Abstract
Acute glaucoma is one of the leading causes of irreversible vision impairment characterized by the rapid elevation of intraocular pressure (IOP) and consequent retinal ganglion cell (RGC) death. Oxidative stress and neuroinflammation have been considered critical for the pathogenesis of RGC death in acute glaucoma. Trimetazidine (TMZ), an anti-ischemic drug, possesses antioxidative and anti-inflammatory properties, contributing to its therapeutic potential in tissue damage. However, the role of TMZ in acute glaucoma and the underlying molecular mechanisms remain elusive. Here, we report that treatment with TMZ significantly attenuated retinal damage and RGC death in mice with acute glaucoma, with a significant decrease in reactive oxygen species (ROS) and inflammatory cytokine production in the retina. Furthermore, TMZ treatment directly decreased ROS production and rebalanced the intracellular redox state, thus contributing to the survival of RGCs in vitro. TMZ treatment also reduced the production of inflammatory cytokines in vitro. Mechanistically, the TMZ-mediated inhibition of apoptosis and inflammatory cytokine production in RGCs occurred via the regulation of the nuclear factor erythroid 2-related factor 2/heme oxygenase 1/caspase-8 pathway. Moreover, the TMZ-mediated neuroprotection in acute glaucoma was abrogated when an HO-1 inhibitor, SnPP, was used. Our findings identify potential mechanisms of RGC apoptosis and propose a novel therapeutic agent, TMZ, which exerts a precise neuroprotective effect against acute glaucoma.
Keywords: Acute glaucoma, inflammation, Oxidation, Retinal ganglion cells, Trimetazidine
Introduction
Acute glaucoma is one of the main causes of irreversible visual impairment and blindness, especially amongst people of Asian descent [1,2]. Acute glaucoma is characterized by considerably high intraocular pressure (IOP), pain and vision loss [3]. The rapid increase and decrease in IOP cause retinal ischemic/reperfusion (I/R) injury, thus resulting in apoptosis of retinal ganglion cells (RGCs). Although the pathogenesis of RGCs death is not fully understood, oxidative stress and neuroinflammation have been considered critical for the pathogenesis for RGCs death in retinal I/R injury during acute glaucoma [4]. Current treatments that lower IOP through medication and surgery cannot completely prevent the progressive death of RGCs in acute glaucoma. Therefore, safe and effective novel alternatives are needed for acute glaucoma treatment.
Trimetazidine (TMZ), a piperazine-derived antianginal agent [5,6], has traditionally been used as an anti-ischemic drug for coronary artery disease. Recently, studies have shown that TMZ possesses antioxidative and anti-inflammatory properties and plays cytoprotective roles in various tissues, including nervous tissue, pancreatic tissue and renal tissue [7,8]. Therefore, TMZ may be a potential therapeutic alternative for acute glaucoma treatment. However, the role of TMZ in acute glaucoma has not yet been explored. Moreover, the mechanisms by which TMZ mediates antioxidative and anti-inflammatory effects remain elusive. Thus, in the present study, we investigated the therapeutic effect of TMZ on experimental acute glaucoma and the antioxidative and anti-inflammatory mechanisms of TMZ.
Materials and methods
Establishment of the retinal I/R model
Six- to eight-week-old C57BL/6J male mice were purchased from Guangdong Medical Laboratory Animal Center. The feeding and administration of mice strictly complied with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
The mice were anesthetized with 100 mg/kg pentobarbital sodium by intraperitoneal injection. Before operation, mice corneas were topically anesthetized with 0.5% Alcaine eye drops, and pupils were dilated with 1% tropicamide. We then conducted cannulation into the anterior chamber of the right eye using a 30-gauge needle supplied with balanced salt solution to maintain the IOP at 70 mmHg for 60 min. Sham operation was performed in the contralateral eye without elevating the IOP to serve as the control. After 60 min, the IOP was normalized by withdrawing the needle, and then tobramycin ointment was used to prevent bacterial infection.
Preparation of TMZ
TMZ with purity higher than 95% was purchased from Sigma Chemical Co. (St. Louis, U.S.A.), dissolved in sterile PBS to a stock concentration of 0.1 mol/l, and stored at 4°C in the dark to be used within 2 days after preparation.
Experiment grouping design
The mice were randomly divided into five groups: normal control group (Normal), Tin protoporphyrin IX dichloride (SnPP) group, I/R group, TMZ (100 μM) group, and TMZ + SnPP group. The mice in I/R, TMZ, and TMZ + SnPP groups were subjected to experimental I/R. The normal control and SnPP groups had sham cannulation performed without elevating the IOP as described above. The eyes of mice in the SnPP or TMZ groups were intravitreally injected with 1 μl TMZ [9] or SnPP solution, respectively, before the onset of reperfusion. The eyes of mice in the TMZ + SnPP group were intravitreally injected with 1 μl TMZ and SnPP solution together before the onset of reperfusion. The eyes of mice in the normal control group were injected with 1 μl of sterile PBS into the vitreous cavity.
RGC labeling and survival quantitation
Mice were anesthetized with 100 mg/kg pentobarbital sodium by intraperitoneal injection and placed in a stereotactic apparatus (Stoelting, U.S.A.). The skull was exposed and cleaned with PVP-J. Bilateral holes were drilled at the surface of superior colliculi. Approximately 1 µl of 4% Fluorogold (FG, hydroxystilbamidine; Fluorochrome, U.S.A.) solution was injected into both superior colliculis. After injection, the microsyringe was kept still for 30 s and then slowly removed. Finally, the dissected scalp was sutured with topical application of tobramycin.
To ensure proper RGC labeling, the animals were allowed 7 days for retrograde transport of FG before killing. FG-positive RGCs were identified with a fluorescence microscope (Axio Imager; Carl Zeiss MicroImaging Inc., U.S.A.) in retinal flat mount. Surviving RGCs (green dots) were counted automatically using ImageJ (LOCI, University of Wisconsin–Madison).
Histological examination
Seven days after I/R treatment, mouse eyes were enucleated and embedded in paraffin. Every paraffin block was sectioned 4-µm thick through the optic nerve. Three sections of each eye were cut and stained with Hematoxylin and Eosin (H&E). The inner plexiform layer (IPL) thickness was measured within 1 mm to the optic nerve center to quantitate retinal damage by Axiovision software (Carl Zeiss MicroImaging Inc.). The data from three sections per eye were averaged.
Primary culture of RGCs
Primary RGCs were obtained according to the protocol published by Winzeler and Wang in 2013 [10]. In brief, retinal samples were separated from neonatal mice to prepare single cell suspensions. The retinal suspension was incubated in rabbit anti-mouse macrophage antibody-coated flasks (Cedarlane, U.S.A.) and goat anti-mouse macrophage antibody-coated flasks (Jackson Immunoresearch, U.S.A.) to remove the adherent macrophages. The non-adherent cells were transferred to Thy1.2 monoclonal antibody-coated flasks (Millipore Chemicon, U.S.A.) to collect adherent cells. The adherent RGCs were incubated at 37°C in 5% CO2 with RGC growth medium containing supplements as described.
Mix culture with BV2 cells
Mouse microglia cell line BV2 (ATCC, U.S.A.) was cocultured with primary RGCs in DMEM (high glucose 4.5 g/ml, Gibco, U.S.A.) supplemented with 10% FBS at 37°C with 5% CO2 and 95% air. RGCs were placed on the upper permeable membrane of a transwell chamber, with BV2 cells grown in the lower well of the plate for 24 h before treatment.
Establishment of the oxygen-glucose deprivation and reperfusion model
The oxygen-glucose deprivation and reperfusion (OGD/R) model was established as follows: the culture medium was replaced with glucose-free DMEM (Gibco) after washing the cells twice with PBS, and then the cells were placed in a modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA) filled with a gas mixture of 5% CO2 and 95% N2 at 37°C for 3 h. The control cells were incubated in serum-free medium with 4.5 g/l D-glucose under normoxic conditions (5% CO2 and 95% air) for the same duration. At the end of the exposure period, the cells were returned to normoxic conditions with glucose and incubated for 12 h.
Cell treatment with TMZ
Primary RGCs were cultured in six-well plates or transwell plates before treatment. TMZ was added at indicated concentrations into cell supernatants 6 h prior to the onset of OGD/R or other assays. TMZ was diluted in DMEM and added at concentrations ranging from 0.1 to 100 μM.
ELISA
The cytokines TNFα and IL1β were measured in the culture supernatant of BV2 cells. Immunochemical analyses were conducted using commercially available ELISA kits (eBioscience, Vienna, Austria). For measuring the level of 3-nitrotyrosine proteins in retinal homogenates, a commercial kit was used (OxiSelect Nitrotyrosine kit; Cell Biolabs, Inc., U.S.A.), following the manufacturer’s instructions.
Viability and apoptosis assays in RGCs
Cell viability was measured using the CCK8 Assay Kit (Beyotime Biotechnology, China) according to the manufacturer’s protocol. CCK8-reduction activity was presented as the percentage of the unexposed control cells (100%). Flow cytometry was also performed to measure OGD/R-induced apoptosis of RGCs by using a propidium iodide (PI) and Annexin V-FITC detection kit (BD Biosciences, New York, U.S.A.) according to the manufacturer’s instructions. Flow cytometric analysis using FlowJo 7.6.2 (FlowJo, LLC) was performed following standard protocols.
Inhibition of Ho-1 activity
Ho-1 activity was inhibited in vivo through an intraperitoneal injection of SnPP (40 mol/kg, Tocris Bioscience, U.S.A.) 0.5 h prior to I/R and once daily for 3 days after I/R. SnPP was dissolved in 0.1 N NaOH and diluted with PBS (pH = 7.4).
Cells were seeded in a six-well plate at approximately 70% confluency. Ten micromolar SnPP or vehicle was added to the culture medium for 6 h. The cells were then treated with TMZ or vehicle for 6 h and subjected to OGD/R.
Detection of intracellular ROS levels
Quantitation of intracellular reactive oxygen species (ROS) accumulation was performed by fluorescence detection as well as flow cytometry using the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA, KeyGEN, China). Primary RGCs were subjected to the appropriate treatments and then incubated for 20 min in the dark at 37°C with 10 µM DCFH-DA solutions. After incubation, the cells were analyzed within 30 min. Mean fluorescence intensity of ROS was measured using a fluorescence microscope, and flow cytometry was performed using a Fortessa system (BD). The data were analyzed using ImageJ and FlowJo software.
Analysis on MMP (JC-1 staining)
The changes in mitochondrial membrane potential (MMP) in RGCs were explored using the 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazolyl carbocyanine iodide (JC-1) probe as described by the manufacturer and then visualized using an inverted fluorescence microscope. The average result of three plates of cells or mice were obtained for each condition.
Quantitative real-time PCR
Total RNA was extracted from the retinal samples and cultured cells by TRIzol reagent (Invitrogen, U.S.A.) following the manufacturer’s protocol. cDNA synthesis was conducted with PrimeScript RT Master Mix (TaKaRa, China). Quantitative analysis was performed with a light cycler 480 real-time PCR system. The expression level of target mRNA was measured and normalized to Gapdh. The primer sequences are as follows: GAPDH forward primer CCGGGAAACTGTGGCGTGATGG; GAPDH reverse primer AGGTGGAGGAGTGGGTGTCGCTGTT; TNFα forward primer GCACCACCATCAAGGACTCAA; TNFα reverse primer TCGAGGCCCAGTGAATTCG; and IL1β forward primer GGGCCTCAAAGGAAAGAATC, IL1β reverse primer CTCTGCTTGTGAGGTGCTGA.
Western blot
Total protein was isolated from the retinal samples and cultured cells according to standard procedures. Nuclear protein was extracted using ProteoExtract® Subcellular Proteome Extraction Kit (Merck Millipore, Germany) following the manufacturer’s protocol. Proteins were run on 12% polyacrylamide gels following standard protocol. The expression of total protein and nuclear protein was normalized to Gapdh and Histone H3, respectively, and then quantitated using ImageJ.
The primary antibodies and dilutions used were as follows: anti-Nrf2 (CST, U.S.A., 1:400), anti-Ho-1 (CST, 1:200), anti-cleaved-Caspase-8 (CST, 1:400), anti-Gapdh (CST, 1:400), anti-Histone H3 (Abcam, U.K., 1:400).
Caspase-8 activity assay
The Caspase-8 activity of retinal tissues and RGCs were performed using the CaspGLOW™ Fluorescein Active Caspase-8 Staining Kit (BioVision, Milpitas, U.S.A.) according to the manufacturer’s instructions. One microliter of FITC-IETD-FMK was added into each tube containing 300 µl of cell suspension and incubated for 1 h at 37°C in 5% CO2. Then, the cells were resuspended, and the fluorescence intensity was measured at Ex/Em = 485/535 nm.
Statistical analysis
The data were presented as the mean ± S.D. One-way ANOVA was performed, followed by Bonferroni’s post hoc test using SPSS software, version 17 (SPSS Inc., Chicago, IL, U.S.A.). All statistical tests were two-tailed, and a P-value below 0.05 was considered statistically significant.
Results
Neuroprotective effects of intravitreal TMZ in IOP-induced retinal damage
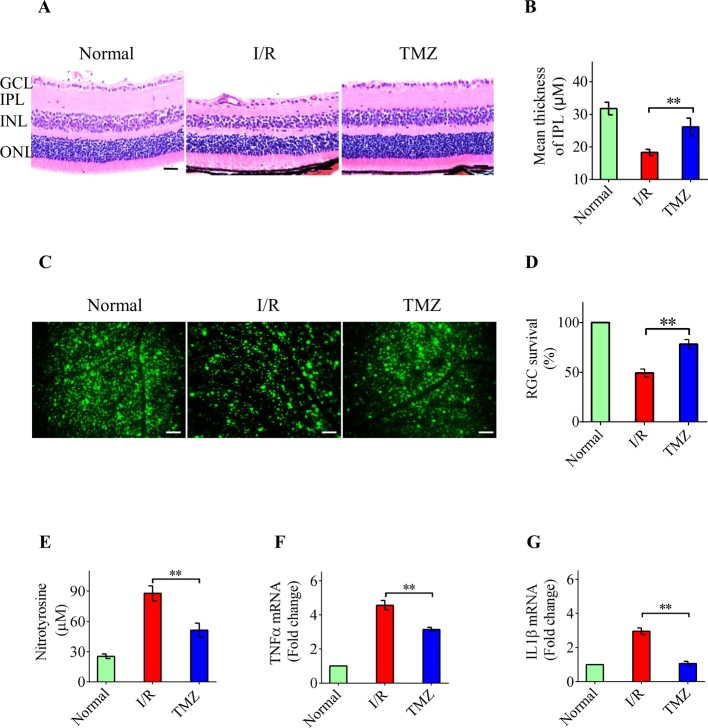
To evaluate the role of TMZ in acute glaucoma, TMZ was administered intravitreally before the acute elevation of IOP. We first investigated the change in retinal thickness in three independent groups of mice. Retinas exposed to I/R displayed a significant decrease in IPL thickness compared with normal retinas 7 days after reperfusion. However, the damage to the retina, especially to the IPL, was significantly ameliorated by TMZ (Figure 1A,B). The irreversible apoptosis of RGCs is another important indicator for the functional damage of the retina. FG analysis demonstrated that the number of RGCs was decreased in the experimental mice at 7 days after reperfusion compared with that observed in control mice (Figure 1C,D). Intravitreal TMZ injections significantly decreased the severity of retinal damage and extent of RGC death (Figure 1C,D). Previous studies have shown that overproduction and accumulation of neurotoxic mediators, including ROS, TNF-α, and IL-1β, play important roles in the primary and secondary waves of RGC apoptosis [11–13]. As shown in Figure 1E, the production of nitrotyrosine (a protein derivative of ROS) was significantly increased in the whole retina at 24 h after reperfusion compared with that in control retinas, and it declined in the retinas treated with TMZ. In addition, intravitreal injection of TMZ significantly suppressed the expression of TNFα and IL1β mRNA (Figure 1F,G). Collectively, these data show that TMZ exerts neuroprotective effects against IOP-induced retinal damage.
Figure 1. Neuroprotective effects of intravitreal TMZ against IOP-induced retinal damage.
(A,B) H&E staining of retinal cross-sections showing that intravitreal TMZ injection significantly inhibited the attenuation of total retinal thickness and IPL thickness in response to I/R damage 7 days after reperfusion. (C,D) FG labeling was performed 7 days after reperfusion. The results indicated that TMZ significantly increased the number of surviving cells in the RGC layer compared with that in the untreated I/R group. (E) The production of nitrotyrosine was significantly decreased by TMZ treatment in the whole retina 24 h after reperfusion compared with that in the untreated retinas. (F,G) As measured by real-time PCR, intravitreal injection of TMZ significantly suppressed the expression of TNFα and IL1β mRNA in retinas. Scale bar =50 μm. The data represents the means ± S.D. (n=6); **P<0.01. Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer 462 nuclear layer.
TMZ directly protects RGC apoptosis induced by OGD/R
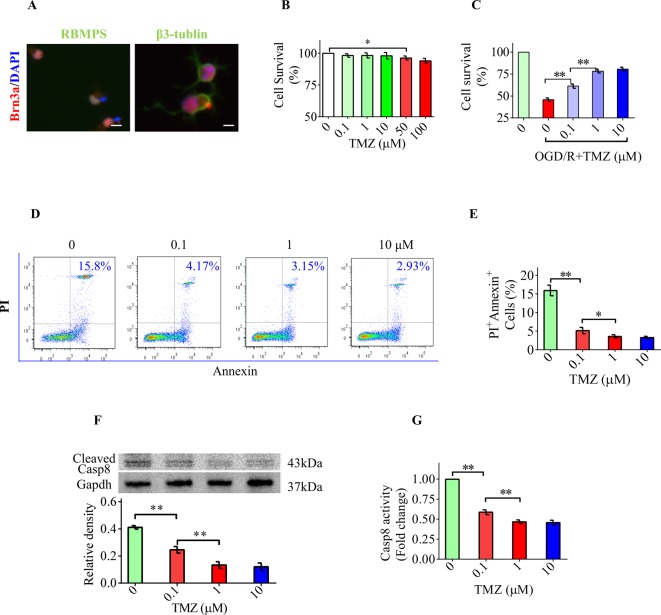
Next, we performed a series of in vitro studies to explore whether TMZ is capable of directly inhibiting the apoptosis of RGCs and the underlying mechanisms. We first identified primary cultured RGCs by characteristic markers. Immunostaining results indicated that primary RGCs indeed expressed Brn-3a, β3-tubulin, and RBPMS (Figure 2A). CCK8 assay was used to test whether TMZ can influence cellular metabolism or proliferation. The results showed that cellular metabolism was affected when TMZ concentration reached 50 μM (Figure 2B). Therefore, we selected the concentration range from 0.1 to 10 μM for subsequent in vitro studies. We then exposed primary RGCs to OGD/R to effectively mimic the IOP-induced I/R damage in vivo [4,14]. OGD/R decreased cell viability and TMZ (0.1–10 μM) treatment significantly increased the number of surviving RGCs (Figure 2C). The flow cytometry results showed that the number of apoptotic cells marked by PI and Annexin V was increased after OGD/R exposure, whereas TMZ treatment significantly decreased the number of PI + Annexin + cells (Figure 2D,E). The TMZ concentration ranging from 1 to 10 μM was found to be the most effective in exerting cytoprotective effects against OGD/R. In addition, TMZ significantly decreased caspase-8 activation as measured by Western blotting and caspase-8 activity assay (Figure 2F,G). These results demonstrate that TMZ decreases OGD/R-induced RGC apoptosis in vitro.
Figure 2. TMZ directly protects RGCs against apoptosis induced by OGD/R.
(A) Immunostaining results indicated that primary RGCs indeed expressed characteristic markers of RGCs including Brn-3a (red), RBPMS (green, left), and β3-tubulin (green, right). (B) CCK8 assay showed that cellular metabolism was affected when TMZ concentration reached 50 µM. (C) CCK8 assay showed that cell survival was significantly increased by TMZ treatment after OGD/R. (D,E) The results of flow cytometry showed that the proportion of apoptotic cells was increased to 15.8% (±3.1%) after OGD/R exposure. TMZ significantly decreased the proportion of PI + Annexin + cells to an average of 2.93%. (F) Western blot analysis demonstrated that TMZ significantly suppressed the level of cleaved caspase-8. (G) The reduction in caspase-8 activity was confirmed by caspase-8 activity assay. Scale bar =20 μm. The data represent the means ± S.D. (n=8); *P<0.05, **P<0.01.
The antioxidative effect of TMZ is involved in the TMZ-mediated neuroprotection in RGCs
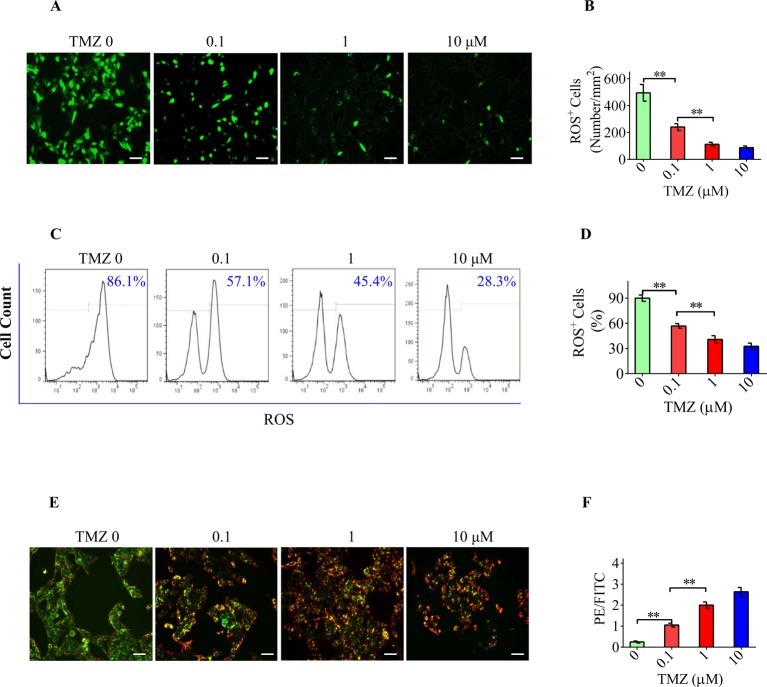
Primary RGCs in the OGD/R group display a greater extent of ROS production than the cells in the control group. TMZ was found to significantly attenuate the overproduction of ROS in RGCs exposed to OGD/R as marked by the reduction in green fluorescence in a concentration-dependent manner (Figure 3A,B). The diminished ROS production in primary RGCs by TMZ could also be observed by flow cytometry. The peak area occupied by FITC+ cells, representing cells with excess ROS, was found to be significantly decreased by TMZ treatment (Figure 3C,D). OGD/R also resulted in a decrease in the MMP in RGCs, which was marked by fluorescence shift from red to green. However, TMZ treatment protected mitochondrial function by restoring the MMP (Figure 3E,F). These results further support the antioxidative effect of TMZ on RGCs in vitro.
Figure 3. The antioxidative effect of TMZ is involved in the TMZ-mediated neuroprotective effect on RGCs.
(A,B) After OGD/R exposure, primary RGCs were subjected to ROS overproduction (green fluorescence). TMZ could significantly decrease ROS levels in RGCs. (C,D) As marked by the peak area of FITC+ population, the proportion of cells with excess ROS was significantly decreased from 80% (±6.1%) to 30% (±1.7%) in the OGD/R + TMZ RGCs. (E,F) OGD/R decreased the MMP, which was marked by a fluorescence shift from red to green in RGCs. TMZ pretreatment elevated the PE/FITC ratio. Scale bar =50 μm. The data represent the means ± S.D. (n=8); **P<0.01.
TMZ conferred protection against apoptosis in RGCs via Nrf2/Ho-1 signaling
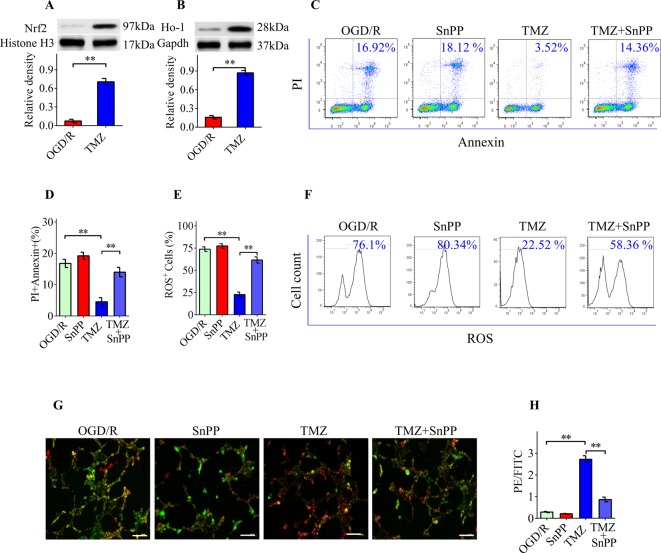
The above results showed that the antioxidative effects of TMZ are actively involved in the TMZ-mediated protection of RGCs. Nrf2, a transcription factor, is regarded as a master regulator of cellular responses to oxidative stress. Thus, we next determined whether Nrf2 and its downstream signaling factor, Ho-1, is associated with the TMZ-mediated protection against RGC apoptosis. Western blotting results showed that TMZ (10 μM) treatment significantly induced the nuclear translocation of Nrf2 in RGCs under OGD/R conditions (Figure 4A). TMZ treatment also increased Ho-1 protein expression compared with vehicle treatment (Figure 4B). To further confirm the role of Nrf2/Ho-1 signaling in the TMZ-mediated neuroprotection of RGCs, SnPP, a Ho-1 inhibitor was used. Our results showed that SnPP treatment significantly but incompletely reversed the TMZ-mediated neuroprotection of RGCs (Figure 4C,D). The reduction in intracellular ROS (Figure 4E,F) and alterations in MMP (Figure 4G,H) induced by TMZ were partially inhibited by SnPP. Taken together, these data suggest that TMZ confers neuroprotection through an Nrf2/Ho-1-dependent mechanism.
Figure 4. TMZ protects RGCs from apoptosis via Nrf2/Ho-1 signaling.
(A,B) Western blot analysis demonstrated that TMZ elevated the nuclear translocation of Nrf2 and total expression of Ho-1 in primary RGCs under OGD/R conditions. (C,D) TMZ suppressed OGD/R-induced apoptosis in primary RGCs. SnPP treatment significantly but incompletely reversed TMZ-mediated cytoprotective effect on RGCs. (E,F) Flow cytometry assay of ROS accumulation (FITC+) in RGCs. SnPP resulted in an increase in the proportion of FITC+ cells and inhibited the TMZ-induced antioxidative effect. (G,H) The decrease in PE/FITC ratio indicated significant disruption of the MMP in the SnPP-treated RGCs, which could not be rescued by TMZ treatment. Scale bar =50 μm. The data represent the means ± S.D. (n=8); **P<0.01.
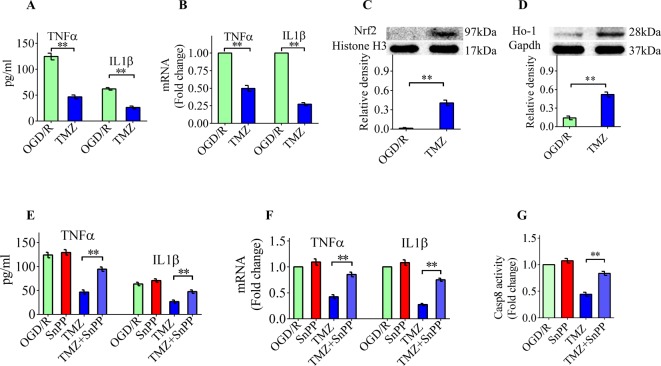
Nrf2/Ho-1 signaling is essential for TMZ-mediated anti-inflammatory effects in vitro
We also performed a series of in vitro experiments to further evaluate the anti-inflammatory effects of TMZ and to identify the underlying mechanisms. For this purpose, BV2 cells, an established mouse microglial cell line was cultured under OGD/R conditions. BV2 cells were plated in medium in the presence or absence of TMZ for 6 h before OGD/R. We used ELISA kits to examine the concentration of TNFα and IL1β in the supernatant of BV2 cells after OGD/R. The OGD/R-induced overproduction of TNFα and IL1β could be inhibited by TMZ (Figure 5A). The real-time PCR results further confirmed the anti-inflammatory effects of TMZ (Figure 5B). Nrf2/Ho-1 signaling plays an important role in the TMZ-mediated neuroprotection of RGCs. Thus, we next assessed whether this pathway is also involved in the anti-inflammatory effects of TMZ. As shown in Figure 5C,D, TMZ significantly induced the nuclear translocation of Nrf2 and increased the protein expression of Ho-1 in BV2 cells under OGD/R conditions. Our results demonstrated that SnPP significantly but incompletely reversed the TMZ-mediated inhibition of TNFα and IL1β production (Figure 5E,F). In addition, the inhibitory effect of TMZ on caspase-8 activation was significantly decreased after SnPP treatment (Figure 5G). These results indicate that TMZ is capable of regulating Nrf2/Ho-1 signaling to exert anti-inflammatory effects in vitro.
Figure 5. Nrf2/Ho-1 signaling is essential for the TMZ-mediated anti-inflammatory effects in vitro.
(A) As measured by ELISA, the concentration of TNFα and IL1β in supernatants was found to be significantly reduced by TMZ. (B) Real-time PCR analysis showed that TMZ decreased TNFα and IL1β mRNA in the TMZ group. (C,D) TMZ significantly induced the nuclear translocation of Nrf2 and increased the protein expression of Ho-1 in BV2 cells under OGD/R conditions. (E,F) SnPP significantly but incompletely reversed the TMZ-mediated inhibition on TNFα and IL1β production as measured by ELISA and real-time PCR. (G) SnPP treatment significantly decreased the inhibitory effect of TMZ on caspase-8 activation. The data represent the means ± S.D. (n=8); **P<0.01.
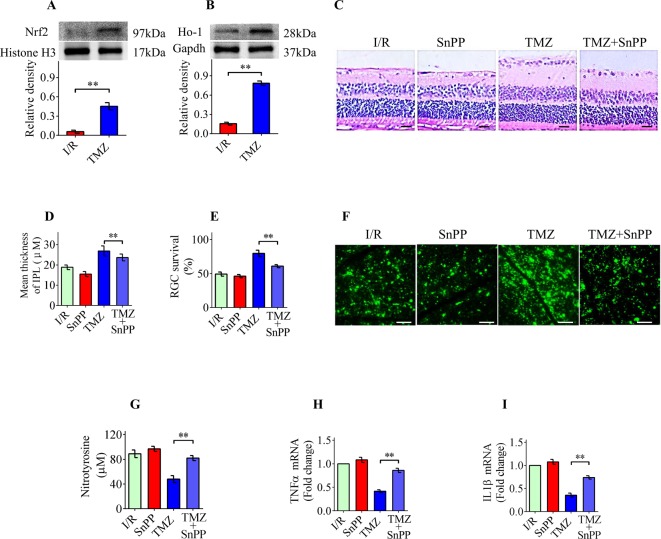
TMZ protects RGCs from IOP-induced damage via Nrf2/Ho-1 signaling
Our in vitro studies showed that Nrf2/Ho-1 signaling plays a critical role in TMZ-mediated antioxidative and anti-inflammatory effects. Therefore, we determined whether Nrf2/Ho-1 signaling was also involved in the TMZ-mediated neuroprotection against IOP-induced retinal damage. Western blotting results showed that TMZ treatment significantly induced the nuclear translocation of Nrf2 and increased the expression of Ho-1 in the retinas (Figure 6A,B). SnPP was intraperitoneally injected (40 mol/kg) 0.5 h prior to I/R and once daily for 3 days after I/R. As shown in Figure 6C–F, treatment with the Ho-1 inhibitor significantly blocked the TMZ-mediated neuroprotection against IOP-induced retinal damage. The Ho-1 inhibitor also significantly decreased the inhibitory effect of TMZ on ROS accumulation and inflammatory cytokine production (Figure 6G–I). These findings suggest that the up-regulation of Nrf2/Ho-1 signaling may contribute, at least in part, to the TMZ-mediated neuroprotection against IOP-induced retinal damage.
Figure 6. TMZ protects RGCs from IOP-induced damage via Nrf2/Ho-1 signaling.
(A,B) Western blot analysis demonstrated that TMZ dramatically induced the nuclear translocation of Nrf2 and increased the expression of Ho-1 in the retinas. (C,D) Treatment with the SnPP significantly blocked the TMZ-mediated neuroprotection in IOP-induced retinal attenuation, especially IPL thickness. (E,F) SnPP dramatically inhibited the TMZ-mediated neuroprotection in IOP-induced RGC apoptosis. (G) SnPP also significantly decreased the inhibitory effect of TMZ on nitrotyrosine production. (H,I) Real-time PCR results confirmed that SnPP significantly suppressed the TMZ-induced decrease in TNFα and IL1β mRNA levels compared with the TMZ only group. The data represent the means ± S.D. (n=8); **P<0.01.
Discussion
Acute glaucoma jeopardizes normal vision due to substantially high IOP. The progressive loss of RGCs caused by rapid increase in IOP is mainly responsible for irreversible blindness in acute glaucoma [15]. Currently, selecting effective drugs to prevent RGC apoptosis in IOP-induced retinal damage has become an attractive alternative for next-generation glaucoma therapy. TMZ, which improves energy metabolism in ischemic myocytes, was first developed as an antianginal drug [16]. However, a growing number of studies suggest that TMZ possesses numerous non-antianginal functions, including immunosuppression [17], antioxidation [18], anti-ischemia [19,20], and anti-apoptosis [21]. The present study demonstrated that treatment with TMZ significantly ameliorated high IOP-induced retinal damage and RGC apoptosis, with a significant decrease in ROS and inflammatory cytokine production in the retina. TMZ primarily exerts its therapeutic efficacy through its antioxidative and anti-inflammatory effects. These results are consistent with those of previous studies, showing that TMZ treatment attenuates acute inflammatory responses and oxidative stress in experimental cardiac remodeling [22] and pancreatitis [23]. Next, we found that the antioxidative and anti-inflammatory effects of TMZ depended primarily on the Nrf2/HO-1 pathway. Interestingly, TMZ treatment in IOP-induced retinal damage resulted in an obvious activation of the Nrf2/HO-1 pathway, which plays a crucial role in TMZ-mediated protection against IOP-induced retinal damage. These findings suggest that TMZ exerts neuroprotective effects against acute glaucoma mainly through antioxidative and anti-inflammatory properties.
The transcription factor Nrf2 is a central regulator of cellular responses to oxidative stress stimulation [24]. Under normal conditions, Nrf2 is an inactive complex in the cytoplasm with Kelch-like ECH-associated protein 1. When cells undergo oxidative stress, the complex is disrupted, and, subsequently, Nrf2 translocates to the nucleus, leading to target gene transcription, including Ho-1, a key heme-degrading enzyme [25,26]. In addition to its well-known antioxidative properties, the Nrf2/Ho-1 signaling pathway also exhibits anti-inflammatory and anti-apoptotic properties [27,28]. In the present study, we demonstrated that TMZ treatment promoted the nuclear translocation of Nrf2 and subsequently increased the expression of HO-1 in primary RGCs and BV2 cells. Furthermore, HO-1 blockade significantly but incompletely reversed the TMZ-mediated antioxidative and anti-inflammatory effects. Importantly, the HO-1 inhibitor also blocked the TMZ-mediated neuroprotection in RGCs in acute glaucoma. Therefore, these compelling findings suggest that Nrf2/HO-1 signaling contributes, at least in part, to the TMZ-mediated neuroprotection in acute glaucoma.
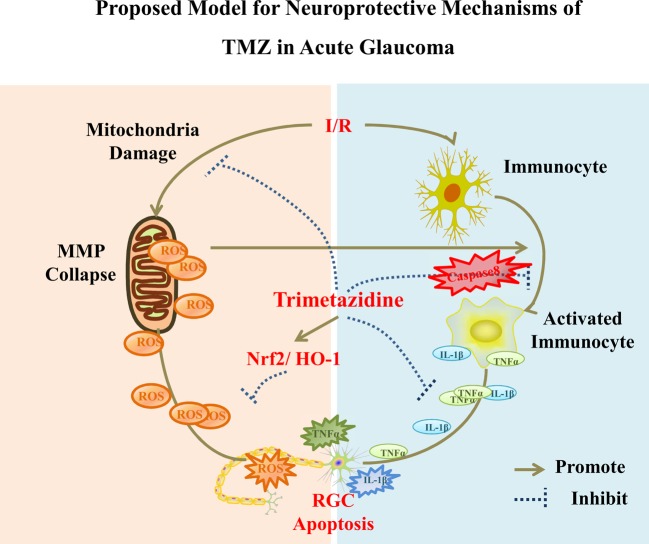
Caspase-8, a well-characterized initiator of apoptotic signaling, has been shown to have multiple non-apoptotic functions. Burguillos et al. reported that caspase-8 promotes microglial activation and neuroinflammation [29]. Our recent study indicated that caspase-8 mediates the production of cytokines, including IL1β and TNFα in immunocytes, which play important roles in high IOP-induced retinal damage [4]. In the present study, we observed that TMZ inhibited caspase-8-mediated RGC apoptosis. Furthermore, TMZ reduced caspase-8-dependent inflammatory reactions both in vivo and in vitro. Therefore, combining our results on the protective role of TMZ in acute glaucoma with existing evidence enables us to propose a mechanistic model that is described in Figure 7 (proposed model for the neuroprotective mechanisms of TMZ in acute glaucoma).
Figure 7. Proposed model for the neuroprotective mechanisms of TMZ in acute glaucoma.
I/R damages the function of mitochondria by disrupting the MMP, which leads to the overproduction of ROS. ROS pushes the cells toward the apoptotic fate and stimulates neuroinflammation. Nrf2/Ho-1 pathway can be activated by TMZ to confer protective effects by inhibiting the accumulation of ROS. Immunocyte activation during injury results in caspase-8-dependent inflammation by increasing TNFα and IL1β production, which also induces RGC apoptosis. Proinflammatory reactions can be effectively suppressed by TMZ. Therefore, RGC apoptosis can be attenuated by pretreatment with TMZ via antioxidative and anti-inflammatory mechanisms in acute glaucoma.
The unique pharmacological properties of TMZ enhance its efficacy and enable it to reach its peak concentration in less than 2 h. Timely treatment is closely associated with efficacy of neuroprotection [1,2]. Hence, TMZ could be used to halt the early stages of RGCs apoptosis in acute glaucoma, which is attributable to its fast mechanism of action. Due to its efficacy and safety, TMZ could be used a promising candidate to treat acute glaucoma and other related ocular diseases.
In summary, our study initially demonstrated the neuroprotective effects of TMZ on experimental acute glaucoma. Our results showed that TMZ inhibited RGC apoptosis and inflammation in vitro and in vivo, possibly by inhibiting caspase-8 signaling. Importantly, we further determined the neuroprotective mechanisms of TMZ by demonstrating that Nrf2-HO-1 signaling is essential for TMZ-mediated neuroprotective effects in vitro and in vivo. These findings provide compelling evidence that TMZ can protect against acute glaucoma and emerge as a promising candidate in the treatment of acute glaucoma.
Clinical perspectives
Acute glaucoma jeopardizes normal vision due to substantially high IOP and consequent RGCs death. Currently, an attractive alternative for next-generation glaucoma therapy is to select an effective drug to prevent RGC apoptosis in IOP-induced retinal damage.
The present study demonstrated that TMZ significantly ameliorated high IOP-induced retinal damage and RGC apoptosis, by exerting therapeutic efficacy through its antioxidative and anti-inflammatory properties via the Nrf2/HO-1 pathway.
Our findings suggested that TMZ can protect against acute glaucoma and emerge as a promising candidate in the treatment of acute glaucoma and other neurological disorders.
Abbreviations
- DCFH-DA
2′,7′-dichlorofluorescein diacetate
- FG
fluorogold
- IOP
intraocular pressure
- IPL
inner plexiform layer
- I/R
ischemic/reperfusion
- MMP
mitochondrial membrane potential
- OGD/R
oxygen-glucose deprivation and reperfusion
- PI
propidium iodide
- RGC
retinal ganglion cell
- ROS
reactive oxygen species
- TMZ
trimetazidine
Author contribution
P.W. and W.S. were responsible for the conception and design, data collection and assembly, manuscript writing, and final manuscript approval. W.S. was also responsible for the financial support. Y.Zhang, Z.L. and C.D. were responsible for data collection and assembly. Y.Zhuo was responsible for conception and design, manuscript writing, and final manuscript approval.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the NSFC [grant number 81470627]. The sponsor of the study had no role in the design of the original study protocol, in the collection, analysis and interpretation of the data, in writing the report, or in the decision to submit the manuscript for publication.
References
- 1.Guo L., Moss S.E., Alexander R.A., Ali R.R., Fitzke F.W. and Cordeiro M.F. (2005) Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest. Ophthalmol. Vis. Sci. 46, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo L., Salt T.E., Maass A., Luong V., Moss S.E., Fitzke F.W. et al. (2006) Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest. Ophthalmol. Vis. Sci. 47, 626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shakya S. and Gupta H.R. (2006) Angle closure glaucoma: a cause for bilateral visual threat. Nepal Med. Coll. J. 8, 153–155 [PubMed] [Google Scholar]
- 4.Chi W., Li F., Chen H., Wang Y., Zhu Y., Yang X. et al. (2014) Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma. Proc. Natl. Acad. Sci. U.S.A. 111, 11181–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopaschuk G.D., Barr R., Thomas P.D. and Dyck J.R. (2003) Beneficial effects of trimetazidine in ex vivo working ischemic hearts are due to a stimulation of glucose oxidation secondary to inhibition of long-chain 3-ketoacyl coenzyme a thiolase. Circ. Res. 93, e33–e37 [DOI] [PubMed] [Google Scholar]
- 6.MacInnes A., Fairman D.A., Binding P., Ja R., Wyatt M.J., Phelan A. et al. (2003) The antianginal agent trimetazidine does not exert its functional benefit via inhibition of mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ. Res. 93, e26–e32 [DOI] [PubMed] [Google Scholar]
- 7.Atilgan D., Parlaktas B.S., Uluocak N., Erdemir F., Markoc F., Saylan O. et al. (2014) The effects of trimetazidine and sildenafil on bilateral cavernosal nerve injury induced oxidative damage and cavernosal fibrosis in rats. ScientificWorldJournal 2014, 970363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serarslan Y., Bal R., Altug M.E., Kontaş T., Keleş O.N., Unal D. et al. (2009) Effects of trimetazidine on crush injury of the sciatic nerve in rats: a biochemical and stereological study. Brain Res. 1247, 11–20 [DOI] [PubMed] [Google Scholar]
- 9.Kay L., Finelli C., Aussedat J., Guarnieri C. and Rossi A. (1995) Improvement of long-term preservation of the isolated arrested rat heart by trimetazidine: effects on the energy state and mitochondrial function. Am. J. Cardiol. 76, 45B–49B [PubMed] [Google Scholar]
- 10.Winzeler A. and Wang J.T. (2013) Purification and culture of retinal ganglion cells from rodents. Cold Spring Harb. Protoc. 2013, 643–652 [DOI] [PubMed] [Google Scholar]
- 11.Li S.Y., Fu Z.J., Ma H., Jang W.C., So K.F., Wong D. et al. (2009) Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Invest. Ophthalmol. Vis. Sci. 50, 836–843 [DOI] [PubMed] [Google Scholar]
- 12.Liu L., Sun Q., Wang R., Chen Z., Wu J., Xia F. et al. (2016) Methane attenuates retinal ischemia/reperfusion injury via anti-oxidative and anti-apoptotic pathways. Brain Res. 1646, 327–333 [DOI] [PubMed] [Google Scholar]
- 13.Yang C., Lafleur J., Mwaikambo B.R., Zhu T., Gagnon C., Chemtob S. et al. (2009) The role of lysophosphatidic acid receptor (LPA1) in the oxygen-induced retinal ganglion cell degeneration. Invest. Ophthalmol. Vis. Sci. 50, 1290–1298 [DOI] [PubMed] [Google Scholar]
- 14.Osborne A., Aldarwesh A., Rhodes J.D., Broadway D.C., Everitt C. and Sanderson J. (2015) Hydrostatic pressure does not cause detectable changes in survival of human retinal ganglion cells. PLoS ONE 10, e0115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Almasieh M., Wilson A.M., Morquette B., Cueva Vargas J.L. and Di Polo A. (2012) The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 31, 152–181 [DOI] [PubMed] [Google Scholar]
- 16.Mehrotra T.N. and Bassadone E.T. (1967) Trimetazidine in the treatment of angina pectoris. Br. J. Clin. Pract. 21, 553–554 [PubMed] [Google Scholar]
- 17.Chen J., Lai J., Yang L., Ruan G., Chaugai S., Ning Q. et al. (2016) Trimetazidine prevents macrophage-mediated septic myocardial dysfunction via activation of the histone deacetylase sirtuin 1. Br. J. Pharmacol. 173, 545–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tselepis A., Doulias P., Lourida E., Glantzounis G., Tsimoyiannis E. and Galaris D. (2001) Trimetazidine protects low-density lipoproteins from oxidation and cultured cells exposed to H2O2 from DNA damage. Free Radic. Biol. Med. 30, 1357–1364 [DOI] [PubMed] [Google Scholar]
- 19.Demir T., Turgut B., Ozercan I., Gul F.C., Ilhan N. and Celiker U. (2010) Trimetazidine for prevention of induced ischemia and reperfusion of guinea pig retina. Clin. Ophthalmol. 4, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozden S., Kildaci B., Muftuoglu S., Cakar N. and Yildirim C. (2001) Effect of trimetazidine on retinal ischemia/reperfusion injury in rats. Ophthalmologica 215, 309–317 [DOI] [PubMed] [Google Scholar]
- 21.Gong X., Fan G., Wang W. and Wang G. (2014) Trimetazidine protects umbilical cord mesenchymal stem cells against hypoxia and serum deprivation induced apoptosis by activation of Akt. Cell. Physiol. Biochem. 34, 2245–2255 [DOI] [PubMed] [Google Scholar]
- 22.Zhou X., Li C., Xu W. and Chen J. (2012) Trimetazidine protects against smoking-induced left ventricular remodeling via attenuating oxidative stress, apoptosis, and inflammation. PLoS ONE 7, e40424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanoglu A., Yazgan Y., Kaplan M., Berber U., Kara M., Demırel D. et al. (2015) Trimetazidine significantly reduces cerulein-induced pancreatic apoptosis. Clin. Res. Hepatol. Gastroenterol. 39, 145–150 [DOI] [PubMed] [Google Scholar]
- 24.Ma Q. (2013) Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53, 401–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loboda A., Damulewicz M., Pyza E., Jozkowicz A. and Dulak J. (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 73, 3221–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Na H.K. and Surh Y.J. (2014) Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic. Biol. Med. 67, 353–365 [DOI] [PubMed] [Google Scholar]
- 27.Li F., Lu S., Zhu R., Zhou Z., Ma L., Cai L. et al. (2011) Heme oxygenase-1 is induced by thyroid hormone and involved in thyroid hormone preconditioning-induced protection against renal warm ischemia in rat. Mol. Cell. Endocrinol. 339, 54–62 [DOI] [PubMed] [Google Scholar]
- 28.Paine A., Eiz-Vesper B., Blasczyk R. and Immenschuh S. (2010) Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 80, 1895–1903 [DOI] [PubMed] [Google Scholar]
- 29.Burguillos M.A., Deierborg T., Kavanagh E., Persson A., Hajji N., Garcia-Quintanilla A., Cano J., Brundin P., Englund E., Venero J.L. and Joseph B. (2011) Caspase signalling controls microglia activation and neurotoxicity. Nature 472, 319–234 doi: 10.1038/nature09788 [DOI] [PubMed] [Google Scholar]