacha M, Pžová T, Kvíćalová M. 2009. Radio frequency magnetic fields disrupt magnetoreception in American cockroach. J. Exp. Biol. 212, 3473–3477. ( 10.1242/jeb.028670) [DOI] [PubMed] [Google Scholar]
- 41.Kavokin K. 2009. The puzzle of magnetic resonance effect on the magnetic compass of migratory birds. Bioelectromagnetics 30, 402–410. ( 10.1002/bem.20485) [DOI] [PubMed] [Google Scholar]
- 42.Mouritsen H, Hore P. 2012. The magnetic retina: light-dependent and trigeminal magnetoreception in migratory birds. Curr. Opin. Neurobiol. 22, 343–352. ( 10.1016/j.conb.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 43.Kavokin K, Chernetsov N, Pakhomov A, Bojarinova J, Kobylkov D, Namozov B.. 2014. Magnetic orientation of garden warblers (Sylvia borin) under 1.4 MHz radiofrequency magnetic field. J. R. Soc. Interface 11, 20140451 ( 10.1098/rsif.2014.0451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emlen ST, Emlen JT. 1966. A technique for recording migratory orientation of captive birds. Auk 83, 361–367. ( 10.2307/4083048) [DOI] [Google Scholar]
- 45.Gwinner E, Wiltschko W. 1978. Endogenously controlled changes in migratory direction of the garden warbler, Sylvia borin. J. Comp. Physiol. 125, 267–273. ( 10.1007/BF00656605) [DOI] [Google Scholar]
- 46.Mouritsen H, Larsen ON. 1998. Migrating young pied flycatchers Ficedula hypoleuca do not compensate for geographical displacements. J. Exp. Biol. 201, 2927–2934. [Google Scholar]
- 47.Batschelet E. 1981. Circular statistics in biology. London, UK: Academic Press. [Google Scholar]
- 48.Fisher NI. 1995. Statistical analysis of circular data. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 49.Alert B, Michalik A, Thiele N, Bottesch M, Mouritsen H.. 2015. Re-calibration of the magnetic compass in hand-raised European robins (Erithacus rubecula). Sci. Rep. 5, 62 ( 10.1038/srep14323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolshakov C, Shapoval A, Zelenova N.. 2001. Results of bird ringing by the Biological Station ‘Rybachy’ on the Courish Spit: long-distance recoveries of birds ringed in 1956–1997. Part 1. Avian Ecol. Behav. Suppl. 5, 1–106. [Google Scholar]
- 51.Pakhomov A, Chernetsov N. 2014. Early evening activity of migratory garden warbler Sylvia borin: compass calibration activity? J. Ornithol. 155, 621–630. ( 10.1007/s10336-014-1044-x) [DOI] [Google Scholar]
- 52.Bolshakov C, Shapoval A, Zelenova N. 2002. Results of bird ringing by the Biological Station ‘Rybachy’ on the Courish Spit: long-distance recoveries of birds ringed in 1956–1997. Part 2. Avian Ecol. Behav. Suppl. 6, 1–126. [Google Scholar]
- 53.Berthold P. 1990. Spatiotemporal programs and genetics of orientation. Experientia 46, 363–371. ( 10.1007/BF01952169) [DOI] [Google Scholar]
- 54.Liechti F, Komenda-Zehnder S, Bruderer B. 2012. Orientation of passerine trans-Sahara migrants: the directional shift (‘Zugknick’) reconsidered for free-flying birds. Anim. Behav. 83, 63–68. ( 10.1016/j.anbehav.2011.10.005) [DOI] [Google Scholar]
- 55.Ottosson U, Waldenström J, Hjort C, Mcgregor R. 2005. Garden warbler Sylvia borin migration in sub-Saharan West Africa: phenology and body mass changes. Ibis 147, 750–757. ( 10.1111/j.1474-919X.2005.00460.x) [DOI] [Google Scholar]
- 56.Engels S, et al. 2014. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature 509, 353–356. ( 10.1038/nature13290) [DOI] [PubMed] [Google Scholar]
- 57.Nießner C, Winklhofer M.. 2017. Radical-pair-based magnetoreception in birds: radio-frequency experiments and the role of cryptochrome. J. Comp. Physiol. A 203, 499–507. ( 10.1007/s00359-017-1189-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kavokin K. 2017. Can a hybrid chemical-ferromagnetic model of the avian compass explain its outstanding sensitivity to magnetic noise? PLoS ONE 12, e0173887 ( 10.1371/journal.pone.0173887) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.