Abstract
Activating mutations in one of the two subunits of the ATP-sensitive potassium (KATP) channel cause neonatal diabetes (ND). This may be either transient or permanent and, in approximately 20% of patients, is associated with neurodevelopmental delay. In most patients, switching from insulin to oral sulfonylurea therapy improves glycemic control and ameliorates some of the neurological disabilities. Here, we review how KATP channel mutations lead to the varied clinical phenotype, how sulfonylureas exert their therapeutic effects, and why their efficacy varies with individual mutations.
Trends
Activating mutations in KATP channel genes lead to ND, which may be permanent (PNDM) or transient (TNDM). Some (∼20%) patients with PNDM also have neurodevelopment problems.
All ND mutations reduce the ability of ATP to close the channel, and so stimulate insulin secretion. The greater the decrease in channel ATP sensitivity, the more severe the clinical phenotype.
Sulfonylurea drugs close most mutant KATP channels and provide better glycemic control compared with insulin. Their efficacy correlates with the specific mutation, being less for highly ATP-insensitive channels. It also decreases with patient age, probably due to the deleterious effects of chronic hyperglycemia on the beta cell.
Introduction
ND (see Glossary) is a rare disorder with a prevalence of around 1 in 100 000–200 000 live births. 1 in 1001 in 100 It is characterized by diabetes that usually presents within the first 6 months of life. ND is caused by mutations in several different genes but those in the genes encoding the pore-forming (Kir6.2, KCNJ11) and regulatory (SUR1, ABCC8) subunits of the KKATPchannel channel are the most common (∼50% of cases) and are the focus of this review.
The KATP channel plays a key role in insulin release from pancreatic beta cells because it links cell metabolism to electrical activity [1]. It does so by regulating the beta cell resting membrane potential. At low glucose levels, channel activity is high, which hyperpolarizes the beta cell and switches off electrical activity and insulin secretion [1]. Elevation of extracellular glucose increases glucose uptake and metabolism and stimulates ATP production at the expense of ADP. These nucleotide changes result in closure of the KATP channel, triggering membrane depolarization, opening of voltage-gated calcium channels, calcium influx, and insulin release. Decreased KATP channel activity not only initiates insulin secretion; its further closure also contributes to the graded increase in action potential firing and insulin secretion at glucose concentrations above threshold [2].
Activating mutations in either Kir6.2 or SUR1 that reduce the ability of metabolically generated ATP to close the channel prevent glucose-induced electrical activity and insulin release, resulting in ND 3, 4, 5. However, most mutant channels can be closed by sulfonylurea drugs, such as glibenclamide (glyburide) [6]. These are now the treatment of choice for ND, because they improve the patient’s clinical condition and quality of life, and reduce medical costs 7, 8.
In this review, we provide an overview of recent advances in our understanding of the molecular mechanisms underlying KATP channel ND, and its treatment with sulfonylureas.
Activating KATP Channel Mutations Cause ND
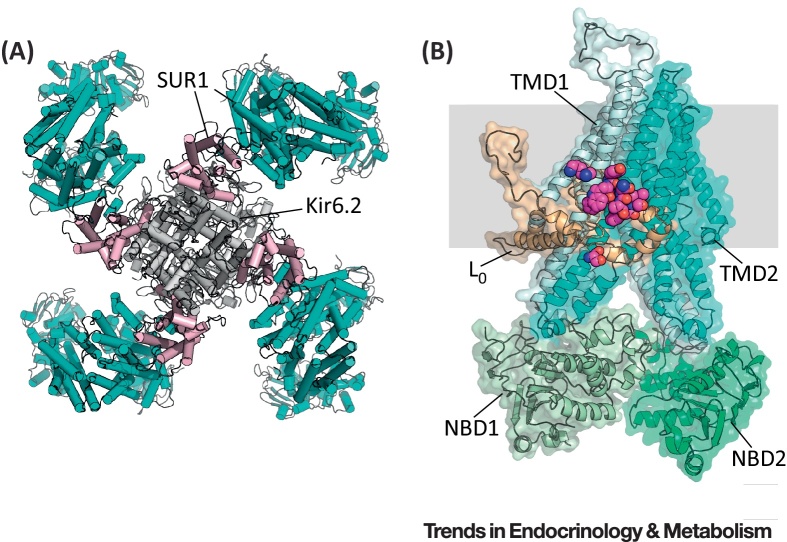
The KATP channel is a large octameric complex comprising a central Kir6.2 tetramer, surrounded by four SUR1 subunits, as can be seen in the recent 6-Å resolution cryo-electron microscopy (EM) structure 9, 10 (Figure 1). Activating mutations in the KATP channel produce a range of phenotypes that correlate with the extent of reduction in channel ATP sensitivity 4, 11. At one end of the spectrum are mutations that cause a small decrease in ATP inhibition. These produce permanent (PNDM) or transient ND (TNDM) 3, 4, 12, 13, lead to diabetes in young adult life 14, 15, 16, or enhance the risk of type 2 diabetes mellitus (T2DM) [17]. At the other end of the spectrum are mutations that render the channel highly insensitive to ATP and cause neurological complications in addition to diabetes 4, 18. Thus, the greater the decrease in channel ATP sensitivity, the more severe the clinical phenotype.
Figure 1.
Structure of the KATP Channel. (A) Structure of the KATP channel, viewed from above, showing four SUR1 subunits (cyan and pink) surrounding a central tetrameric Kir6.2 pore (gray) (Protein Data Bank: 5TWV) [10]. SUR1 interacts with Kir6.2 via its first set of transmembrane domains (pink). (B) Side view of a single SUR1 subunit, showing its position in the membrane (gray), and the putative location of the sulfonylurea-binding site. The nucleotide-binding domains (NBDs) are shown in green, TMD0 in beige, TMD1 in light blue, and TMD2 in cyan. Residues surrounding the putative binding pocket are shown in magenta. (C) Close-up of the putative sulfonylurea-binding pocket.
Most ND mutations occur spontaneously. Kir6.2 mutations are almost all heterozygous and most frequently occur at residues R201 and V59 [11], whereas SUR1 mutations are genetically more heterogeneous 11, 19. Although diabetes usually develops within 6 months after birth, it is now clear that some patients, including those with intermediate developmental delay and ND(iDEND) syndrome 20, 21, gestational diabetes, adult-onset diabetes 14, 15, 16, or PNDM [22] may present significantly later in life. Why diabetes does not occur immediately after birth in all patients remains something of a puzzle.
All ND mutations reduce the ability of ATP to cause channel closure. However, mechanistically, they act in a variety of ways. They may impair ATP binding at Kir6.2 or the way in which ATP binding is translated into pore closure 23, 24, 25. They may enhance MgADP activation at SUR1 by increasing the affinity of the nucleotide-binding domains (NBDs) for nucleotides [26]. In addition, mutations in either Kir6.2 or SUR1 may increase the unliganded channel open probability, which leads to a decrease in both ATP and sulfonylurea block 18, 27.
Sulfonylurea Therapy in ND
Sulfonylureas bind to SUR1 and induce KATP channel closure, thereby triggering membrane depolarization, electrical activity, calcium influx, and insulin release. They have been used for >50 years to treat T2DM [6], and are now the therapy of choice for KATP channel-dependent ND 7, 28. Higher drug concentrations are often needed in patients with ND than in those with T2DM: the usual dose for an adult with T2DM is 0.08–0.25 mg/kg/day, whereas patients with ND, on average, require 0.5 mg/kg [29]. Patients with functionally severe mutations may need even higher doses to ameliorate their neurological complications; up to 2.3 mg/kg/day has been used 29, 30. The greater drug dose required by patients with ND may be attributed to the increased KATP channel activity and, in some cases, a reduced sensitivity of the mutant channel to sulfonylureas. Patients with SUR1 mutations often require a lower drug dose compared with those with Kir6.2 mutations, consistent with the fact that SUR1 mutations have less functional impact. Most patients with ND have been treated with glibenclamide, although a few have been treated with other sulfonylureas, such as gliclazide [7].
Sulfonylurea therapy has considerable therapeutic benefits. First, the marked fluctations in blood glucose characteristic of insulin therapy are absent 31, 32. Second, control of glucose homeostasis dramatically improves, as indicated by a lower HbA1c level [7]. Conseqently, it is expected that patients will be less likely to develop diabetic complications. Third, despite the improved glucose control, there is no change in incidence of hypoglycemic events [6] and reports of hypoglycemic events are rare [28]. Finally, sulfonylurea therapy is far easier for the patient (or carer) to manage. Despite the high doses needed to treat ND, sulphonylureas have few side effects (apart from potential hypoglycemia). Transient gastric problems (e.g., diarrhea or abdominal pain) may occur, but generally quickly resolve 28, 33. Staining of tooth enamel by glibenclamide has also been reported, usually in patients who chew their tablets before swallowing 33, 34.
The ability of most patients with ND to transfer to sulfonylurea therapy implies they retain significant numbers of beta cells, often despite many years of diabetes. This is consistent with post-mortem EM studies of the pancreas from patients with ND 35, 36. However, islets were smaller and reduced in number, and the percentage of cells staining for insulin was decreased. Similar features are found in mouse models of ND 37, 38, 39, 40. It is likely they result from poor glucose homeostasis.
In marked contrast to T2DM, where sulfonylureas eventually fail to control glycemia, a common feature of sulfonylurea therapy in ND is that the drug dose declines with time after transfer from insulin [6]. This may reflect a time-dependent improvement in beta cell function and/or mass, because glycemia is better controlled by sulfonylureas. Support for this idea comes from studies of an inducible mouse model of diabetes, where diabetes of longer duration required higher glibenclamide doses to achieve euglycemia [38]. This arises because hyperglycemia causes marked downregulation of insulin content, numerous changes in gene expression, and impaired beta cell metabolism 37, 38, 39, 40. Many (but not all) of these changes are prevented or reversed by restoration of euglycemia [38]. A time-dependent improvement in beta cell mass and/or function may explain why drug dose declines with time in many patients with ND.
Insights into the Molecular Mechanism of Sulfonylurea Action
Sulfonylureas interact with two sites on the KATP channel, a high-affinity site on SUR1 and a low-affinity site on Kir6.2 6, 41, but only the former is of therapeutic importance. The location of the sulfonylurea-binding site on SUR1 is still unknown, but mutagenesis studies indicate that it involves residues in the third and eighth cytosolic loops. These regions come into close apposition in the recent 6-Å cryoEM structure of the KATP channel 9, 10, suggesting a possible site for the sulfonylurea-binding pocket (Figure 1B).
It is important to recognize that sulfonylureas act as partial antagonists at the high-affinity site, and that KATP channels with sulfonylurea bound to SUR1 are still able to open, albeit with lower open probability [42]. As a consequence, high-affinity inhibition is not complete, but reaches a maximum of approximately 50–80%, producing a pedestal in the concentration–response curve when studied in excised inside-out membrane patches 41, 43. Surprisingly, however, channel inhibition by sulfonylureas is complete when studied in intact cells, using the whole-cell configuration [43]. Resolution of this paradox comes from the finding that sulfonylurea block is modulated by intracellular adenine nucleotides 41, 43. This arises from a combination of a mutual antagonism between sulfonylurea binding and Mg2+ nucleotide binding at the NBDs of SUR1, and the complex effects of nucleotides on the KATP channel.
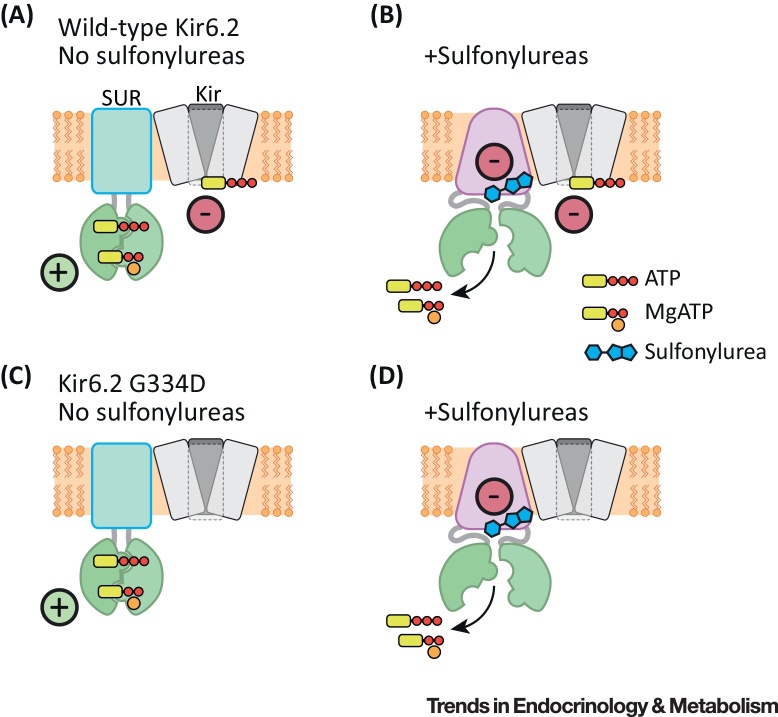
Mg2+ nucleotides both inhibit and stimulate KATP channel activity by interaction with Kir6.2 and SUR1, respectively [1] (Figure 2A). Thus, channel activity is governed by the relative intensity of these two effects. Inhibition can be studied in isolation by expressing Kir6.2 in the absence of SUR1. Conversely, activation can be isolated by expressing SUR1 with an ATP-insensitive Kir6.2 mutant [44]. This reveals that sulfonylureas impair both nucleotide binding to SUR1 and its transduction into channel activation, as exemplified by a decrease in both the EC50 for activation and its maximal amplitude [43]. Consequently, channel activation is markedly decreased by sulfonylureas. Therefore, in wild-type channels, nucleotide inhibition predominates and this adds to the sulfonylurea block to produce almost complete channel inhibition at physiological nucleotide levels (Figure 2B).
Figure 2.
Schematic Showing How Nucleotides and Sulfonylureas Interact with SUR1. In wild-type channels, MgATP stimulates channel activity at SUR1 and inhibits it at Kir6.2. Thus, channel activity is a balance between these inhibitory and stimulatory effects (A). Sulfonylureas both block channel activity directly (by a maximum of 50–80%) and prevent MgATP activation (B). Consequently, ATP inhibition is no longer counteracted by MgATP activation. Thus, ATP and sulfonylurea block now summate to produce complete channel inhibition (B). Channels with mutations that reduce ATP block (e.g., G334D) are activated, but not blocked, by MgATP (C). Sulfonylureas still both block channel activity and prevent MgATP activation (D). However, the overall block of channel activity is less than for wildtype channels because of lack of ATP block at Kir6.2.
Sulfonylurea inhibition (or, more correctly, apparent inhibition) will therefore be influenced by the extent of ATP block at Kir6.2. It will be less for channels with mutations that reduce ATP inhibition (Figure 2C,D), leading to a pedestal of unblocked current whose magnitude varies with the ATP sensitivity of the channel 45, 46. This probably explains why sulfonylureas are ineffective in patients with ND who carry mutations that give rise to channels that are very ATP insensitive [24].
The fact that sulfonylurea inhibition is incomplete for channels containing severe ND mutations (due to their impaired ATP sensitivity), explains why patients with these mutations are less susceptible to severe hypoglycemia [8]. Given that channel activity is never completely abolished [46], maximal insulin secretion will be limited. However, patients with mutations that only produce a mild reduction in ATP sensitivity can be expected to have channels that are completely blocked by sulfonylureas and, therefore, may experience hypoglycemia if they take too much drug.
In addition to inhibition of the KATP channel, certain sulfonylureas (such as glibenclamide and tolbutamide, but, interestingly, not gliclazide) stimulate insulin secretion via an interaction with Epac2 47, 48. Therefore, it appears possible that Epac2 mediates the reported effects of sulfonylureas on beta cell exocytosis [49]. However, it is important to emphasize that KATP channel inhibition is a prerequisite for sulfonylurea-stimulated insulin secretion, in order to elevate intracellular calcium.
Why Is Glucose Homeostasis Better on Sulfonylurea Therapy?
Wild-type KATP channels are largely blocked at resting blood glucose concentrations (5–7 mM) 50, 51, so that the beta cell sits poised on the cusp of secretion and only a small reduction in channel activity is needed to trigger electrical activity and insulin secretion. In patients with ND, it appears likely that circulating sulfonylureas close the KATP channel to a similar extent, sufficient to maintain blood glucose at resting levels, but not enough to cause hypoglycemia.
Transfer to sulfonylurea therapy restores meal-stimulated insulin release [7] and decreases fluctuations in blood glucose 31, 32. Restoration of the beta cell incretin response and the amplifying effects of glucose likely contribute to these important effects. Incretins are released in response to the presence of food in the gut and exert a stimulatory effect on insulin secretion, reducing the excursion in blood glucose. However, both incretins (e.g., GLP-1) and paracrine hormones (e.g., glucagon) only potentiate insulin secretion induced by glucose. They do not initiate insulin release because they are only effective once intracellular calcium has been elevated by glucose-stimulated KATP channel closure [3]. Therefore, they are ineffective in patients with insulin-treated ND, but become effective following transfer to sulfonylureas [7]. Likewise, the amplifying effects of glucose on insulin secretion [52] will only be enabled following KATP channel closure and membrane depolarization.
It is well recognized that the ability of sulfonylureas to enhance insulin secretion in patients with with T2DM rapidly wanes. By contrast, to date, patients with ND have not shown secondary failure to sulfonylureas, although many have been treated for at least 8–10 years [53]. This argues that the origin of the beta cell defect in T2DM is not the same as in ND.
Given that their diabetes is so well controlled, some patients with ND elect to remain on drug therapy when pregnant. Good glycemic control is important for both mother and fetus. However, glibenclamide can cross the placenta and, as a consequence, fetuses who do not carry a ND mutation may require early delivery, and develop fetal macrosomia and/or postnatal hyperinsulinemic hypoglycemia 54, 55, 56. Thus, sulfonylurea therapy should be avoided (at least during the third trimester) if prenatal genetic testing has not been performed or if the fetus is found not to carry the mutation. Non-invasive prenatal genetic testing in the offspring of a father with ND has been successfully performed [57]. By contrast, if the fetus does carry the mutation, sulfonylureas may be beneficial, because they can be expected to prevent the low birth weight 7, 19, caused by reduced fetal insulin secretion in utero that is characteristic of ND neonates.
Why Do Some Patients Not Respond to Sulfonylureas?
Not all patients respond to sulfonylurea therapy. Whether they do so depends on the specific mutation they possess and the duration of their diabetes.
Some mutations cause a marked increase in channel activity in the absence of nucleotide [18]: this produces a marked decrease in sulfonylurea inhibition [58]. Other mutations do not alter channel gating but produce a dramatic reduction in ATP block at Kir6.2 [24], and thereby decrease sulfonylurea block (as described above). All patients with these mutations fail to transfer [59] and it is unlikely that they would be able to transition even if the drug dose were greatly increased.
There are also several mutations for which some patients transferred and others failed to do so, despite taking an adequate dose of sulfonylureas 33, 59. This appears to be related to the duration of diabetes. Almost all individuals who started taking sulfonylureas during the first 5 years after diabetes diagnosis transferred successfully, whereas only 65% of those over the age of 18 were able to do so [59]. This emphasizes the need to implement sulfonylurea therapy as early as possible.
One explanation for the better outcome when sulfonylureas are started early is that the patient’s beta cells have been exposed to chronic hyperglycemia for a shorter time. Mouse models of ND reveal that chronic hyperglycemia markedly impairs beta cell function and reduces beta cell mass 37, 38, 39, 40. Nevertheless, even when the patient fails to transfer fully, it is recommended they continue sulfonylureas (as well as insulin) because of the improvement in glycemic control and neurological function this can provide.
Why Is ND Sometimes Transient?
In some patients with ND, diabetes is transient, remitting within a few months or years of birth 4, 11, 12. Why diabetes remits is unclear. One possibility is that it reflects a reduced insulin demand or improved beta cell function at the time of remission. Why many TNDM patients subsequently relapse also remains unknown, but might, in part, result from the increase in insulin resistance that normally develops at puberty 60, 61.
Interestingly, approximately 30% of mice expressing an activating KATP channel mutation that were treated with glibenclamide at disease onset for just 6 days, were subsequently able to maintain euglycemia without drug therapy [62]. Quite why this happens is unclear. Because the mutation was targeted specifically to the beta cell, one possible explanation is that new beta cells are generated (subsequent to gene induction) from progenitor cells. As these will lack the mutant KATP channel, they will be essentially normal (provided hyperglycemia is prevented). Thus, a gradual increase in the number of beta cells lacking the mutation might underlie the impoved glucose control. Lineage tracking could determine if this idea is correct.
That early glibenclamide therapy can ‘cure’ human ND appears unlikely. It has been reported that aggessive glibenclamide therapy immediately following diagnosis can cause diabetes remission, with patients subsequently requiring no drug or a subtherapeutic dose 63, 64. However, because of their young age, it is difficult to know whether these individuals are in fact patients with TNDM who have remitted but not yet relapsed. Nevertheless, this is an interesting area that deserves futher study.
It is worth noting that, because ND may be transient, iatrogenic hyperglycemia may occur if sulfonylurea therapy is not discontinued.
ND Mutations Can Cause Neurological Problems
KATP channels (Kir6.2/SUR1) are not confined to beta cells; they are found in many other tissues, including muscle and brain. This accounts for the fact that approximately 20% of patients with ND have profound neurological deficits. These include delayed motor and mental development, attention deficit, hyperactivity, autism, aggression, intellectual disability, and (occasionally) seizures 4, 65, 66. Hand–eye coordination is also impaired [67]. This spectrum of disorders is known as iDEND syndrome. In 3% of cases, epilepsy is also present (DEND syndrome). In general, these features are confined to patients with the most functionally severe mutations and are mainly (but not invariably [68]) associated with mutations in Kir6.2. The motor and behavioral problems are usually ameliorated by sulfonylurea therapy, in some cases markedly so 65, 69, and seizures may cease 13, 30, However, the neurological problems often persist or are only partially improved 65, 66, 70. The motor problems of patients with iDEND/DEND are recapitulated in mice expressing an activating KATP channel mutation specifically in their neurones (nV59M mice), but no effect was seen when the same mutation was expressed in muscle [71]. Furthermore, muscle function tests were normal in patients with ND [65] and gliclazide (which acts on neuronal but not muscle KATP channels [6]) enhanced motor function [25]. This argues that the motor deficit is neuronal in origin. This reflects, at least in part, impaired cerebellar activity, because nV59M mice show decreased electrical activity in their cerebellar Purkinje cells [72] and SPECT scanning suggests that patients with iDEND also have reduced cerebellar activity [73]. Interestingly, the latter is improved by glibenclamide, which accords with the improved motor function observed after transfer to sulfonylurea therapy in patients with iDEND.
Anxiety disorders, attention deficit hyperactivity disorder (ADHD), and autism are frequently observed in patients with iDEND (especially those with the V59M mutation) [66]. Similarly, nV59M mice show hyperactivity and increased exploratory behavior 72, 74. However, in contrast to patients with iDEND, nV59M mice show reduced anxiety [74]. Why this is the case is unclear, but it is worth noting it is difficult to disentangle effects on anxiety and locomotor activity in mice. Interestingly, nV59M mice also exhibit reduced sensitivity to common inhalation anaesthetics, such as isofluorane [75], but whether this happens in humans is unknown.
The failure of sulfonylureas to fully reverse the neurological deficits of patients with iDEND may result from a failure of the drug to reach a therapeutic concentration in the brain. In rats, sulfonylureas are rapidly pumped out of the brain [75]. Whether other drugs that inhibit KATP channels (e.g., carbemazepine [76]) might be more effective remains to be tested. Given that KATP channel activation appears to decrease inhibitory tone, drugs that decrease GABA uptake or prolong its action might also be worth exploring.
An alternative explanation for the poor effects of sulfonylureas on cognitive function is that KATP channel overactivity in the developing brain produces irreversible structural effects. Although gross changes in brain anatomy are not observed, it is possible that the enhanced KATP current prevents synaptic connections from forming correctly. Because the brain continues to develop after birth, this may explain why early treatment affords the best outcome, with some patients carrying severe mutations who were treated at diagnosis meeting their developmental milestones, at least initially 13, 69.
There are several reports that some patients with SUR1 mutations that cause transient ND also have neurological symptoms 5, 77, 78. By contrast, this is not observed in patients with transient ND due to Kir6.2 mutations. This is clearly an area where more research is necessary. If the neurological symptoms are confirmed to be a consequence of the SUR1 mutation, it would suggest that SUR1 may have an additional role in the brain, distinct from its ability to modulate the KATP channel. In beta cells, SUR1 is also found in the insulin granule (where its role remains to be defined) [79] and has been proposed to act as a scaffold for exocytotic proteins in beta cells [80]. This raises the question of whether SUR1 serves a similar function at the neuronal synapse.
Why Do ND Mutations Not Cause Cardiac Problems?
Surprisingly, despite the fact that Kir6.2 forms the pore of both beta cell and cardiac KATP channels, there are no reported cardiac effects in patients with ND. Similarly, mice in which an activating Kir6.2 mutation is expressed in the heart (cV59M mice) show no cardiac defects [71]. This is probably because most cardiac KATP channels contain SUR2A rather than SUR1 subunits. The Kir6.2/SUR2A channel shows a markedly lower sensitivity to activation when cytosolic ATP falls 72, 81. Why this is the case is not fully established. In isolated membrane patches, ATP inhibits (and MgADP activates) Kir6.2/SUR2A and Kir6.2/SUR1 channels to similar extents [72]. However, MgADP markedly reduces the inhibitory effect of ATP on Kir6.2/SUR1, but not on Kir6.2/SUR2A 72, 82. This probably explains the different metabolic sensitivites of beta cell and cardiac KATP channels, and protects patients with ND from deleterious cardiac effects.
This interpretation rests on the assumption that cardiac KATP channels are primarly composed of SUR2A, not SUR1, subunits. Interestingly, SUR1 is expressed in mouse atrial myocytes [83], and its activation contributes to action potential shortening during metabolic inhibition [84]. However, no change in heart rate is seen in cV59M mice [71]. Furthermore, the functional importance of SUR1 in the human heart remains unclear, and the lack of obvious changes in cardiac function in patients with ND suggests that its contribution is small.
Does ND Offer Insight into T2DM Etiology?
Given that activating KATP mutations cause ND, can mutations that produce a lesser decease in ATP sensitivity cause diabetes in later life? The answer appears to be yes.
First, mutations in SUR1 are associated with adult-onset diabetes, with some patients exhibiting glucose intolerance, some having clinical features of maturity-onset diabetes of the young (MODY), and others being diagnosed with T2DM 14, 15, 16, 85, 86. In a few cases, functional studies [85] or the existence of an ND relative (or other patient) carrying the same mutation 12, 15, 16, 86, confirmed that the mutation was pathogenic. Similarly, KCNJ11 mutations can cause young-onset diabetes or gestational diabetes 87, 88. However, the frequency of such cases is low.
Second, a common variant in Kir6.2 (E23K) is associated with a 40% reduction in glucose-stimulated insulin secretion in people with normal glucose tolerance [89], and a slightly increased risk of T2DM [17]. The effects on KATP channel function are somewhat controversial, but most studies have found a (very) small decrease in ATP inhibition 89, 90. Others have suggested that it is a polymorphism in SUR1 (A1369S), linked to Kir6.2-E23K, that accounts for the lower ATP sensitivity [91]. Whichever subunit is involved, the crucial question is whether the reduction in ATP sensitivity is sufficient to increase diabetes risk. This is not easy to address, given that the increased disease risk only achieves significance in population studies comprising thousands of subjects [17]. It is clearly not possible to perform functional experiments on this scale.
However, some insight into the puzzle is offered by a recessive Kir6.2 mutation (G324R) that causes TNDM. The difference in ATP sensitivity between heterozygous and homozygous Kir6.2-G324R channels is tiny (IC50 of 30 μM versus 38 μM) yet the homozygous proband had TNDM, while his heterozygous parents were unaffected [92]. Furthermore, the ATP sensitivity of heterozygous Kir6.2-G324R channels was less than that of homozygous Kir6.2-E23K channels (IC50 ∼20 μM) 89, 90, 92. This suggests that the heterozygous Kir6.2-G324R parents may have an increased risk of T2DM in later life. It also demonstrates that tiny differences in ATP sensitivity can markedly impact insulin secretion (Box 1).
Box 1. Small Changes in KATP Current Markedly Impact Insulin Secretion.
It is important to appreciate that, because the KATP channel dominates the beta cell membrane potential, its closure causes a large increase in the input resistance of the membrane. Ohm’s law dictates that, when the membrane resistance is high, tiny changes in current will lead to marked changes in membrane potential. One should also bear in mind that, below the threshold for electrical activity, there will be no insulin secretion. Above it, insulin release will be initiated. Thus, even a tiny change in KATP current may suffice to make the difference between electrical silence and action potential firing (depending on the input resistance and the membrane potential). This explains why tiny changes in KATP current (or ATP sensitivity), which can be very hard to measure, can markedly affect insulin secretion.
Alt-text: Box 1
Concluding Remarks and Future Perspectives
ND provides a paradigm for how understanding the pathophysiology of a disease can improve therapy for affected patients. It is an excellent example of pharmacogenomics, illuminating the beneficial effects of tailoring therapy to a patient’s individual genetic make-up. Functional studies have revealed why some mutations produce a more severe clinical phenotype and why some patients respond to drug therapy whereas others do not. Conversely, ND has provided novel insights into the physiological role of the KATP channel. Nevertheless, many questions remain (see Outstanding Questions). We still lack an understanding of precisely how nucleotides and drugs regulate channel activity, how chronic hyperglycemia impairs beta cell mass and/or function, why diabetes is transient is some patients, and what causes the neurological deficits. Answers to these questions will illuminate our understanding not only of neonatal diabetes, but also of its far more common relative, T2DM. They will also provide fresh insight into the role of the KATP channel in tissues other than the beta cell.
Outstanding Questions.
What is the atomic (high-resolution) structure of the KATP channel complex?
How does nucleotide interaction with the NBDs of SUR1 regulate the gating of the Kir6.2 pore?
Why does diabetes remit in TNDM and why does it then relapse?
Why does hyperglycemia decrease beta cell mass and function, and is this fully reversed by good glucose control? For how high and how long must glucose be elevated to cause irreversible beta cell damage?
What causes the neurological complications in iDEND/DEND syndrome and how can these best be managed?
Acknowledgments
The research from our laboratory described here was funded by the Wellcome Trust (grant no. 089795), the European Research Council (ERC, grant no. 332620), and the Royal Society. F.M.A. holds a Royal Society/Wolfson Merit Award and an ERC Advanced Investigatorship.
Glossary
- ATP-sensitive potassium (KATP) channel)
a hetero-octomeric complex of four Kir6.2 and four SUR subunits that functions as a metabolically sensitive potassium channel.
- Developmental delay, epilepsy and neonatal diabetes (DEND) syndrome
caused by activating mutations in KCNJ11 and (more rarely) in ABCC8.
- Intermediate DEND syndrome:
a syndrome, with features similar to DEND syndrome but lacking epilepsy; caused by activating mutations in KCNJ11 and (more rarely) ABCC8.
- Kir6.2
the pore-forming subunit of the KATP channel (encoded by the KCNJ11 gene). ATP binding to this subunit inhibits channel activity.
- Neonatal diabetes (ND)
diabetes that usually presents within the first 6 months of life. It is caused by mutations in several genes, but, in this review, ‘ND’ refers solely to that caused by mutations in KCNJ11 or ABCC8.
- Sulfonylureas
hypoglycemic drugs used to treat T2DM for >60 years and, more recently, ND. They act by binding to the SUR1 subunit of the KATP channel and inducing its closure.
- SUR1
the regulatory subunit of the beta cell and neuronal KATP channel (encoded by the ABCC8 gene); so-called because sulfonylureas bind to this subunit to cause channel closure. Mg2+ nucleotide
- SUR2A
the regulatory subunit of the cardiac and skeletal muscle KATP channel (encoded by the ABCC9 gene).
References
- 1.Nichols C.G. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft F.M., Rorsman P. Electrophysiology of the pancreatic b-cell. Prog. Biophys. Mol. Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 3.Gloyn A.L. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N. Engl. J. Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- 4.Hattersley A.T., Ashcroft F.M. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–2513. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- 5.Babenko A.P. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N. Engl. J. Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- 6.Gribble F.M., Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 7.Pearson E.R. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N. Engl. J. Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- 8.Greeley S.A. Neonatal diabetes mellitus: a model for personalized medicine. Trends Endocrinol. Metab. 2010;21:464–472. doi: 10.1016/j.tem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N. Structure of a pancreatic ATP-sensitive potassium channel. Cell. 2017;168:101–110. doi: 10.1016/j.cell.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Martin G.M. Cryo-EM structure of the ATP?sensitive potassium channel illuminates mechanisms of assembly and gating. Elife. 2017;6:024149. doi: 10.7554/eLife.24149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan S.E. Update of mutations in the genes encoding the pancreatic b-cell KATP channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 2009;30:170–180. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]
- 12.Flanagan S.E. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes. 2007;56:1930–1937. doi: 10.2337/db07-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto Y. Molecular and clinical features of KATP-channel neonatal diabetes mellitus in Japan. Pediatr. Diabetes. 2016 doi: 10.1111/pedi.12447. Published online September 29, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Bowman P. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia. 2012;55:123–127. doi: 10.1007/s00125-011-2319-x. [DOI] [PubMed] [Google Scholar]
- 15.Riveline J.P. Clinical and metabolic features of adult-onset diabetes caused by ABCC8 mutations. Diabetes Care. 2012;35:248–251. doi: 10.2337/dc11-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klupa T. Mutations in the ABCC8 (SUR1 subunit of the KATP channel) gene are associated with a variable clinical phenotype. Clin. Endocrinol. 2009;71:358–362. doi: 10.1111/j.1365-2265.2008.03478.x. [DOI] [PubMed] [Google Scholar]
- 17.Gloyn A.L. Large-scale association studies of variants in genes encoding the pancreatic b-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. 2003;52:568–572. doi: 10.2337/diabetes.52.2.568. [DOI] [PubMed] [Google Scholar]
- 18.Proks P. Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17539–17544. doi: 10.1073/pnas.0404756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellard S. Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am. J. Hum. Genet. 2007;81:375–382. doi: 10.1086/519174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamadi A. Medical and developmental impact of transition from subcutaneous insulin to oral glyburide in a 15-yr-old boy with neonatal diabetes mellitus and intermediate DEND syndrome: extending the age of KCNJ11 mutation testing in neonatal DM. Pediatr. Diabetes. 2010;11:203–207. doi: 10.1111/j.1399-5448.2009.00548.x. [DOI] [PubMed] [Google Scholar]
- 21.Battaglia D. Glyburide ameliorates motor coordination and glucose homeostasis in a child with diabetes associated with the KCNJ11/S225T, del226-232 mutation. Pediatr. Diabetes. 2012;13:656–660. doi: 10.1111/j.1399-5448.2012.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio-Cabezas O. KATP channel mutations in infants with permanent diabetes diagnosed after 6 months of life. Pediatr. Diabetes. 2012;13:322–325. doi: 10.1111/j.1399-5448.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- 23.Shimomura K. The first clinical case of a mutation at residue K185 of Kir6.2 (KCNJ11): a major ATP-binding residue. Diabet. Med. 2010;27:225–229. doi: 10.1111/j.1464-5491.2009.02901.x. [DOI] [PubMed] [Google Scholar]
- 24.Masia R. An ATP-binding mutation (G334D) in KCNJ11 is associated with a sulfonylurea-insensitive form of developmental delay, epilepsy, and neonatal diabetes. Diabetes. 2007;56:328–336. doi: 10.2337/db06-1275. [DOI] [PubMed] [Google Scholar]
- 25.Koster J.C. The G53D mutation in Kir6.2 (KCNJ11) is associated with neonatal diabetes and motor dysfunction in adulthood that is improved with sulfonylurea therapy. J. Clin. Endocrinol. Metab. 2008;93:1054–1061. doi: 10.1210/jc.2007-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortiz D. Two neonatal diabetes mutations on transmembrane helix 15 of SUR1 increase affinity for ATP and ADP at nucleotide binding domain 2. J. Biol. Chem. 2012;287:17985–17995. doi: 10.1074/jbc.M112.349019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babenko A.P., Vaxillaire M. Mechanism of KATP hyperactivity and sulfonylurea tolerance due to a diabetogenic mutation in L0 helix of sulfonylurea receptor 1 (ABCC8) FEBS Lett. 2011;585:3555–3559. doi: 10.1016/j.febslet.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rafiq M. Effective treatment with oral sulfonylureas in patients with diabetes due to sulfonylurea receptor 1 (SUR1) mutations. Diabetes Care. 2008;31:204–209. doi: 10.2337/dc07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiabetesGenes DiabetesGenes.org. http://www.diabetesgenes.org/ (accessed January 2017).
- 30.Shimomura K. A novel mutation causing DEND syndrome: a treatable channelopathy of pancreas and brain. Neurology. 2007;69:1342–1349. doi: 10.1212/01.wnl.0000268488.51776.53. [DOI] [PubMed] [Google Scholar]
- 31.Zung A. Glibenclamide treatment in permanent neonatal diabetes mellitus due to an activating mutation in Kir6.2. J. Clin. Endocrinol. Metab. 2004;89:5504–5507. doi: 10.1210/jc.2004-1241. [DOI] [PubMed] [Google Scholar]
- 32.Verstappen S., Mul D. A ‘picturesque’ case of transition from subcutaneous to oral treatment in neonatal diabetes. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2013-202912. bcr2013202912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thurber B.W. Age at the time of sulfonylurea initiation influences treatment outcomes in KCNJ11-related neonatal diabetes. Diabetologia. 2015;58:1430–1435. doi: 10.1007/s00125-015-3593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumaraguru J. Tooth discoloration in patients with neonatal diabetes after transfer onto glibenclamide: a previously unreported side effect. Diabetes Care. 2009;32:1428–1430. doi: 10.2337/dc09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greeley S.A. Case report: preservation of reduced numbers of insulin-positive cells in sulfonylurea-unresponsive KCNJ11-related diabetes. J. Clin. Endocrinol. Metab. 2016;2016:jc20162826. doi: 10.1210/jc.2016-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busiah K. Human pancreas endocrine cell populations and activating ABCC8 mutations. Horm. Res. Paediatr. 2014;82:59–64. doi: 10.1159/000360004. [DOI] [PubMed] [Google Scholar]
- 37.Brereton M.F. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat. Commun. 2014;5:4639. doi: 10.1038/ncomms5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brereton M.F. Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic b-cells. Nat. Commun. 2016;7:13496. doi: 10.1038/ncomms13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z. Pancreatic b cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 2014;19:872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remedi M.S. Secondary consequences of b cell inexcitability: identification and prevention in a murine model of KATP-induced neonatal diabetes mellitus. Cell Metab. 2009;9:140–151. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gribble F.M. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J. Physiol. 1997;504:35–45. doi: 10.1111/j.1469-7793.1997.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrett-Jolley R., Davies N.W. Kinetic analysis of the inhibitory effect of glibenclamide on KATP channels of mammalian skeletal muscle. J. Membr. Biol. 1997;155:257–262. doi: 10.1007/s002329900178. [DOI] [PubMed] [Google Scholar]
- 43.Proks P. Sulfonylureas suppress the stimulatory action of Mg-nucleotides on Kir6.2/SUR1 but not Kir6.2/SUR2A KATP channels: a mechanistic study. J. Gen. Physiol. 2014;144:469–486. doi: 10.1085/jgp.201411222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proks P. Activation of the KATP channel by Mg-nucleotide interaction with SUR1. J. Gen. Physiol. 2010;136:389–405. doi: 10.1085/jgp.201010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drain P. ATP and sulfonylurea linkage in the KATP channel solves a diabetes puzzler. Diabetes. 2013;62:3666–3668. doi: 10.2337/db13-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Proks P. Molecular mechanism of sulphonylurea block of KATP channels carrying mutations that impair ATP inhibition and cause neonatal diabetes. Diabetes. 2013;62:3909–3919. doi: 10.2337/db13-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C.L. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science. 2009;325:607–610. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi H. Role of Epac2A/Rap1 signaling in interplay between incretin and sulfonylurea in insulin secretion. Diabetes. 2015;64:1262–1272. doi: 10.2337/db14-0576. [DOI] [PubMed] [Google Scholar]
- 49.Renstrom E. Sulfonylurea-mediated stimulation of insulin exocytosis via an ATP-sensitive K+ channel-independent action. Diabetes. 2002;51(Suppl. 1):S33–S36. doi: 10.2337/diabetes.51.2007.s33. [DOI] [PubMed] [Google Scholar]
- 50.Tarasov A.I. ATP sensitivity of the ATP-sensitive K+ channel in intact and permeabilized pancreatic b-cells. Diabetes. 2006;55:2446–2454. doi: 10.2337/db06-0360. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q. Cell coupling in mouse pancreatic b-cells measured in intact islets of Langerhans. Philos. Trans. A Math. Phys. Eng. Sci. 2008;366:3503–3523. doi: 10.1098/rsta.2008.0110. [DOI] [PubMed] [Google Scholar]
- 52.Henquin J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 53.Iafusco D. No beta cell desensitisation after a median of 68 months on glibenclamide therapy in patients with KCNJ11-associated permanent neonatal diabetes. Diabetologia. 2011;54:2736–2738. doi: 10.1007/s00125-011-2273-7. [DOI] [PubMed] [Google Scholar]
- 54.Myngheer N. Fetal macrosomia and neonatal hyperinsulinemic hypoglycemia associated with transplacental transfer of sulfonylurea in a mother with KCNJ11-related neonatal diabetes. Diabetes Care. 2014;37:3333–3335. doi: 10.2337/dc14-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klupa T. The first case report of sulfonylurea use in a woman with permanent neonatal diabetes mellitus due to KCNJ11 mutation during a high-risk pregnancy. J. Clin. Endocrinol. Metab. 2010;95:3599–3604. doi: 10.1210/jc.2010-0096. [DOI] [PubMed] [Google Scholar]
- 56.Gaal Z. Sulfonylurea use during entire pregnancy in diabetes because of KCNJ11 mutation: a report of two cases. Diabetes Care. 2012;35:e40. doi: 10.2337/dc12-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Franco E. Analysis of cell-free fetal DNA for non-invasive prenatal diagnosis in a family with neonatal diabetes. Diabet. Med. 2016 doi: 10.1111/dme.13180. Published online June 29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sumnik Z. Sulphonylurea treatment does not improve psychomotor development in children with KCNJ11 mutations causing permanent neonatal diabetes mellitus accompanied by developmental delay and epilepsy (DEND syndrome) Diabet. Med. 2007;24:1176–1178. doi: 10.1111/j.1464-5491.2007.02228.x. [DOI] [PubMed] [Google Scholar]
- 59.Babiker T. Successful transfer to sulfonylureas in KCNJ11 neonatal diabetes is determined by the mutation and duration of diabetes. Diabetologia. 2016;59:1162–1166. doi: 10.1007/s00125-016-3921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelsey M.M., Zeitler P.S. Insulin resistance of puberty. Curr. Diab. Rep. 2016;16:64. doi: 10.1007/s11892-016-0751-5. [DOI] [PubMed] [Google Scholar]
- 61.Goran M.I., Gower B.A. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 62.Remedi M.S. Acute sulfonylurea therapy at disease onset can cause permanent remission of KATP-induced diabetes. Diabetes. 2011;60:2515–2522. doi: 10.2337/db11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marshall B.A. Remission of severe neonatal diabetes with very early sulfonylurea treatment. Diabetes Care. 2015;38:e38–e39. doi: 10.2337/dc14-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wambach J.A. Successful sulfonylurea treatment of an insulin-naive neonate with diabetes mellitus due to a KCNJ11 mutation. Pediatr. Diabetes. 2010;11:286–288. doi: 10.1111/j.1399-5448.2009.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beltrand J. Sulfonylurea therapy benefits neurological and psychomotor functions in patients with neonatal diabetes owing to potassium channel mutations. Diabetes Care. 2015;38:2033–2041. doi: 10.2337/dc15-0837. [DOI] [PubMed] [Google Scholar]
- 66.Bowman P. Psychiatric morbidity in children with KCNJ11 neonatal diabetes. Diabet. Med. 2016;33:1387–1391. doi: 10.1111/dme.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McTaggart J.S. Gain-of-function mutations in the KATP channel (KCNJ11) impair coordinated hand–eye tracking. PLoS One. 2013;8:e62646. doi: 10.1371/journal.pone.0062646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Proks P. Mechanism of action of a sulphonylurea receptor SUR1 mutation (F132L) that causes DEND syndrome. Hum. Mol. Genet. 2007;16:2011–2019. doi: 10.1093/hmg/ddm149. [DOI] [PubMed] [Google Scholar]
- 69.Shah R.P. Visuomotor performance in KCNJ11-related neonatal diabetes is impaired in children with DEND-associated mutations and may be improved by early treatment with sulfonylureas. Diabetes Care. 2012;35:2086–2088. doi: 10.2337/dc11-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slingerland A.S. Sulphonylurea therapy improves cognition in a patient with the V59M KCNJ11 mutation. Diabet. Med. 2008;25:277–281. doi: 10.1111/j.1464-5491.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- 71.Clark R. Mice expressing a human KATP channel mutation have altered channel ATP sensitivity but no cardiac abnormalities. Diabetologia. 2012;55:1195–1204. doi: 10.1007/s00125-011-2428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clark R.H. Muscle dysfunction caused by a KATP channel mutation in neonatal diabetes is neuronal in origin. Science. 2010;329:458–461. doi: 10.1126/science.1186146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fendler W. Switching to sulphonylureas in children with iDEND syndrome caused by KCNJ11 mutations results in improved cerebellar perfusion. Diabetes Care. 2013;36:2311–2316. doi: 10.2337/dc12-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lahmann C. A mutation causing increased KATP channel activity leads to reduced anxiety in mice. Physiol. Behav. 2014;129:79–84. doi: 10.1016/j.physbeh.2014.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lahmann C. Systemic administration of glibenclamide fails to achieve therapeutic levels in the brain and cerebrospinal fluid of rodents. PLoS One. 2015;10:e0134476. doi: 10.1371/journal.pone.0134476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen P.C. Carbamazepine as a novel small molecule corrector of trafficking-impaired ATP-sensitive potassium channels identified in congenital hyperinsulinism. J. Biol. Chem. 2013;288:20942–20954. doi: 10.1074/jbc.M113.470948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaxillaire M. New ABCC8 mutations in relapsing neonatal diabetes and clinical features. Diabetes. 2007;56:1737–1741. doi: 10.2337/db06-1540. [DOI] [PubMed] [Google Scholar]
- 78.Batra C.M. Transient neonatal diabetes due to activating mutation in the ABCC8 gene encoding SUR1. Indian J. Pediatr. 2009;76:1169–1172. doi: 10.1007/s12098-009-0222-y. [DOI] [PubMed] [Google Scholar]
- 79.Geng X. The insulin secretory granule is the major site of K(ATP) channels of the endocrine pancreas. Diabetes. 2003;52:767–776. doi: 10.2337/diabetes.52.3.767. [DOI] [PubMed] [Google Scholar]
- 80.Shibasaki T. Integration of ATP, cAMP, and Ca2+ signals in insulin granule exocytosis. Diabetes. 2004;53(Suppl. 3):S59–S62. doi: 10.2337/diabetes.53.suppl_3.s59. [DOI] [PubMed] [Google Scholar]
- 81.Li C.G. Sensitivity of KATP channels to cellular metabolic disorders and the underlying structural basis. Acta Pharmacol. Sin. 2016;37:134–142. doi: 10.1038/aps.2015.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuoka T. C-terminal tails of sulfonylurea receptors control ADP-induced activation and diazoxide modulation of ATP-sensitive K+ channels. Circ. Res. 2000;87:873–880. doi: 10.1161/01.res.87.10.873. [DOI] [PubMed] [Google Scholar]
- 83.Flagg T.P. Differential structure of atrial and ventricular KATP: atrial KATP channels require SUR1. Circ. Res. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glukhov A.V. Functional roles of KATP channel subunits in metabolic inhibition. J. Mol. Cell. Cardiol. 2013;62:90–98. doi: 10.1016/j.yjmcc.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tarasov A.I. A rare mutation in ABCC8/SUR1 leading to altered ATP-sensitive K+ channel activity and b-cell glucose sensing is associated with type 2 diabetes in adults. Diabetes. 2008;57:1595–1604. doi: 10.2337/db07-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klee P. A novel ABCC8 mutation illustrates the variability of the diabetes phenotypes associated with a single mutation. Diabetes Metab. 2012;38:179–182. doi: 10.1016/j.diabet.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 87.D’Amato E. Variable phenotypic spectrum of diabetes mellitus in a family carrying a novel KCNJ11 gene mutation. Diabet. Med. 2008;25:651–656. doi: 10.1111/j.1464-5491.2008.02443.x. [DOI] [PubMed] [Google Scholar]
- 88.Bonnefond A. Whole-exome sequencing and high throughput genotyping identified KCNJ11 as the thirteenth MODY gene. PLoS. 2012;7:e37423. doi: 10.1371/journal.pone.0037423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Villareal D.T. Kir6.2 variant E23K increases ATP-sensitive K+ channel activity and is associated with impaired insulin release and enhanced insulin sensitivity in adults with normal glucose tolerance. Diabetes. 2009;58:1869–1878. doi: 10.2337/db09-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schwanstecher C. Kir6.2 polymorphism predisposes to type 2 diabetes by inducing overactivity of pancreatic β-cell ATP-sensitive K+ channels. Diabetes. 2002;51:875–879. doi: 10.2337/diabetes.51.3.875. [DOI] [PubMed] [Google Scholar]
- 91.Hamming K.S. Coexpression of the type 2 diabetes susceptibility gene variants KCNJ11 E23K and ABCC8 S1369A alter the ATP and sulfonylurea sensitivities of the ATP-sensitive K+ channel. Diabetes. 2009;58:2419–2424. doi: 10.2337/db09-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vedovato N. Neonatal diabetes caused by a homozygous KCNJ11 mutation demonstrates that tiny changes in ATP sensitivity markedly affect diabetes risk. Diabetologia. 2016;59:1430–1436. doi: 10.1007/s00125-016-3964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]