Abstract
The periodontal ligament (PDL) is the connective tissue between tooth root and alveolar bone containing mesenchymal stem cells (MSC). It has been suggested that human periodontal ligament stem cells (hPDLSCs) differentiate into osteo/cementoblast and ligament progenitor cells. The periodontitis is a representative oral disease where the PDL tissue is collapsed, and regeneration of this tissue is important in periodontitis therapy. Fibroblast growth factor-2 (FGF-2) stimulates proliferation and differentiation of fibroblastic MSCs into various cell lineages. We evaluated the dose efficacy of FGF-2 for cytodifferentiation of hPDLSCs into ligament progenitor. The fibrous morphology was highly stimulated even at low FGF-2 concentrations, and the expression of teno/ligamentogenic markers, scleraxis and tenomodulin in hPDLSCs increased in a dose dependent manner of FGF-2. In contrast, expression of the osteo/cementogenic markers decreased, suggesting that FGF-2 might induce and maintain the ligamentogenic potential of hPDLSCs. Although the stimulation of tenocytic maturation by TGF-β1 was diminished by FGF-2, the inhibition of the expression of early ligamentogenic marker by TGF-β1 was redeemed by FGF-2 treatment. The stimulating effect of BMPs on osteo/cementogenesis was apparently suppressed by FGF-2. These results indicate that FGF-2 predominantly differentiates the hPDLSCs into teno/ligamentogenesis, and has an antagonistic effect on the hard tissue differentiation induced by BMP-2 and BMP-4.
Keywords: bone morphogenic protein (BMP), human fibroblast growth factor 2 (hFGF-2), ligamentogenesis, osteogenesis, periodontal ligament stem cells (PDLSCs), TGF-β1
INTRODUCTION
The PDL is a specialized soft connective tissue, which connects the tooth root and the alveolar bone, and mechanically supports teeth (Beertsen et al., 1997). Once the PDL tissue is destroyed, regeneration of damaged PDL is very limited, since little is known about how the ligament tissue develops during tooth eruption and is maintained after that. For successful regeneration of the lost PDL tissue, it is important to secure cells containing multipotential stemness to differentiate into PDL. It has been revealed that the PDL tissue possesses MSCs which contain the multi-lineage differentiation potential. These cells can differentiate into osteoblasts, cementoblasts, and PDL fibroblasts, and are a promising source for regeneration of periodontium (Maeda et al., 2011). The MSC markers such as CD44, CD73, CD90, CD146, CD166, and STRO-1, are highly expressed in these cells, whereas the population of hematopoietic marker (CD34)-positive cells is extremely small (Choi et al., 2015; Seo et al., 2004). Although PDL stem cells (PDLSCs) are thought to play key roles for not only bone remodeling but also wound healing and regeneration of the tissues (Lekic and McCulloch, 1996), the regulation of differentiation mechanism are not fully understood yet and remain unclear. It is important for stimulation of the postnatal stem cells within the PDL tissue to differentiate into osteoblast, cementoblast, and periodontal ligament progenitor in clinically regenerating damaged periodontal tissue. The treatment with growth factors and morphogens can be efficient for stimulating these cell types (Kao et al., 2009). Rather than the treatment with a single cytokine, the combinatorial treatment with multiple factors induces proper stimulation of specific progenitor cells for development and regeneration of functional periodontal tissues. During the stimulation process, cytokines influence proliferation and differentiation of stem/progenitor cells, and control the gene expression required for damage repair and regeneration of PDL tissue (Terranova, 1993). FGF-2 is a member of the fibroblast growth factor family, and mediates signal transduction for stimulation of growth through mitogen activated protein kinase pathway (Yu et al., 2007). It maintains the differentiation potential, such as chondrogenic and adipogenic differentiation of mesenchymal stem cells (Neubauer et al., 2004; Solchaga et al., 2005). However, in case of mouse mesenchymal stem cells, FGF-2 reversibly inhibits multilineage differentiation through the suppression of ERK-1 and -2 activation and the upregulation of Twist2 and Sprey4, which are negative regulators of cell differentiation (Lai et al., 2011). In periodontal ligament cells, FGF-2 is involved in the process of wound healing and periodontal regeneration, such as cell migration and regulation of extracellular matrix production, in a cooperative manner with vascular endothelial growth factors (Shimabukuro et al., 2011; Yanagita et al., 2014). Based on these reports, FGF-2 exhibits positive and negative effects on growth and differentiation of mesenchymal stem cells as well as periodontal ligament stem cells, suggesting that still little known about the clear effect of its differentiation potential. Transforming growth factor-beta-1 (TGF-β1) is an abundant fibrogenic mitogen, and is a potent stimulator of tissue regeneration (Shi and Massague, 2003). It has been known that TGF-β1 improves bone regeneration on guided tissue regeneration in animal models (Wikesjo et al., 1998). Conversely, in a recent report, TGF-β1 was shown to inhibit osteogenic and cementogenic differentiation of the primary cell line of PDL by competing with the effects of BMP-2 (Kawahara et al., 2015). In fact, TGF-β1 has shown a differential effect on chondrogenesis, osteogenesis, and fibrogenesis, when comparing in vitro and in vivo experiments, implicating that the exact function of this factor in periodontal differentiation remains ambiguous (de Gorter et al., 2011; Lorda-Diez et al., 2009). Bone morphogenic proteins (BMPs), originally identified within osteoinductive extracts derived from bone (Urist, 1965), are involved in the development of hard tissues (both bones and teeth), as well as soft tissue types such as cartilage. BMP-2 and BMP-4 function in growth control in the developing vertebrate limb (Francis et al., 1994), and appear to mediate mesenchymal epithelial interactions during odontogenesis (Vainio et al., 1993). In this study, we examined an efficient cytodifferentiation of human PDLSCs into periodontal ligament progenitor by concerted application of FGF-2, TGF-β1, and BMP-2/-4, which contain differential or biphasic potentials in proliferation and differentiation of various tissues.
MATERIALS AND METHODS
Cell culture
Periodontal ligament tissues were collected from third molar teeth extracted from dental surgery patients of 20–29 years old under guidelines approved by the IRB of the Dankook Dental Hospital (H-1506/006/001). Healthy periodontal tissue was separated from the surface of the center of the tooth root with a surgical scalpel. The tissues were digested by 3 mg/ml collagenase (Sigma) and 4 mg/ml Dispase (Sigma) at 37°C for 1 h with shaking. Cell suspension was incubated in α-MEM (Hyclone) containing 20% FBS (Hyclone) and antibiotics (Lonza) at 37°C in 5% CO2. All experiments were carried out with cells from the third passages.
Cytokine treatment and Osteogenic induction
To assess the effects of cytokines on cytodifferentiation, hPDLSCs were cultured in α-MEM containing 20% FBS at first. When cell density became 40–50% confluence, media was changed with α-MEM containing 2% FBS. After preincubation for 2 days, cells were cultured in α-MEM containing 5% FBS and various concentration of FGF-2 (0, 3, 5, 10, 50, 100, 200, and 500 ng/ml) for the indicated time. For cotreatment of FGF-2 and TGF-β1, TGF-β1 was pre-treated for 2 days, followed by FGF-2 treatment for 5 days. For cotreatment of FGF-2 and BMPs, FGF-2 and BMPs were treated simultaneously for the indicated time after pretreatment with FGF-2 for 2 days. For osteogenic induction, cells were incubated in osteogenic additive medium containing 5 mM β-glycerophosphate, 500 nM dexamethasone, and 100 μM ascorbic acid with cytokines for 14 days with changing of medium every 2 or 3 days.
Western blot analysis
Cell extracts were separated on SDS-PAGE, transferred to a PVDF membrane (Millipore), and then probed with the anti-vimentin antibody (Santa Cruz), followed by treatment with the secondary antibody conjugated with HRP (GE Healthcare). The protein signal was visualized by using ECL-Detection Kit (GE Healthcare), and exposed under X-ray film.
Quantitative real-time PCR
Total RNA was isolated from the cells using the Easy-Spin™ Kit (iNtRON) in accordance with the manufacturer’s protocol. The cDNA was synthesized by using a ReverTra Ace qPCR RT Mix (TOYOBO). The qRT-PCR was performed by using iTaq™ Universal SYBR™ Green Supermix (Bio-Rad). Used Primers are listed in Table 1. During PCR, a dissociation curve was constructed in the range of 65°C to 95°C, and the cycling parameters of qPCR were followed; 1 cycle for 1 min at 95°C, 40 cycles for 15 s at 95°C, and 1 min at 60°C. The GAPDH was used as an internal control to normalize the variability in target gene expression. Statistical analyses on three readings were carried out using Student’s t-test, and p values of less than 0.05 were considered significant.
Table 1.
The list of primers and sequences for the quantitative RT-PCR.
| Target gene | Primer sequences |
|---|---|
| Collagen type-I | Forward-5′-GGAGGAGAGTCAGGAAGG-3′ |
| Reverse-5′-TCAGCAACACAGTTACACAA-3′ | |
| Periostin | Forward-5′-GGGACAACTTGGATTCTGAT-3′ |
| Reverse-5′-CCATTTGTTGCAATCTGGTT-3′ | |
| PDLs17 | Forward-5′-ATGGAACTATTATTATTAGAAG-3′ |
| Reverse-5′-ACCTTTCAAAACATGGAGTAA-3′ | |
| Runx2 | Forward-5′-GTCTCACTGCCTCTCACT-3′ |
| Reverse-5′-TACACACATCTCCTCCCT TC-3′ | |
| Scleraxis | Forward-5′-AGAAAGTTGAGCAAGGACC-3′ |
| Reverse-5′-CTGTCTGTACGTCCGTCT-3′ | |
| Osterix | Forward-5′-TTGACATGTACCCCTTTCTG-3′ |
| Reverse-5′-CAATACCCCTGATGAAGAGG-3′ | |
| Tenomodulin | Forward-5′-GATGGCTCTTTGGAAGATGACGAT-3′ |
| Reverse-5′-GTCTTCACATCAATGCTCTGCCAA-3′ | |
| BSP | Forward-5′-TACCGAGCCTATGAAGATGA-3′ |
| Reverse-5′-CTTCCTGAGTTGAACTTCGA-3′ | |
| CP23 | Forward-5′-GATGGCAGGATGACACTAAT-3′ |
| Reverse-5′-CAGCGTTCTGTTTTCTCTTC-3′ | |
| Sca-1 | Forward-5′- CGAAATTCAAAGGATGGCTC -3′ |
| Reverse-5′- TGAAAAGTAGCGTCAAAGGA -3′ | |
| Sox9 | Forward-5′- TCAGGCTTTGCGATTTAAGGA -3′ |
| Reverse-5′- AGTGAACAAGCAAAGGCAGGA -3′ | |
| GAPDH | Forward-5′-GTATGACAACAGCCTCAAGAT-3′ |
| Reverse-5′-CCTTCCACGATACCAAAGTT-3′ |
Alizarin red staining
For quantification of mineralization, cells were fixed by 70% cold ethanol for 30 min and dried completely. Then, cells were treated with 2% Alizarin red S (pH4.5) for 30 min in dark room at room temperature. After washing with distilled water, alizarin red stained on the mineralized cells was dissolved in 10% acetic acid for 30 min with shaking, followed by heating at 85°C for 10 min with mineral oil. After neutralization by adding 10% ammonium hydroxide, the staining intensity was analyzed at 405 nm.
RESULTS
FGF-2 treatment induces a fibroblastic phenotype in hPDLSCs
hPDLSCs have a potential for teno/ligamentogenesis as well as osteogenesis/cementogenesis. Because cells differentiated into ligament are histologically shown as fibroblasts, we initially investigated whether FGF-2 stimulates cytodifferentiation of hPDLSCs into the ligament progenitors. Previously, we had reported a dose dependent effect of the purified human recombinant FGF-2 on cell growth in stem cells originating from periodontal ligament tissues of both adult and baby supernumerary teeth (Lee et al., 2015); about 40 ng/ml of FGF-2 was the optimal concentration for maximal proliferation activity. Actively growing hPDLSCs were an asynchronous and wide-spreading phenotype (Figs. 1A and 1B). When hPDLSCs were treated with FGF-2 at various concentrations from 3 ng/ml to 500 ng/ml, cellular phenotypes dramatically changed to fibrous form in low density cell cultures (Figs. 1A,b–1A,h). In confluent cultures, the cell phenotypes changed to fusiform, which aligned tightly and paralleled under the treatment with various concentration of FGF-2 (Figs. 1B,b–1B,h). Expression of the PDL fibroblast-specific marker, PDLs17, increased than in control, when treated with 3 ng/ml of FGF-2 (Fig. 1C). Expression level of PDLs17 was maintained in the increased status in cells treated with FGF-2, although slightly decreased in cells treated with high concentrations (200–500 ng/ml) of FGF-2 (Fig. 1C). Vimentins is type-III intermediate filaments found in various non-epithelial cells, and is highly expressed in fibroblastic cell lines (Olson and Capetanaki, 1989). When FGF-2 was treated during cultivation of hPDLSCs, the total amount of vimentin proteins of 43–57 kDa increased in comparison with those in actively growing cells without treatment of FGF-2 (Fig. 1D), suggested that FGF-2 treatment stimulates and maintains the fibroblastic feature in hPDLSCs.
Fig. 1.
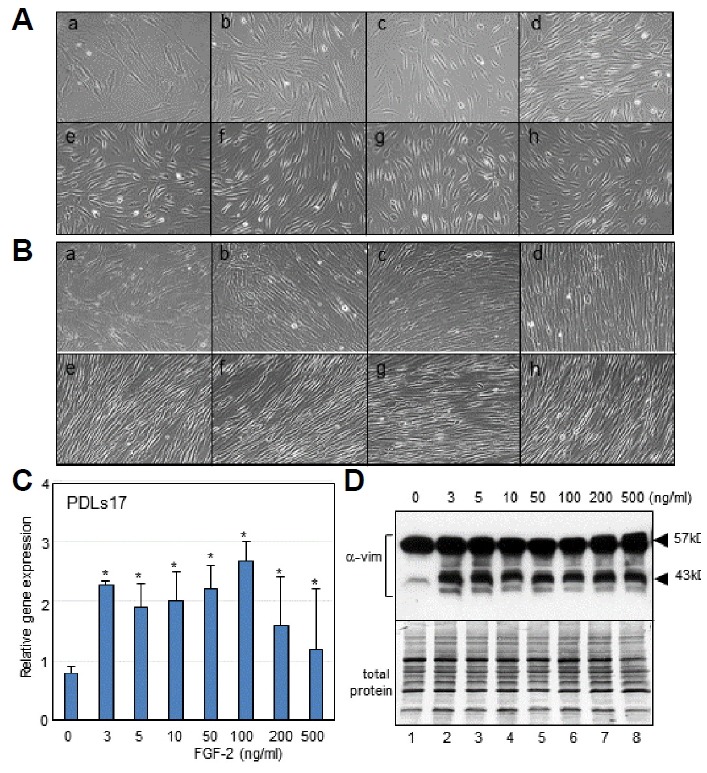
FGF-2 treatment induces and maintains the fibroblastic feature in hPDLSCs.
(A, B) Cellular phenotypes of hPDLSCs treated with various concentration of FGF-2 in low density of cell culture (A) and in confluent culture (B). (a), no treatment; (b), 3 ng/ml; (c), 5 ng/ml; (d), 10 ng/ml; (e), 50 ng/ml; (f), 100 ng/ml; (g), 200 ng/ml; (h), 500 ng/ml. (C) Evaluation of mRNA expression of PDLs17 by qRT-PCR. Significant differences from statistical analysis were indicated as *P < 0.01 versus 0 ng/ml. (D) Evaluation of vimentin expression by western analysis. Endogenous vimentin was detected as multiple bands on SDS-PAGE of 43–57 kDa (α-vim). Used protein amount was indicated in lower panel (total protein).
Differential effects of FGF-2 in cytodifferentiation of hPDLSCs
Although periodontal ligament derived stem cells can differentiate into ligament progenitor and osteo/cementoblast, the induction mechanism of each differentiation remains unclear. Cytokines and growth factors play important roles in the regulation of specific cytodifferentiation. To evaluate the dose dependent effect of FGF-2 on expression of teno/ligamentogenic markers, hPDLSCs were subjected to various concentrations of FGF-2. Increasing concentrations of FGF-2 in the culture medium stimulated the gene expression of scleraxis, an early ligament progenitor marker, which almost doubled when exposed to 100 ng/ml or more of FGF-2 (Fig. 2A,a). Although there are serious deviations in gene expression for each culture from tissue samples, the expression of tenomodulin, one of the late tenocyte markers was increased according to the increment of concentration of FGF-2 (Fig. 2A,b). Conversely, the expression of osteoblast markers showed a different aspect as compared to the teno/ligamentogenic markers. The gene expression of three early osteoblast factors (osterix, runx2 and periostin) and a bone marker (bone sialoprotein, BSP) decreased with increasing concentrations of FGF-2 (Figs. 2B,a–2B,e). These data suggested that FGF-2 stimulates cytodifferentiation into ligamento/tenogenic progenitor at relatively higher concentration of more than 50 ng/ml. Teno/ligamentocyte have been known to arise from scleraxis+/sox9+ progenitors, and sox9 and scleraxis are essentially increased during ligamentogenic differentiation (Sugimoto et al., 2013). Indeed, sox9 expression was slightly increased by concentrations of FGF-2 (Fig. 2C,a), suggested that FGF-2 treatment might induce and maintain ligamentogenetic potential of hPDLSCs. Based on these data, although hPDLSCs contain the teno/ligamentogenic potential as their own, this potential could not be consistently maintained without stimulation by cytokines. Treatment with 50–200 ng/ml of FGF-2 on hPDLSCs consistently stimulate ligamentogenic cytodifferentiation, and induce the expression of early teno/ligament progenitor markers. Stem cells antigen-1 (Sca-1) is the common biological marker, which has been used to identify hematopoietic stem cells along with other markers (Bradfute et al., 2005). As expected, Sca-1 expression decreased in FGF-2 treatment (Fig. 2C,b).
Fig. 2.
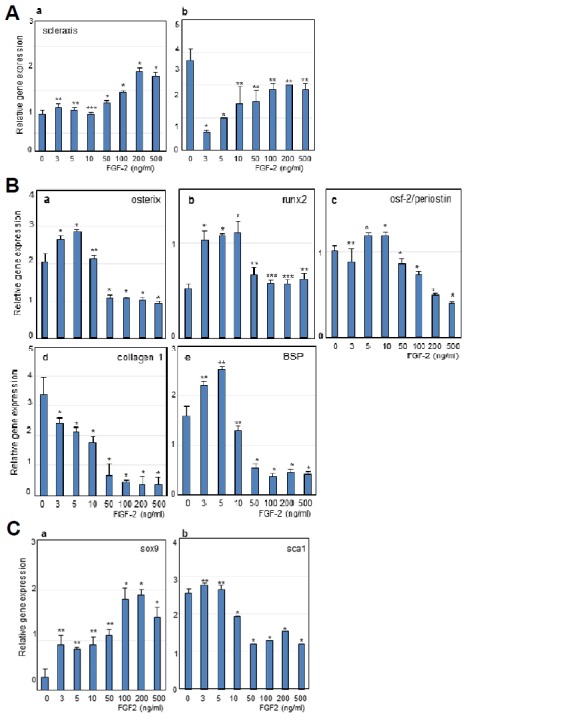
Relative mRNA expressions of representative markers involved in differentiation of hPDLSCs treated with FGF-2.
(A) Expression of early and late teno/ligamentogenic markers, scleraxis (a) and tenomodulin (b). (B) Expression of early osteoblast factors and bone markers. a, osterix; b, runx2; c, osteoblast factor-2; d, collagen type-1; e, BSP. (C) Expression of chondrogenic marker, Sox9 (a) and hematopoietic stem cell marker, Sca-1 (b). Significant differences from statistical analysis were indicated as *P < 0.01 versus 0 ng/ml, **P < 0.05 versus 0 ng/ml, and ***P < 0.1 versus 0 ng/ml.
FGF-2 redeems the inhibitory effect of TGF-β1 on the gene expression of scleraxis, the early teno/ligamentogenic marker
TGF-β1 is one of the cytokines known to stimulate tenocytic differentiation (Shi and Massague, 2003). In immature PDL cells, TGF-β1 contributes to the differentiation into mature tenocyte (Fujii et al., 2010). Indeed, the expression of mature tenocyte marker, tenomodulin was highly increased by treatment with TGF-β1 than those in the untreated hPDLSCs (Fig. 3, bars 2nd in a), whereas the expression of the early ligamentogenic marker, scleraxis was dramatically decreased by treatment with TGF-β1 (Fig. 3, bar 2nd in b). To investigate the co-effect of FGF-2 and TGF-β1, FGF-2 was added after 2days of TGF-β1 pre-treatment. The expression of mature tenocyte markers stimulated by TGF-β1 treatment was down-regulated by the treatment with FGF-2 (Fig. 3, bars 3rd–4th in a). Unlike mature tenocyte markers, the decrease of scleraxis expression was redeemed by treatment with FGF-2 (Fig. 3, bars 3rd–4th in b). These data indicated that FGF-2 stimulates the initial ligamentogenic cytodifferentiaton of hPDLSCs, and might have a dominant effect on the TGF-β1 function in late tenogenic differentiation.
Fig. 3.
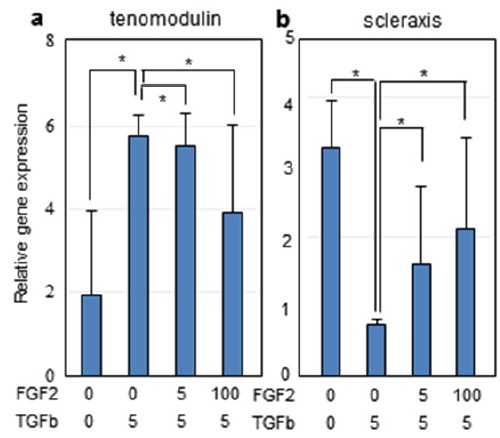
Relative mRNA expressions of representative markers involved in teno/ligamentogenic differentiation of hPDLSCs co-treated with FGF-2 and TGF-β1.
Cells were treated with TGF-β1 (5 ng/ml) for 2 days, followed by treatment with FGF-2 (5 and 100ng/ml) for 5 days. (a), tenomodulin; (b), scleraxis. Significant differences from statistical analysis were indicated as *P < 0.01. **P < 0.05, and ***P < 0.1.
FGF-2 has the antagonistic effect on osteo/cementogenic differentiation stimulated by BMPs in hPDLSCs
To investigate the osteo/cementogenic differentiation of hPDLSCs, FGF-2 and BMP-2/-4 were treated simultaneously for 7 days. When hPDLSCs were exposed to BMPs, the expressions of tenomodulin were decreased in comparison to those in cells treated with FGF-2 (Fig. 4A,a). Scleraxis expression was slightly increased by co-treatment with FGF-2 and BMP in comparison to cells treated with FGF-2 alone (Fig. 4A,b); however, variations were seen for each experiment. As expected, osteogenic and cementogenic markers were highly increased by treatment with BMPs. Indeed, the expression of the osteoblastic markers such as BSP, runx-2, and osterix dramatically increased in cells treated with BMP-2 or BMP-4 (Fig. 4B, bars 2nd–3rd in a-c). However, cotreatment with FGF-2 and BMPs in hPDLSCs inhibited the expression of these genes (Fig. 4B, bars 5th–6th in a–c). Similarly, the expression of cementoblast marker CP23 was greatly increased in cells treated with BMPs (Fig. 4B, 2nd–3rd in d), and this inducible effect of BMPs was deflected by cotreatment with FGF-2 (Fig. 4B, bars 5th–6th in d). Stimulation of osteoblast markers (such as BSP and Runx2) by BMP-2 was more effective than BMP-4, whereas BMP-4 was more effective in expression of cementoblast marker CP23 (Fig. 4B, bar 3rd in d). These data indicated that osteo/cementogenic potential might be induced by treatment with BMP-2 or BMP-4 exclusively, but this inducible effect was dramatically diminished by co-treatment with FGF-2.
Fig. 4.
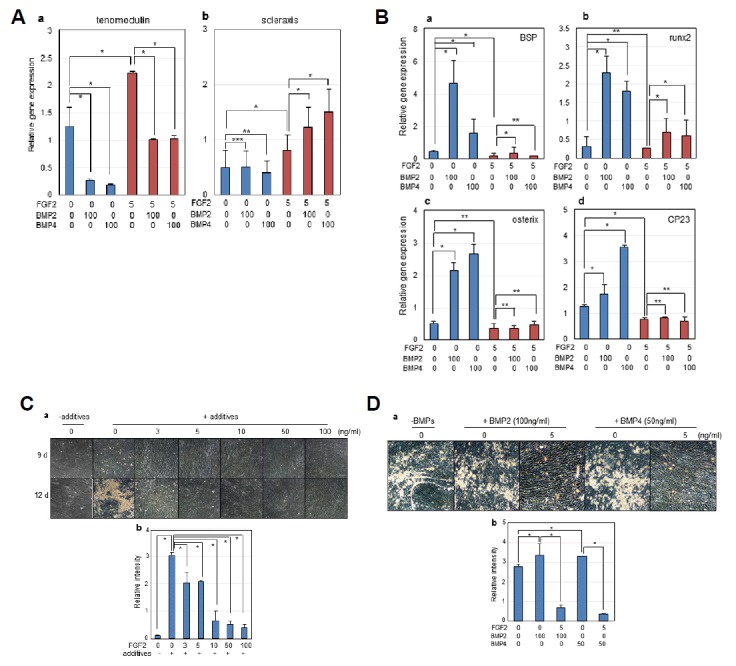
Inhibitory effect of FGF-2 on osteo/cementogenic cytodifferentiation and mineralization in hPDLSCs.
Cells were treated with FGF-2 (5 ng/ml) and BMP-2 or BMP-4 (100 ng/ml each) in α-MEM medium containing 5% FBS for 7 days. (A) Relative mRNA expressions of teno/ligamentogenic markers. (a), tenomodulin; (b), scleraxis. (B) Relative mRNA expressions of osteo/cementogenic markers. (a), BSP; (b), runx2; (c), osterix; (d), CP23. For mineralization of PDLSCs, Cells were incubated in α-MEM medium containing 5% FBS, osteogenic additives, and cytokines for the indicated time. (C) Effects of various FGF-2 concentrations on mineralization. 1, 0 ng/ml of FGF-2 & without additives; 2, 0 ng/ml of FGF-2 & with additives; 3, 3 ng/ml of FGF-2 & with additives; 4, 5 ng/ml of FGF-2 & with additives; 5, 10 ng/ml of FGF-2 & with additives; 6, 50 ng/ml of FGF-2 & with additives; 7, 100 ng/ml of FGF-2 & with additives. (D) Effects of FGF-2 on mineralization stimulated by BMP-2 or BMP-4. 1, no cytokines; 2, 100 ng/ml of BMP-2; 3, 5 ng/ml of FGF-2 & 100 ng/ml of BMP-2; 4, 50 ng/ml of BMP-4; 5, 5 ng/ml of FGF-2 & 50 ng/ml of BMP-4. After mineralization induction for the indicated time, the mineral granules accumulated by mineralization were detected by microscope (a in C&D) and then mineralization amounts were quantified by alizarin red staining (b in C&D). Significant differences from statistical analysis were indicated as *P < 0.01, **P < 0.05, and ***P < 0.1.
Mineralization was totally inhibited by FGF-2 treatment in hPDLSCs
To investigate the antagonistic effect of FGF-2 and BMPs in mineralization, hPDLSCs were cultured in medium containing osteogenic additives and cytokines for 12 days. Mineralization was analyzed both microscopically and by alizarin red staining. Without osteogenic additives, no mineral granules was detected on the surface of hPDLSCs (Fig. 4C, panels 1 in a and bar 1st in b), whereas a great quantity of mineral granules were detected on cells treated with additives (Fig. 4C, panels 2 in a and bar 2nd in b). Interestingly, this mineralization dramatically decreased and was totally inhibited by treatment with FGF-2 (Fig. 4C, panels 3–7 in a and bars 3rd–7th in b). In addition, although BMP-2 and BMP-4 stimulated mineralization in hPDLSCs under the addition of osteogenic additives (Fig. 4D, panels 2 & 4 in a and bars 2nd & 4th in b), this induction effect was totally diminished by treatment with FGF-2 (Fig. 4D, panels 3 & 5 in a and bars 3rd & 5th in b). These data suggested that FGF-2 inhibits the osteogenic differentiation in hPDLSCs and is a dominant antagonist of BMPs, which stimulate osteo/cementgenesis and mineralization.
DISCUSSION
PDL-derived cells contain stem cell population, originating from neural crest-derived ectomesenchymal cells. Human PDL-derived cells contain the undifferentiated phenotypes and heterogeneous characters, and have a potential to express both osteo/cementogenesis-related genes and teno/ligamentogenesis-related genes (Itaya et al., 2009). It is considered that cytodifferentiation of hPDLSC occurs by the function of various growth factors and cytokines, exclusively or simultaneously. FGF-2 is a key factor that promotes wound healing and regeneration, and has been used for clinical application. In this study, we focused on the possible involvement of FGF-2 in ligamentogenesis of hPDLSCs. For the stimulation of fibroblastic cell growth of hPDLSCs, 3–5 ng/ml of FGF-2 was sufficient (Fig. 1). With increasing concentrations of FGF-2, the expression of teno/ligamentogenic markers was proportionately stimulated (Fig. 2A). The expression of the mature tenocyte markers, tenomodulin, apparently increased with the concentrations of FGF-2 (Fig. 2A,b). However, the expression of this gene in control cells was decreased by addition of low concentration of FGF-2. The reason for this could be explained that expression level varies in each culture batch, and that some PDL cells from the primary culture had a mature fibrogenic feature, whereas some were in the undifferentiated state. In contrast to teno/ligamentogenic markers, the expression of osteo/cementogenic markers decreased according to FGF-2 concentrations (Fig. 2B), and mineralization was also dramatically inhibited by treatment with FGF-2 (Fig. 4C). These data indicated that FGF-2 treatment inhibit osteo/cementogenesis of hPDLSCs. The expression of teno-moduin was induced by treatment with TGF-β1 in comparison with control (Fig. 3, bars 2nd in a), and TGF-β1 treatment decreased the scleraxis expression (Fig. 3b). However, FGF-2 treatment diminished the effect of TGF-β1 on tenocytic maturation (Fig. 3, bars 3rd–4th in a), indicating that TGF-β1 induces tenocyte differentiation, and that FGF-2 controls the mature ligament differentiation and maintains the potential for initial ligamentogenesis. TGF-β1 is known as a factor for stimulation of teno/ligamentogenic phenotype, which is involved in ECM production and collagen type-1 stimulation (Asano et al., 2005; Dangaria et al., 2009). Indeed, the expression of collagen type-1 also stimulated by TGF-β1 in antagonistic way to FGF-2 treatment (Supplementary Fig. 1). The expression pattern of collagen type-1 by TGF-β1 was similar to that of tenomodulin, suggested that TGF-β1 might stimulate the mature ligamentogenesis. The general problem in induction of ligamentogenic cytodifferentiation in hPDLSCs is that the expression level of these genes was not always consistent and more or less heterogeneous in crude hPDL-derived cells cultured from each tissue sample. In other words, there are individual differences in differentiation state among the primary cultures of hPDLSC; age and genotypic variation of patients, and culture condition of density and passage number. Therefore, for comparison of gene expressions under the treatment with various cytokines and growth factors, the expression level of genes in crude hPDL-derived cells used as control was not always a good standard in experiments. In many cases, it was detected that the expression levels of tenomodulin and scleraxis were already high or low even without treatment with FGF-2 or TGF-β1 (data not shown). FGF-2 has the opposite effect with BMPs on osteo/cementogenic differentiation (Figs. 4A and 4B) and totally inhibited the mineralization, which was induced by BMP-2 and BMP-4 (Figs. 4C and 4D), indicating that FGF-2 played a dominant role as an antagonist to BMPs, and maintained the potential for the early stage of ligamentogenic differentiation in hPDLSCs.
Supplementary Information
ACKNOWLEDGMENTS
This research was supported by the Bio & Medical Technology Development Program (NRF-2015M3A9C6029130) & by the Basic Science Research Program (NRF-2016R1A1A3A04 005189) of the NRF funded by the Korean government, MSIP.
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Asano M, Kubota S, Nakanishi T, Nishida T, Yamaai T, Yosimichi G, Ohyama K, Sugimoto T, Murayama Y, Takigawa M. Effect of connective tissue growth factor (CCN2/CTGF) on proliferation and differentiation of mouse periodontal ligament-derived cells. Cell Commun Signal. 2005;3:11. doi: 10.1186/1478-811X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontology 2000. 1997;13:20–40. doi: 10.1111/j.1600-0757.1997.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Bradfute SB, Graubert TA, Goodell MA. Roles of Sca-1 in hematopoietic stem/progenitor cell function. Exp Hematol. 2005;33:836–843. doi: 10.1016/j.exphem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Choi JK, Hwang HI, Jang YJ. The efficiency of the in vitro osteo/dentinogenic differentiation of human dental pulp cells, periodontal ligament cells and gingival fibroblasts. Int J Mol Med. 2015;35:161–168. doi: 10.3892/ijmm.2014.1986. [DOI] [PubMed] [Google Scholar]
- Dangaria SJ, Ito Y, Walker C, Druzinsky R, Luan X, Diekwisch TG. Extracellular matrix-mediated differentiation of periodontal progenitor cells. Differentiation. 2009;78:79–90. doi: 10.1016/j.diff.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gorter DJ, van Dinther M, Korchynskyi O, ten Dijke P. Biphasic effects of transforming growth factor beta on bone morphogenetic protein-induced osteoblast differentiation. J Bone Miner Res. 2011;26:1178–1187. doi: 10.1002/jbmr.313. [DOI] [PubMed] [Google Scholar]
- Francis PH, Richardson MK, Brickell PM, Tickle C. Bone morphogenetic proteins and a signalling pathway that controls patterning in the developing chick limb. Development. 1994;120:209–218. doi: 10.1242/dev.120.1.209. [DOI] [PubMed] [Google Scholar]
- Fujii S, Maeda H, Tomokiyo A, Monnouchi S, Hori K, Wada N, Akamine A. Effects of TGF-beta1 on the proliferation and differentiation of human periodontal ligament cells and a human periodontal ligament stem/progenitor cell line. Cell Tissue Res. 2010;342:233–242. doi: 10.1007/s00441-010-1037-x. [DOI] [PubMed] [Google Scholar]
- Itaya T, Kagami H, Okada K, Yamawaki A, Narita Y, Inoue M, Sumita Y, Ueda M. Characteristic changes of periodontal ligament-derived cells during passage. J Periodontal Res. 2009;44:425–433. doi: 10.1111/j.1600-0765.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- Kao RT, Murakami S, Beirne OR. The use of biologic mediators and tissue engineering in dentistry. Periodontology 2000. 2009;50:127–153. doi: 10.1111/j.1600-0757.2008.00287.x. [DOI] [PubMed] [Google Scholar]
- Kawahara T, Yamashita M, Ikegami K, Nakamura T, Yanagita M, Yamada S, Kitamura M, Murakami S. TGF-β negatively regulates the BMP2-dependent early commitment of periodontal ligament cells into hard tissue forming cells. PloS one. 2015;10:e0125590. doi: 10.1371/journal.pone.0125590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai WT, Krishnappa V, Phinney DG. Fibroblast growth factor 2 (Fgf2) inhibits differentiation of mesenchymal stem cells by inducing Twist2 and Spry4, blocking extracellular regulated kinase activation, and altering Fgf receptor expression levels. Stem Cells. 2011;29:1102–1111. doi: 10.1002/stem.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Kim WT, Ryu CJ, Jang YJ. Optimization of treatment with recombinant FGF-2 for proliferation and differentiation of human dental stem cells, mesenchymal stem cells, and osteoblasts. Biochem Cell Biol. 2015;93:298–305. doi: 10.1139/bcb-2014-0140. [DOI] [PubMed] [Google Scholar]
- Lekic P, McCulloch CA. Periodontal ligament cell population: the central role of fibroblasts in creating a unique tissue. Anat Rec. 1996;245:327–341. doi: 10.1002/(SICI)1097-0185(199606)245:2<327::AID-AR15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Lorda-Diez CI, Montero JA, Martinez-Cue C, Garcia-Porrero JA, Hurle JM. Transforming growth factors beta coordinate cartilage and tendon differentiation in the developing limb mesenchyme. J Biol Chem. 2009;284:29988–29996. doi: 10.1074/jbc.M109.014811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Tomokiyo A, Fujii S, Wada N, Akamine A. Promise of periodontal ligament stem cells in regeneration of periodontium. Stem Cell Res Ther. 2011;2:33. doi: 10.1186/scrt74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer M, Fischbach C, Bauer-Kreisel P, Lieb E, Hacker M, Tessmar J, Schulz MB, Goepferich A, Blunk T. Basic fibroblast growth factor enhances PPARgamma ligand-induced adipogenesis of mesenchymal stem cells. FEBS Lett. 2004;577:277–283. doi: 10.1016/j.febslet.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Olson EN, Capetanaki YG. Developmental regulation of intermediate filament and actin mRNAs during myogenesis is disrupted by oncogenic ras genes. Oncogene. 1989;4:907–913. [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shimabukuro Y, Terashima H, Takedachi M, Maeda K, Nakamura T, Sawada K, Kobashi M, Awata T, Oohara H, Kawahara T, et al. Fibroblast growth factor-2 stimulates directed migration of periodontal ligament cells via PI3K/AKT signaling and CD44/hyaluronan interaction. J Cell Physiol. 2011;226:809–821. doi: 10.1002/jcp.22406. [DOI] [PubMed] [Google Scholar]
- Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Takimoto A, Akiyama H, Kist R, Scherer G, Nakamura T, Hiraki Y, Shukunami C. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. 2013;140:2280–2288. doi: 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- Terranova VP. Biologically active factors in the treatment of periodontal disease. Curr Opin Periodontol. 1993;1993:129–135. [PubMed] [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- Wikesjo UM, Razi SS, Sigurdsson TJ, Tatakis DN, Lee MB, Ongpipattanakul B, Nguyen T, Hardwick R. Periodontal repair in dogs: effect of recombinant human transforming growth factor-beta1 on guided tissue regeneration. J Clin Periodontol. 1998;25:475–481. doi: 10.1111/j.1600-051x.1998.tb02476.x. [DOI] [PubMed] [Google Scholar]
- Yanagita M, Kojima Y, Kubota M, Mori K, Yamashita M, Yamada S, Kitamura M, Murakami S. Cooperative effects of FGF-2 and VEGF-A in periodontal ligament cells. J Dent Res. 2014;93:89–95. doi: 10.1177/0022034513511640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PJ, Ferrari G, Galloway AC, Mignatti P, Pintucci G. Basic fibroblast growth factor (FGF-2): the high molecular weight forms come of age. J Cell Biochem. 2007;100:1100–1108. doi: 10.1002/jcb.21116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


