Abstract
Regular monitoring on experimental animal management found the fluctuation of ART outcome, which showed a necessity to explore whether superovulation treatment is responsible for such unexpected outcome. This study was subsequently conducted to examine whether superovulation treatment can preserve ultrastructural integrity and developmental competence of oocytes following oocyte activation and embryo culture. A randomized study using mouse model was designed and in vitro development (experiment 1), ultrastructural morphology (experiment 2) and functional integrity of the oocytes (experiment 3) retrieved after PMSG/hCG injection (superovulation group) or not (natural ovulation; control group) were evaluated. In experiment 1, more oocytes were retrieved following superovulation than following natural ovulation, but natural ovulation yielded higher (p < 0.0563) maturation rate than superovulation. The capacity of mature oocytes to form pronucleus and to develop into blastocysts in vitro was similar. In experiment 2, a notable (p < 0.0186) increase in mitochondrial deformity, characterized by the formation of vacuolated mitochondria, was detected in the superovulation group. Multivesicular body formation was also increased, whereas early endosome formation was significantly decreased. No obvious changes in other microorganelles, however, were detected, which included the formation and distribution of mitochondria, cortical granules, microvilli, and smooth and rough endoplasmic reticulum. In experiment 3, significant decreases in mitochondrial activity, ATP production and dextran uptake were detected in the superovulation group. In conclusion, superovulation treatment may change both maturational status and functional and ultrastuctural integrity of oocytes. Superovulation effect on preimplantation development can be discussed.
Keywords: artificial reproductive Technology (ART), development, gonadotrophins, microorganelle function, oocyte maturation
INTRODUCTION
Superovulation treatment has been used for retrieving large numbers of mature oocytes (Fowler and Edwards, 1957; Nagy, 2003), which triggers enormous improvements in artificial reproductive technology (ART) of both human and domestic animal species. The safety of superovulation treatment has been confirmed, which generally preserves oocyte morphology and functional integrity (Munoz et al., 1994; Nagy, 2003). The procedure of superovulation treatment has continuously been optimized (Combelles and Albertini, 2003; Edwards and Gates, 1959; Fowler and Edwards, 1960; Hamberger and Wikland, 1993; Legge and Sellens, 1994; Miller and Armstrong, 1981; Steward et al., 2014; Templeton and Morris, 1998), which leads to successful In vitro fertilization (IVF) and embryo transfer (ET) program (Hamberger and Wikland, 1993; Templeton and Morris, 1998).
In several reports, however, it has been reported that superovulation causes perturbation of maternal and paternal imprinted methylation (Fortier et al., 2008; Market-Velker et al., 2010), reduced intrafollicular estradiol and progesterone levels (Assey et al., 1994) and delayed embryonic and fetal development (Van der Auwera and D’Hooghe, 2001). Injection of hormones at supra-physiological levels may cause ultrastructure deformities of oocytes, which leads precocious maturation with functional aberrations (Hasegawa et al., 2015; Moor et al., 1985; Yun et al., 1989). As a matter of fact, occasional fluctuation of ART outcome may be experienced even after employing the optimized superovulation protocol in field trials, and various environmental factors including seasonal variation and miscellaneous changes in oocyte quality may be responsible for such perturbation.
Consequently, we examined how superovulation treatment influences oocyte maturity and development, and their ultrastructural and function integrity. Mature (metaphase II stage) oocytes were provided following superovulation treatment with pregnant mare’s serum gonadotropin (PMSG)/human chorionic gonadotropin (hCG) or not (natural ovulation). Initial observation on retrieval, maturation, pronuclear formation of oocytes and preimplantation development of activated oocytes was monitored. Next, morphological observation of microorganelles such as mitochondria, multivesicular bodies was undertaken using transmission electron microscope (TEM) analysis. Since mitochondria have an important role in various cellular processes (Balaban et al., 2005; Salminen et al., 2014) and since they have particular patterns of distribution during development (Van Blerkom, 1991; Van Blerkom and Runner, 1984), adenosine triphosphate (ATP) production by mitochondria (Kulkarni et al., 1998; Mancini et al., 1997) and early endocytosis (Moore et al., 1997; West et al., 2004) were compared between the superovulation and natural ovulation groups.
MATERIALS AND METHODS
Experimental design
F1 hybrid mice of the non-block B6CBAF1 strain were hysperstimulated with PMSG, and superovulated oocytes following hCG injection were retrieved. As a control treatment, oocytes were retrieved after natural ovulation. In experiment 1, oocyte retrieval, maturation and developmental competence were monitored. Both superovulated and naturally ovulated oocytes were either activated parthenogenetically or fertilized in vitro and were subsequently cultured for 120 h. Number of oocytes retrieved and developed to the metaphase II (mature), pronuclear, 2-cell, 4-cell, morula and blastocyst stages was monitored. In experiment 2, ultrastructural normality of oocytes were monitored with TEM, which includes mitochondria, cortical granules, multivesicular bodies, lipid droplets, microvilli, and the endoplasmic reticulum system. In experiment 3, ATP generation representing mitochondrial function and endocytosis in mature oocytes retrieved from superovulation or natural ovulation were evaluated by JC-1 staining and dextran uptake, respectively.
Experimental animals
B6CBAF1 hybrid mice were employed as experimental animals. Parental C57BL6 female and CBA/caJ male mice were purchased from Jackson Laboratory. Mature oocytes were harvested from 8-week-old female mice after experimental treatments. All animals were maintained under conditions of controlled lightening (14 h of light/10 h of darkness), temperature (20–22°C), and humidity (40–60%). All animal management, breeding, and euthanasic procedures were performed according to the standard protocols of Seoul National University. The experimental protocols were approved by the Institutional Animal Care and Use Committee (approval number SNU-091028-4). Additionally, experimental samples were managed appropriately, and quality control of the laboratory facility and equipment were conducted.
Collection of mature oocytes
Naturally ovulated oocytes were collected by oviduct flushing of F1 female mice in estrus 16 h after they were mated with a vasectomized male mice. Vaginal smears were performed to check the estrous cycle stage of the female donors. The flushing medium was M2 medium (Sigma-Aldrich, St Louis, MO). For ovarian hyperstimulation, 5 IU of PMSG (Folligon™; Intervet International, Boxmeer, the Netherlands) was injected intraperitoneally and ovulation was induced by intraperitoneal injection of 5 IU of hCG (Pregnyl™; Organon, Oss, the Netherlands) 48 h later (Jang et al., 2007). Oocytes were recovered 16 h post-hCG. After hormonal treatments females were sacrificed by cervical dislocation. Oocyte maturation at the metaphase II stage was verified by extrusion of the first polar body in the perivitelline space. For this analysis, oocytes were released from cumulus cells through incubation in M2 medium supplemented with hyaluronidase (Sigma-Aldrich; 200 IU/ml) for 5 min at 37°C.
Parthenogenetic activation and IVF of oocytes
To activate oocytes parthenogenetically, oocytes released from cumulus cells were cultured in calcium-free potassium simplex optimized medium (KSOM) supplemented with 10 mM SrCl2 and 5 μg/ml cytochalasin B for 4 h. For IVF, cumulus-enclosed oocytes retrieved after either natural ovulation or ovarian hyperstimulation were fertilized through incubation with epididymal semen in KSOM for 4–6 h (Byers et al., 2006). Oocytes that had been activated parthenogenetically or inseminated in vitro were then cultured constantly in 5 μl droplets of modified Chatot, Ziomek, and Bacister (CZB) medium for a further 120 h at 37°C under an atmosphere of 5% CO2 in air. Pronucleus formation, cleavage, and development to the 4-cell, morula, and blastocyst stages were monitored under an IX70 inverted microscope (Olympus, Japan) at 6, 24, 48, 72 and 120 h after activation or fertilization.
TEM analysis
Mature oocytes retrieved after experimental treatment were incubated in modified Karnovsky’s solution overnight and were subsequently fixed through incubation with osmium tetroxide for 2 h. The fixed oocytes were dehydrated in a graded ethanol solution. Then, en bloc staining was conducted overnight. The specimens were then embedded in Spurr resin. Ultra-thin sections (60 nm thick) were prepared with an ultramicrotome. The sections were then mounted on grids and stained with uranyl acetate and Reynolds’ lead citrate (Kim, 2008). They were subsequently examined with a LIBRA 120 energy-filtering TEM (Carl Zeiss, Germany). Images of ooplasm (area: 100 μm2) at magnifications of 6,000 to 25,000 were used.
The area of section being observed was randomly chosen, which resulted randomly distributed from the middle to the peripheral areas. The area of section was selected randomly, so all levels had comparative value between experimental groups. Total number of each cellular organelle in single TEM image was calculated and based on the scale bar being designated, number of each cellular organelles per unit area were counted. At least ten images were used for undertaking numerical analysis.
Evaluation of the ratio of activated to less-activated mitochondria by confocal microscopy
We used the potential-sensitive fluorescent dye JC-1 (Molecular Probes, USA) to examine the activity of mitochondria in the ovulated oocytes. JC-1 was dissolved to a stock concentration of 0.2 mM in DMSO and diluted to a final concentration of 2 μM in M2 medium. Oocytes were exposed to JC-1 at 37.5°C under an atmosphere containing 5% CO2 in a humidified incubator for 1 h and then washed three times in M2 medium to remove any surface fluorescence. Stained oocytes were transferred to a confocal dish containing M2 medium. A laser scanning confocal microscope was used to examine mitochondrial activity. The excitation laser line was set to 488 nm, and the emission wavelengths were separated by a 530 nm diachronic mirror, with further filtering through a 515–530 nm band-pass filter (green emission) or a 585 nm long-pass filter (red emission) filter. Fluorescence intensity ratios were measured from the confocal images using NIS-Elements BR 3.0 imaging software (Nikon, Japan). Mitochondria with high membrane potentials (activated) were visualized in red, whereas those with low membrane potentials were in green.
Quantification of ATP synthesis
ATP levels in ovulated oocytes were measured using a Bioluminescent Somatic Cell Assay Kit (FL-ASC; Sigma-Aldrich), which makes use of the luciferin–luciferase reaction. Briefly, oocytes (18 oocytes from natural ovulation and 39 oocytes from hyperstimulation) were placed into 100 μl of ice-cold somatic cell reagent (FL-SAR; Sigma-Aldrich) for 5 min. They were then incubated with 100 μl of diluted ice-cold assay mix [FL-AAM reagent, diluted 1:25 in ATP Assay Mix Dilution Buffer (FL-AAB reagent; Sigma-Aldrich)] for an additional 5 min. The solution was then transferred to an AutoLumat LB 953 Luminometer (EG&G Berthold) for the measurement of luminescence. A 10-point standard curve (0–10 pmol/tube) was constructed for every 20 oocytes, and ATP content was calculated using the formula from linear regression of the standard curve.
Endocytosis assay
To assess dextran uptake, ovulated oocytes were retrieved, and the zona pellucida was removed using acid Tyrode solution. For the analysis of endocytic activity in ovulated oocytes, each group was incubated with FITC-dextran (10,000 MW; Molecular Probes) for 30 min at 37°C. As a positive control, oocytes were cooled to 4°C prior to incubation with dextran at 4°C for 30 min. Oocytes were washed three times and immediately transferred to a confocal dish containing M2 medium, which was kept on ice at all times. To obtain confocal images, we used a krypton–argon mixed-gas laser. The excitation laser line was set to 494 nm, and the emission wavelength was set to 521 nm using a filter. Fluorescence intensity ratios were measured from the confocal images using NIS-Elements BR 3.0 imaging software (Nikon).
Statistical analysis
All experiments were replicated more than three times, and the data obtained were subjected to statistical analysis. Not all oocytes retrieved from each experiment were provided because the superovulation treatment yielded different number of oocytes retrieved. To compensate this discrepancy, randomly selected oocytes were allotted to several treatment groups. A generalized linear model (PROC-GLM) created using Statistical Analysis System (SAS) software version 9.1 (SAS Institute, USA) was used to analyze the data. When a significant model effect was detected, comparisons among groups were subsequently conducted using the least-squares or Duncan methods. A p-value of less than 0.05 indicated a significant difference.
RESULTS
Experiment 1
As shown in Fig. 1, more (32.6 vs. 9.8 oocytes/head; p < 0.0001) oocytes were retrieved after superovulation treatment than after natural ovulation. However, significant (p < 0.0563) decrease in maturation rate was detected in the superovulation group, compared with the control (100% vs. 74%). Both IVF and parthenogenesis of mature oocytes triggered pronuclear formation of oocytes (85–100%), which did not differ significantly (p < 0.4863) among groups. As shown in Table 1, the model effect of superovulation on preimplantation development was not (p > 0.4) detected. The developments to the 2-cell, 4-cell, morula and blastocyst stages were 94 to 100%, 87 to 96%, 87 to 93% and 72 to 82%, respectively.
Fig. 1.
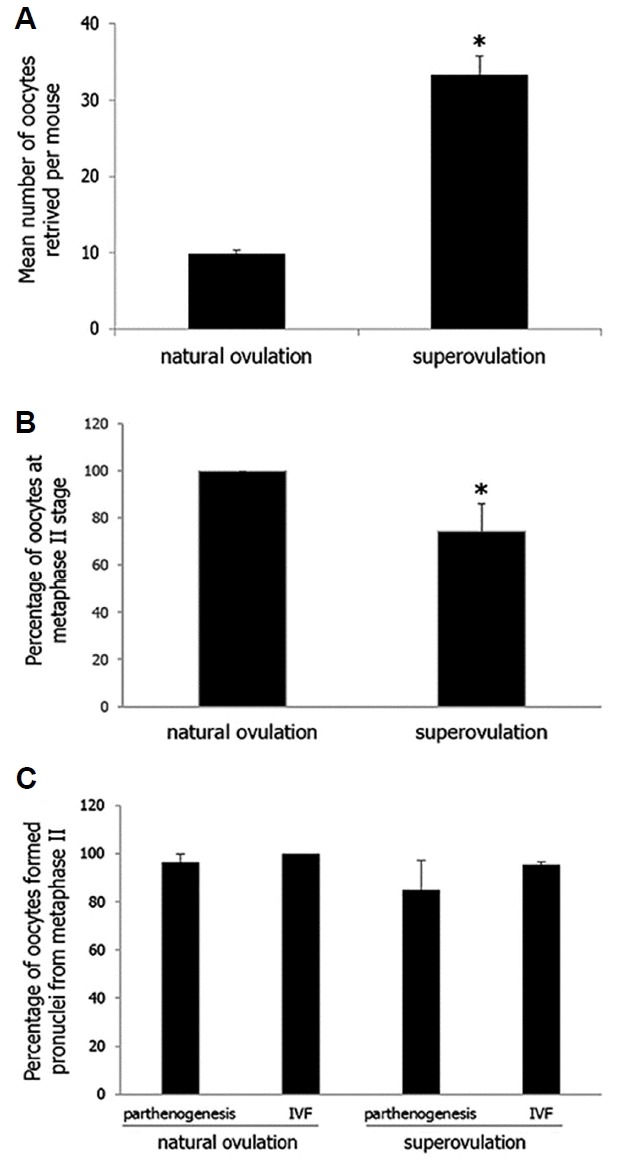
Retrieval, maturation and activation of oocytes derived from B6CBAF1 either superovulated or not (natural mating).
For numerical comparison, number of oocytes retrieved per mouse either superovulated or not (A), percentage of metaphase II stage oocytes to retrieved oocytes (B), percentage of pronuclear stage oocytes to metaphase II stage oocytes (C) were counted. Statistical comparison employing PLOC-GLM model was conducted with numerical values and data are presented as mean ± SE. Model effects (p value) of each parameter were less than 0.0001 (A), 0.0563 (B) and 0.4863 (C). Asterisk indicated statistical siginficance with other treatments (p < 0.0563).
Table 1.
Developmental competence of B6CBAF1 oocytes retrieved after natural ovulation or ovarian hyperstimulation with PMSG and hCG
| Treatments | Methods of oocyte activation | No. of oocytes activated | No. (%)a of oocytes developing to | |||
|---|---|---|---|---|---|---|
| 2-cell | 4-cell | Morula | Blastocyst | |||
| None (natural mating) | Partheno genesis | 31 | 29 (94) | 27 (87) | 27 (87) | 23 (74) |
| IVF | 28 | 28 (100) | 27 (96) | 26 (93) | 23 (82) | |
| Hyperstimulation | Partheno genesis | 61 | 60 (98) | 56 (92) | 54 (89) | 44 (72) |
| IVF | 86 | 82 (95) | 80 (93) | 78 (91) | 65 (76) | |
Model effects of treatment that were indicated as p value were 0.4169, 0.5993, 0.8299 and 0.7066 in the number of oocytes developing to the 2- cell, 4-cell, morula and blastocyst stages, respectively.
Percentage of the number of oocytes cultured.
PMSG, pregnant mare serum gonadotrophin; hCG, human chorionic gonadotrophin; IVF, in vitro fertilization.
Experiment 2
Total 11 mice were euthanized for observing oocyte ultrastructure and, 3 and 8 mice were allotted into the superovulation and the natural cycle group, respectively. The number of oocytes examined was 110 for the superovulation and 109 for the natural cycle groups. However, only 10 images of different oocytes in each group were randomly selected and subsequently employed for the analysis. As shown in Fig. 2A2, a significant increase in mitochondrial deformity was detected (p = 0.0186). The percentage of mitochondria that were vacuolated was significantly increased after superovulation treatment (39% vs. 30%), and normal mitochondria with well-developed cristae and without vacuoles were concomitantly observed. The overall number of mitochondria per mature oocyte did not differ significantly (p > 0.8999) between the two groups (20.1 vs. 20.8). There were more multivesicular bodies in the superovulation group than in the natural ovulation group (1.44 vs. 4.27; p = 0.0221; Fig. 2D). Apart from these microorganelles, no other deformities were found (Figs. 2B and 2C). Cortical granules were found in the area beneath the plasma membrane. Whereas the granules were located just beneath the oolemma in naturally ovulated oocytes, they were located a little farther underneath the oolemma in the superovulation group (0.4–0.6 μm beneath the oolemma). The perivitelline spaces were slightly larger in the superovulation group than in the natural ovulation group. Well-developed smooth endoplasmic reticulum was observed in both groups.
Fig. 2.
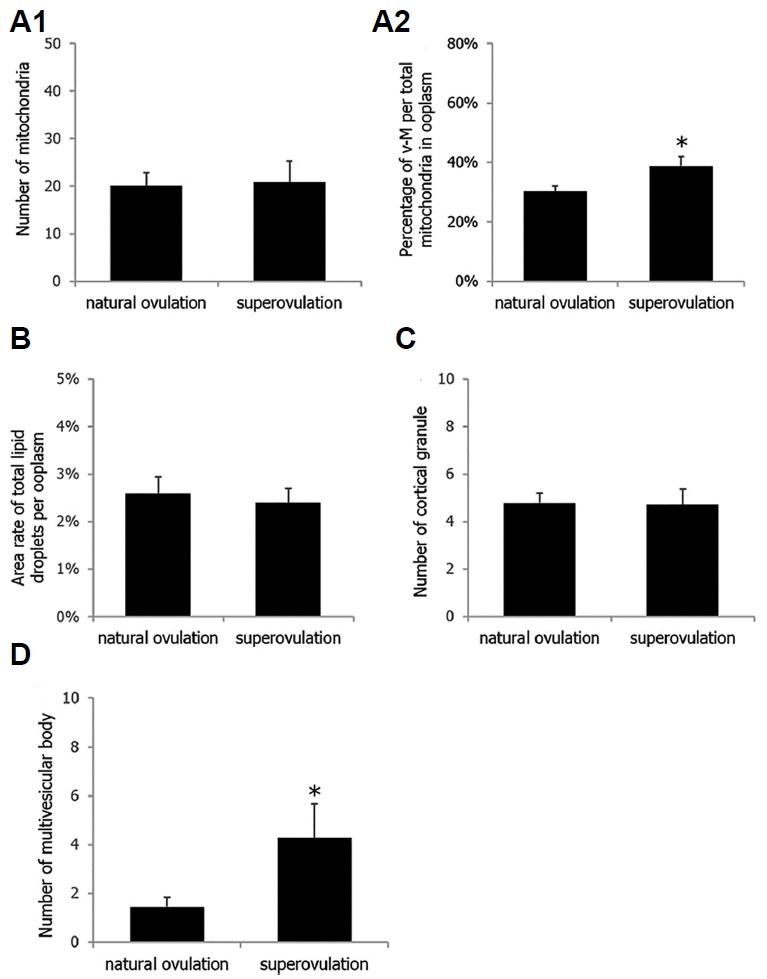
Differences in the number of microorganelles in mature (metaphase II stage) oocytes retrieved after superovulation treatment or natural ovulation.
For numerical comparison, number of mitochondria per 100 μm2 of ooplasm (A1), percentage of vacuolated-mitochondria per total mitochondria in 100 μm2 of ooplasm (A2), percentage of total lipid droplets per 100 μm2 of ooplasm (B), number of cortical granules per 10 μm of oolemma (C), and number of multivesicular bodies per 200 μm2 of ooplasm (D) were counted. Statistical comparison employing PLOC-GLM model was conducted with numerical values and data are presented as mean ± SE. *Statistical siginficance in A2 (p = 0.0186) and D (p = 0.0221)
Experiment 3
Nine mice were provided for JC-1 staining (96 oocyes) and ATP measurement (57 oocytes). As shown in Fig. 4, activated mitochondria in the superovulation group (Fig. 4D2) were distributed in a semi-peripheral pattern as compared with the control group (Fig. 4C2). There was a significant difference in mitochondrial activity between two groups (p = 0.0179; Fig. 4E). Moreover, the ratio of activated mitochondria to inactivated mitochondria was lower after superovulation (1.17 vs. 0.97). As shown in Fig. 4F, superovulation treatment had a significant effect on ATP synthesis activity (p = 0.0002), which was lower in non-stimulated oocytes than in hyperstimulated oocytes (3.66 vs. 0.74 pmol/oocyte). Dextran uptake was measured in 87 oocytes. As shown in Fig. 5, dextran uptake, measured as a marker of early endocytosis, was lower in the hyperstimulation group compared with the control group (65.14 vs. 5.32; p = 0.004), although there was an increase in the number of multivesicular bodies, indicating that late endocytosis was increased.
Fig. 4.
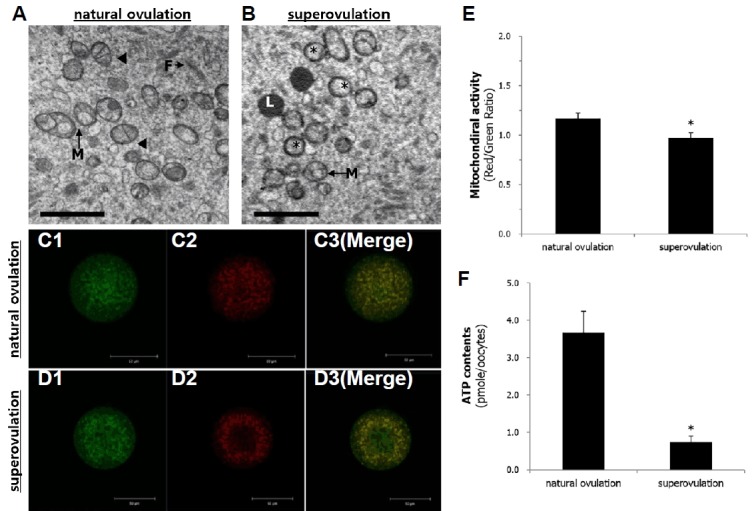
Deformity and functional activity of mitochondria in mature (metaphase II stage) oocytes retrieved after superovulation treatment or natural ovulation.
(A) (natural ovulation) and (B) (superovulation) show morphological difference of mitochondria. Confocal images of mitochondria in oocytes retrieved from natural ovulation (C) or superovulation after JC-1 staining (D). (E) Statistical comparison of mitochondrial activity (mean ± SE) with using of confocal images. Significant (p = 0.0179) difference between two treatments. (F) Quantitative comparison of ATP generation (mean ± SE) in oocytes retrieved from after superovulation or retrieved from natural ovulation. Significantly (p = 0.0002) different. Arrowhead, Well-developed mitochondrial cristae; asterisk, vacuolated mitochondria; F, Filament; L, Lipid droplet; M, Mitochondria. Magnification = ×12,000. Scale bars = 2 μm (A, B)
Fig. 5.

Uptake of dextran-FITC by oocytes retrieved after natural ovulation (A) or after superovulation treatment (B).
Confocal image was employed for monitoring dextran uptake as the representative of endocytosis (mean ± SE). Intensity of signal in confocal images was quantitatively compared (C) and significant (p = 0.004) difference was detected. *Statistical siginficance.
DISCUSSION
The results of this study demonstrated that compared with natural ovulation, the superovulation treatment with PMSG and hCG decreases the proportion of mature oocytes to retrieved oocytes. In some oocytes being superovulated, ultrastructural alterations in mitochondria and lysosomal complexes, characterized by increased numbers of vacuolated mitochondria and multivesicular bodies were observed. ATP synthesis became lower in the superovulation group, which might be related to the mitochondrial deformity, and early endocytosis was also influenced by the superovulation. However, the observation of these experimentations do not reduce the feasibility of superovulation in ART program activation and development. While superovulation treatment has become the basic methodology of human and animal ART programs, it has been reported that decreased quality of superovulated oocytes before and after IVF resulted the reduced fecundity. Increased preimplantation mortality after superovulation has been reported in mice (Beaumont and Smith, 1975; Ertzeid and Storeng, 1992; Kalthur et al., 2015) and rats (Miller and Armstrong, 1981). Ovarian hyperstimulation can increase chromosomal abnormalities in murine embryos (Elbling and Colot, 1985; Huffman et al., 2015; Luckett and Mukherjee, 1986; Ozturk et al., 2016), and mitochondrial deformity further induced oocyte aneuploidy (Plachot, 2003; Takeuchi et al., 2005). Gonadotrophin-induced superovulation induces the proliferation of ovarian surface epithelial cells, which results cell transformation into cancer cells (Burdette et al., 2006). Nevertheless, lots of oocytes retrieved after superovulation treatment have superb capacity of maturation and absolute number of degeneration-fated oocytes take an opportunity to mature by superovulation, which overwhelms adverse effect of superovulation. As a matter of fact, no significant difference on developmental competence of oocytes between the natural cycle and the superovulation groups confirmed normality of mature oocytes retrieved after superovulation (Table 1). The results of these data can contribute not only to obtaining toxicological data, but also to improving ART efficiency by decreasing potential adverse effect of superovulation.
Number of toxicological data could be retrieved from the results of this study. Even under this limited condition of evaluation on ultrastructural morphology, not only structural changes but also functional alterations were detectable after superovulation. Semi-peripheral ring-like distribution of metachromatic organelles of superovulated oocytes showed structural and functional changes of oocytes simultaneously (Fig. 4). These changes were prominent in mitochondria and multivesicular bodies. Significant decrease in ATP generation observed in this study may adversely affect embryonic development via altering chromosomal segregation, meiotic spindle adjunction (Takeuchi et al., 2005), microtubule polymerization (Eichenlaub-Ritter et al., 2004; Plachot, 2003) and calcium oscillations during oocyte activation and embryogenesis (Calarco, 1995). On the other hand, mitochondrial deformity induces oocyte aneuploidy (Field et al., 1999) via predivision or nondisjunction of chromatids (Guerin et al., 2001).
Superovulation treatment also alters endocytotic function of the oocytes, which is critical for metabolic coupling and synthetic activity (Chi et al., 2011; Dobrowolski and De Robertis, 2011; Eden et al., 2009). As a results above, early endocytosis represented by dextran uptake were extremely down-regulated but multivesicular bodies in ultrastructure found more in the superovulation group (Figs. 2 and 5). Increased influx of excessive gonadotropin may result the presence of multivesicular bodies, which changes lots of metabolic pathways (Katzmann et al., 2002). Increased endocytosis at the late stage followed by increased multivesicular bodies may couple with decreasing of metabolic uptake, which cause severe deficiencies of metabolic substrates and cytological stress. In this study, the ring-like distribution of organelles was found in JC-1 staining, which could be related with those stresses. Decreased activities of mitochondria at this stage represented by low endocytosis and mitochondrial membrane potential could be one of cause for those stress for oocyte (Figs. 4 and 5). Mitochondrial damages observed in ultrastructural level connoted developmental disadvantages (Ghavami et al., 2015), which may increase the susceptibility of oocytes to physiological or environmental stress leading to fluctuation of ART outcome.
Various exogenous causes such as temperature, photoperiod and/or other environmental variables may combine with the effect of superovulation treatment, which may result the fluctuation of ART outcome. As a matter of fact, most of studies on superovulation in humans were designed to evaluate the optimal gonadotrophin dosage for retrieving excellent quality of oocytes (Hohmann et al., 2003). Otherwise, relationship between hormonal treatment and aneuploidy rate of embryos (Baart et al., 2007) were examined. Comparing superovulation treatment with natural ovulation in human, however, no obvious difference were found in embryo development, (Gras et al., 1992; Ziebe et al., 2004) and aneuploidy rate (Labarta et al., 2012; Qin et al., 2013). Optimization of microenvironment can decreases adverse effect of superovulation, which further improve ART outcome.
Fig. 3.
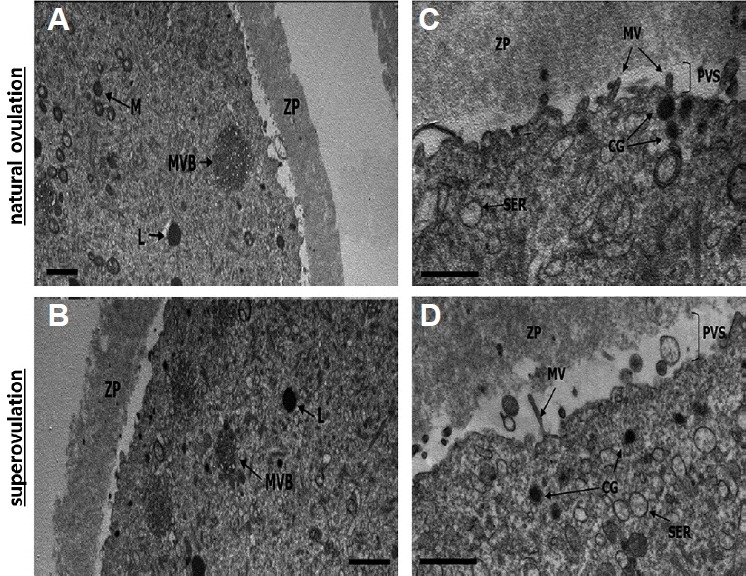
Ultrastructure of mature (metaphase II stage) oocytes retrieved after superovulation treatment or natural ovulation.
Transmission electron microscope was employed for monitoring morphologic analysis. Micrograph A (natural ovulation) and B (superovulation) show multivesicular bodies. Micrograph C (natural ovulation) and D (superovulation) show the morphology and distribution of cortical granules, microvilli and smooth endoplasmic reticulum. L: lipid; M: Mitochondria; MVB: Multivesicular body; ZP: Zona pellucida; CG: Cortical granule; MV: Microvilli; PVS: Perivitelline space; SER: smooth endoplasmic reticulum; ZP: Zona pellucida. Magnification = ×6,000 (A), ×8,000 (B) and ×25,000 (C, D). Scale bars=2 μm (A, B) and 1 μm (C, D).
ACKNOWLEDGMENTS
This research was supported by Bio-industry Technology Development Program [IPET312060-5], Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea and by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIP) [No. 2015R1C1A2A01055746].
REFERENCES
- Assey RJ, Hyttel P, Roche JF, Boland M. Oocyte structure and follicular steroid concentrations in superovulated versus unstimulated heifers. Mol Reprod Dev. 1994;39:8–16. doi: 10.1002/mrd.1080390103. [DOI] [PubMed] [Google Scholar]
- Baart EB, Martini E, Eijkemans MJ, Van Opstal D, Beckers NG, Verhoeff A, Macklon NS, Fauser BC. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Beaumont HM, Smith AF. Embryonic mortality during the pre- and post-implantation periods of pregnancy in mature mice after superovulation. J Reprod Fertil. 1975;45:437–448. doi: 10.1530/jrf.0.0450437. [DOI] [PubMed] [Google Scholar]
- Burdette JE, Kurley SJ, Kilen SM, Mayo KE, Woodruff TK. Gonadotropin-induced superovulation drives ovarian surface epithelia proliferation in CD1 mice. Endocrinology. 2006;147:2338–2345. doi: 10.1210/en.2005-1629. [DOI] [PubMed] [Google Scholar]
- Byers SL, Payson SJ, Taft RA. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs) Theriogenology. 2006;65:1716–1726. doi: 10.1016/j.theriogenology.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Calarco PG. Polarization of mitochondria in the unfertilized mouse oocyte. Dev Genet. 1995;16:36–43. doi: 10.1002/dvg.1020160108. [DOI] [PubMed] [Google Scholar]
- Chi S, Cao H, Wang Y, McNiven MA. Recycling of the epidermal growth factor receptor is mediated by a novel form of the clathrin adaptor protein Eps15. J Biol Chem. 2011;286:35196–35208. doi: 10.1074/jbc.M111.247577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combelles CM, Albertini DF. Assessment of oocyte quality following repeated gonadotropin stimulation in the mouse. Biol Reprod. 2003;68:812–821. doi: 10.1095/biolreprod.102.008656. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, De Robertis EM. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat Rev Mol Cell Biol. 2011;13:53–60. doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden ER, White IJ, Futter CE. Down-regulation of epidermal growth factor receptor signalling within multivesicular bodies. Biochem Soc Trans. 2009;37:173–177. doi: 10.1042/BST0370173. [DOI] [PubMed] [Google Scholar]
- Edwards RG, Gates AH. Timing of the stages of the maturation divisions, ovulation, fertilization and the first cleavage of eggs of adult mice treated with gonadotrophins. J Endocrinol. 1959;18:292–304. doi: 10.1677/joe.0.0180292. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online. 2004;8:45–58. doi: 10.1016/s1472-6483(10)60497-x. [DOI] [PubMed] [Google Scholar]
- Elbling L, Colot M. Abnormal development and transport and increased sister-chromatid exchange in preimplantation embryos following superovulation in mice. Mutat Res. 1985;147:189–195. doi: 10.1016/0165-1161(85)90057-3. [DOI] [PubMed] [Google Scholar]
- Ertzeid G, Storeng R. Adverse effects of gonadotrophin treatment on pre- and postimplantation development in mice. J Reprod Fertil. 1992;96:649–655. doi: 10.1530/jrf.0.0960649. [DOI] [PubMed] [Google Scholar]
- Field C, Li R, Oegema K. Cytokinesis in eukaryotes: a mechanistic comparison. Curr Opin Cell Biol. 1999;11:68–80. doi: 10.1016/s0955-0674(99)80009-x. [DOI] [PubMed] [Google Scholar]
- Fortier AL, Lopes FL, Darricarrere N, Martel J, Trasler JM. Superovulation alters the expression of imprinted genes in the midgestation mouse placenta. Hum Mol Genet. 2008;17:1653–1665. doi: 10.1093/hmg/ddn055. [DOI] [PubMed] [Google Scholar]
- Fowler RE, Edwards RG. Induction of superovulation and pregnancy in mature mice by gonadotrophins. J Endocrinol. 1957;15:374–384. doi: 10.1677/joe.0.0150374. [DOI] [PubMed] [Google Scholar]
- Fowler RE, Edwards RG. Effect of progesterone and oestrogen on pregnancy and embryonic mortality in adult mice following superovulation treatment. J Endocrinol. 1960;20:1–8. doi: 10.1677/joe.0.0200001. [DOI] [PubMed] [Google Scholar]
- Ghavami M, Mohammadnejad D, Beheshti R, Solmani-Rad J, Abedelahi A. Ultrastructural and Morphalogical Changes of Mouse Ovarian Tissues Following Direct Cover Vitrification with Different Cryoprotectants. J Reprod Infertil. 2015;16:138–147. [PMC free article] [PubMed] [Google Scholar]
- Gras L, McBain J, Trounson A, Kola I. The incidence of chromosomal aneuploidy in stimulated and unstimulated (natural) uninseminated human oocytes. Hum Reprod. 1992;7:1396–1401. doi: 10.1093/oxfordjournals.humrep.a137581. [DOI] [PubMed] [Google Scholar]
- Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the preimplantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Hamberger L, Wikland M. Regulations and results concerning assisted reproduction in Sweden. J Assist Reprod Genet. 1993;10:243–245. doi: 10.1007/BF01204934. [DOI] [PubMed] [Google Scholar]
- Hasegawa A, Takahashi T, Igarashi H, Amita M, Matsukawa J, Nagase S. Predictive factors for oocyte retrieval failure in controlled ovarian hyperstimulation protocols: a retrospective observational cohort study. Reprod Biol Endocrinol. 2015;13:53. doi: 10.1186/s12958-015-0052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann FP, Macklon NS, Fauser BC. A randomized comparison of two ovarian stimulation protocols with gonadotropin-releasing hormone (GnRH) antagonist cotreatment for in vitro fertilization commencing recombinant follicle-stimulating hormone on cycle day 2 or 5 with the standard long GnRH agonist protocol. J Clin Endocrinol Metab. 2003;88:166–173. doi: 10.1210/jc.2002-020788. [DOI] [PubMed] [Google Scholar]
- Huffman SR, Pak Y, Rivera RM. Superovulation induces alterations in the epigenome of zygotes, and results in differences in gene expression at the blastocyst stage in mice. Mol Reprod Dev. 2015;82:207–217. doi: 10.1002/mrd.22463. [DOI] [PubMed] [Google Scholar]
- Jang M, Lee EJ, Lee ST, Cho M, Lim JM. Preimplantation and fetal development of mouse embryos cultured in a protein-free, chemically defined medium. Fertil Steril. 2007;87:445–447. doi: 10.1016/j.fertnstert.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Kalthur G, Salian SR, Nair R, Mathew J, Adiga SK, Kalthur SG, Zeegers D, Hande MP. Distribution pattern of cytoplasmic organelles, spindle integrity, oxidative stress, octamer-binding transcription factor 4 (Oct4) expression and developmental potential of oocytes following multiple superovulation. Reprod Fertil Dev. 2015 doi: 10.1071/RD15184. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Kim KW. Visualization of micromorphology of leaf epicuticular waxes of the rubber tree Ficus elastica by electron microscopy. Micron. 2008;39:976–984. doi: 10.1016/j.micron.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Kulkarni GV, Lee W, Seth A, McCulloch CA. Role of mitochondrial membrane potential in concanavalin A-induced apoptosis in human fibroblasts. Exp Cell Res. 1998;245:170–178. doi: 10.1006/excr.1998.4245. [DOI] [PubMed] [Google Scholar]
- Labarta E, Bosch E, Alama P, Rubio C, Rodrigo L, Pellicer A. Moderate ovarian stimulation does not increase the incidence of human embryo chromosomal abnormalities in in vitro fertilization cycles. J Clin Endocrinol Metab. 2012;97:E1987–1994. doi: 10.1210/jc.2012-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge M, Sellens MH. Optimization of superovulation in the reproductively mature mouse. J Assist Reprod Genet. 1994;11:312–318. doi: 10.1007/BF02215719. [DOI] [PubMed] [Google Scholar]
- Luckett DC, Mukherjee AB. Embryonic characteristics in superovulated mouse strains. Comparative analyses of the incidence of chromosomal aberrations, morphological malformations, and mortality of embryos from two strains of superovulated mice. J Hered. 1986;77:39–42. doi: 10.1093/oxfordjournals.jhered.a110164. [DOI] [PubMed] [Google Scholar]
- Mancini M, Anderson BO, Caldwell E, Sedghinasab M, Paty PB, Hockenbery DM. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J Cell Biol. 1997;138:449–469. doi: 10.1083/jcb.138.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Market-Velker BA, Zhang L, Magri LS, Bonvissuto AC, Mann MR. Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet. 2010;19:36–51. doi: 10.1093/hmg/ddp465. [DOI] [PubMed] [Google Scholar]
- Miller BG, Armstrong DT. Effects of a superovulatory dose of pregnant mare serum gonadotropin on ovarian function, serum estradiol, and progesterone levels and early embryo development in immature rats. Biol Reprod. 1981;25:261–271. doi: 10.1095/biolreprod25.2.261. [DOI] [PubMed] [Google Scholar]
- Moor RM, Osborn JC, Crosby IM. Gonadotrophin-induced abnormalities in sheep oocytes after superovulation. J Reprod Fertil. 1985;74:167–172. doi: 10.1530/jrf.0.0740167. [DOI] [PubMed] [Google Scholar]
- Moore A, Weissleder R, Bogdanov A., Jr Uptake of dextran-coated monocrystalline iron oxides in tumor cells and macrophages. J Magn Reson Imaging. 1997;7:1140–1145. doi: 10.1002/jmri.1880070629. [DOI] [PubMed] [Google Scholar]
- Munoz I, Rodriguez de Sadia C, Gutierrez A, Blanquez MJ, Pintado B. Comparison of superovulatory response of mature outbred mice treated with FSH or PMSG and developmental potential of embryos produced. Theriogenology. 1994;41:907–914. doi: 10.1016/0093-691x(94)90506-e. [DOI] [PubMed] [Google Scholar]
- Nagy A, GM, Vintersten K, Behringer T. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor; 2003. [Google Scholar]
- Ozturk S, Yaba-Ucar A, Sozen B, Mutlu D, Demir N. Superovulation alters embryonic poly(A)-binding protein (Epab) and poly(A)-binding protein, cytoplasmic 1 (Pabpc1) gene expression in mouse oocytes and early embryos. Reprod Fertil Dev. 2016;28:375–383. doi: 10.1071/RD14106. [DOI] [PubMed] [Google Scholar]
- Plachot M. Genetic analysis of the oocyte--a review. Placenta. 2003;24(Suppl B):S66–69. doi: 10.1016/s0143-4004(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Pang LH, Li MQ, Xu J, Zhou X. Risk of chromosomal abnormalities in early spontaneous abortion after assisted reproductive technology: a meta-analysis. PLoS One. 2013;8:e75953. doi: 10.1371/journal.pone.0075953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Hiltunen M, Kauppinen A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process. Cell Signal. 2014;26:1598–1603. doi: 10.1016/j.cellsig.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, Muasher SJ. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. 2014;101:967–973. doi: 10.1016/j.fertnstert.2013.12.026. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Neri QV, Katagiri Y, Rosenwaks Z, Palermo GD. Effect of treating induced mitochondrial damage on embryonic development and epigenesis. Biol Reprod. 2005;72:584–592. doi: 10.1095/biolreprod.104.032391. [DOI] [PubMed] [Google Scholar]
- Templeton A, Morris JK. Reducing the risk of multiple births by transfer of two embryos after in vitro fertilization. N Engl J Med. 1998;339:573–577. doi: 10.1056/NEJM199808273390901. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Microtubule mediation of cytoplasmic and nuclear maturation during the early stages of resumed meiosis in cultured mouse oocytes. Proc Natl Acad Sci USA. 1991;88:5031–5035. doi: 10.1073/pnas.88.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J, Runner MN. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am J Anat. 1984;171:335–355. doi: 10.1002/aja.1001710309. [DOI] [PubMed] [Google Scholar]
- Van der Auwera I, D’Hooghe T. Superovulation of female mice delays embryonic and fetal development. Hum Reprod. 2001;16:1237–1243. doi: 10.1093/humrep/16.6.1237. [DOI] [PubMed] [Google Scholar]
- West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG, Prescott AR, Watts C. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- Yun YW, Yu FH, Yuen BH, Moon YS. Effects of a superovulatory dose of pregnant mare serum gonadotropin on follicular steroid contents and oocyte maturation in rats. Gamete Res. 1989;23:289–298. doi: 10.1002/mrd.1120230306. [DOI] [PubMed] [Google Scholar]
- Ziebe S, Bangsboll S, Schmidt KL, Loft A, Lindhard A, Nyboe Andersen A. Embryo quality in natural versus stimulated IVF cycles. Hum Reprod. 2004;19:1457–1460. doi: 10.1093/humrep/deh264. [DOI] [PubMed] [Google Scholar]


