Abstract
Human mesenchymal stem cells (MSCs) are currently being evaluated as a cell-based therapy for tissue injury and degenerative diseases. Recently, several methods have been suggested to further enhance the therapeutic functions of MSCs, including genetic modifications with tissue- and/or disease-specific genes.
The objective of this study was to examine the efficiency and stability of transduction using an adenoviral vector in human MSCs. Additionally, we aimed to assess the effects of transduction on the proliferation and multipotency of MSCs. The results indicate that MSCs can be transduced by adenoviruses in vitro, but high viral titers are necessary to achieve high efficiency. In addition, transduction at a higher multiplicity of infection (MOI) was associated with attenuated proliferation and senescence-like morphology. Furthermore, transduced MSCs showed a diminished capacity for adipogenic differentiation while retaining their potential to differentiate into osteocytes and chondrocytes. This work could contribute significantly to clinical trials of MSCs modified with therapeutic genes.
Keywords: adenoviral vector, mesenchymal stem cells, stemness
INTRODUCTION
Mesenchymal stem cells (MSCs) are self-renewing multipotent stem cells capable of differentiating into adipocytes, chondrocytes, and osteocytes (Pittenger et al., 1999). These cells have been widely explored as a therapeutic agent for various disorders ranging from tissue injury to degenerative diseases because of their regenerative properties (Boregowda and Phinney, 2012; Larsen and Lewis, 2011). The therapeutic mechanism of MSCs is currently thought to occur via paracrine signaling, where cells secrete biologically active molecules that exert beneficial effects on injured tissues (Chen et al., 2008) by promoting angiogenesis and regeneration while inhibiting fibrosis, apoptosis and inflammation (Hocking and Gibran, 2010; Meirelles Lda et al., 2009). Currently, there are more than 700 ongoing or completed clinical trials worldwide using MSCs, highlighting their potential as a cell-based therapy (www.clinicaltrials.gov, 2017).
Numerous clinical and preclinical studies have reported on the safety of MSCs as a cell-based therapy; however, the efficacy of these cells in vivo is limited because of their poor survival, engraftment, and signaling (Deng et al., 2016; Robey et al., 2008). Several approaches have been investigated to overcome these issues, as well as reduce the immune response and optimize factor secretion (Deuse et al., 2009; Kim et al., 2014; Moon et al., 2014; Wang et al., 2015).
Genetic engineering is a common approach to improve the in vivo performance of MSCs by enhancing their innate properties, such as migration, neuro-protection, lineage differentiation and therapeutic factor production (Chan-Il et al., 2013; Chang et al., 2010; Kim et al., 2008). Research demonstrates that MSCs can be modified to enhance their therapeutic effects using plasmid DNA (Byun et al., 2005; Haleem-Smith et al., 2005; Moutsatsos et al., 2001), retrovirus (Chan-Il et al., 2013; Chang et al., 2010; Kim et al., 2008), lentivirus (Lee et al., 2004; Totsugawa et al., 2002; Zhang et al., 2004), adeno-associated virus (Ju et al., 2004; Kumar et al., 2004; Lu et al., 2005), and adenovirus(Lou et al., 1999; Musgrave et al., 1999; Peterson et al., 2005). Nonviral plasmid DNA transfection method is clinically safe but associated with higher cytotoxicity and low transfection efficiency (Park et al., 2015). In comparison, retroviral and lentiviral transduction provide long-term transgene expression but are considered to be less safe because of their propensity to integrate into the host genome. Recombinant adenoviral transduction is a safe, efficient method to transiently over-express a high level of exogenous therapeutic genes in human cells both in vivo and in vitro (Breyer et al., 2001; Crystal, 1999; Garza-Veloz et al., 2013; Tsuda et al., 2003; Vorburger and Hunt, 2002). Adenoviral infection is mediated by the binding of viral fibers to Coxackie virus and adenovirus receptor (CAR) molecules expressed by host cells (Chen and Lee, 2014; Zhang and Bergelson, 2005). However, human MSCs express the relatively low levels of CAR molecules (Suzuki et al., 2014), and thus are fairly resistant to adenoviral infection. Increased viral titers can enhance transduction efficiency, but potentiates dose-dependent cytotoxicity (McMahon et al., 2006). Moreover, adenovirus has been shown to hinder the adipogenic differentiation of transduced MSCs; therefore, it is important to systematically assess the characteristics of transduced MSCs and transgene stability prior to in vivo application.
The present study investigated key MSC characteristics in cells transduced with human adenovirus serotype 5 harboring a GFP reporter, including surface marker expression, proliferative ability, and differentiation capacity, as well as exogenous gene expression stability.
MATERIALS AND METHODS
Isolation and culture of human MSCs
Human MSCs were derived from bone marrow isolated from the iliac crest of healthy 10- to 15-year-old donors undergoing bone marrow aspiration for future allogeneic transplantation as previously described (Kim et al., 2005). Briefly, mono-nucleated adherent cells were collected and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 mg/ml streptomycin and 10 ng/ml basic fibroblast growth factor (b-FGF, Thermo Fisher Scientific, USA). All MSCs used in experiments were from passage 5. All study protocols were approved by the Institutional Review Board of Ajou University, Medical Center (Korea).
Adenoviral transduction of hMSCs
Replication-defective recombinant human adenovirus type 5 harboring a GFP reporter under the control of an elongation factor 1 alpha-promoter (Ad-GFP) was purchased from Vector Biolabs (USA. For transduction experiments, MSCs were plated at a density of 5 × 104 cells per 100 mm dish in the presence of standard MSC growth media. After reaching 70–80% confluency, cells were transduced with adenovirus in 6 ml of growth media at 0, 1, 10, 25, 50, 100, and 200 MOI. After a 2 h incubation at 37°C, the transduction media was replaced with standard MSC growth media. Cells were then analyzed for GFP expression at 48 h post-infection by fluorescence microscopy using an Olympus 1×71 microscope (Olympus Optical Co. Ltd., Japan) at 200× magnification and flow cytometry with a BD FACS Vantage (BD Biosciences, USA). For flow cytometry, 10,000 events were acquired for each condition and the data analyzed using with Cell Quest Pro software (BD Biosciences). To assess GFP stability and cell proliferation after long-term culture, cells were cultured with a standard conditions (plating MSCs at density of 1 × 105 cells per 100 mm dish and sub-culturing upon 70–80% confluency) for 2 subsequent passages. At the end of each passages, cell proliferation was examined by trypan blue exclusion assay. In addition, the percentage of GFP-positive cells and the mean fluorescence intensity (MFI) was analyzed by flow cytometry.
Senescence associated β-galactosidase assay
MSCs transduced with Ad-GFP at 50 MOI were cultured for 1 passage under standard conditions and then plated at 1 × 105 cells per well in 6-well plates. The next day, cells were washed with phosphate-buffered saline (PBS), fixed in 10% formalin for 10 min at room temperature, rinsed twice with PBS, and incubated with senescence-associated β-galactosidase staining solution containing 5 mM potassium ferricyanide (K3[Fe(CN)6]), 5 mM potassium ferrocyanide (K4[Fe(CN)6]), 2 mM MgCl2 in 0.2M citric acid/PBS (pH6.0), and 0.5 mg/mL X-gal solution. Cells were incubated in a 37°C incubator for 12–16 h. X-gal staining was observed under phase contrast microscopy at 200× and merged with corresponding fluorescence images to identify double-positive cells.
Surface antigen analysis
Ad-GFP-transduced MSCs (50 MOI) or normal MSCs were harvested with 0.25% Trypsin/EDTA (Invitrogen), and resuspended in PBS containing 1% bovine serum albumin (BSA), and then stained with fluorochrome-conjugated antibodies against CD90, HLA-ABC, CD45, CD29, CD73, HLA-DR or isotype controls (Biolegend, USA) for 15 min at room temperature (RT). After washing with PBS containing 1% BSA, cells were analyzed with a BD FACS vantage. All assays included unstained and isotype controls.
Multi-lineage differentiation
Ad-GFP MSCs (50 MOI) were harvested 2 days post-transduction and subjected to osteogenic, adipogenic, and chondrogenic differentiation with a human MSC differentiation kit (Thermo Fisher). Normal, untransduced cells and non-induction media were used as controls. Briefly, cells were plated at 4 × 104 cells in 4-well plates in normal MSC culture medium and grown to confluency. The culture medium was then replaced with adipogenic or osteogenic differentiation media (STEMPRO, Thermo Fisher) for 12 days. Adipogenic and osteogenic differentiation was verified by oil-red O and Alizarin Red S staining, respectively. Alternatively, chondrogenic differentiation was induced by culturing 3 × 105 pelleted cells in differentiation medium (STEMPRO Chondrogenesis Differentiation Kit, Thermo Fisher) for 4 weeks. Paraffin embedded chondrogenic differentiated MSCs and Adeno-GFP-transduced MSCs pellets were sectioned at 5 μm thickness and mounted to coated slides for further GFP immunohistochemistry and Alcian blue staining procedures. Briefly, the sections were incubated with 1% H2O2 in PBS for 20 min, blocked with 10% normal goat serum for 1 h at room temperature, and then probed with a chicken anti-GFP antibody (1: 500, Abcam, UK) at 4°C overnight. After washing the primary antibody, the sections were incubated with biotinylated goat anti-chicken secondary antibody (1:200, Vector Laboratories) for 1 h and avidin-peroxidase conjugate (ABC Kit, Vector Laboratories, USA) solution for 30 min. The horseradish peroxidase reaction was detected with 0.05% diaminobenzidine (DAB) and 0.03% H2O2 in 50 mM Tris-HCl (pH 7.0). Sections were subsequently incubated with Alcian blue staining solution for 45 min at room temperature. Stained images were acquired using a Zeiss Axiophot microscope (Carl Zeiss, Germany).
Transgene stability under growth-restrictive conditions
Ad-GFP MSCs at 50 MOI were cultured under growth-restrictive conditions (i.e. high-density plating without passaging). Briefly, cells were harvested 2 days post-transduction with EDTA/trypsin and counted by trypan blue exclusion method. A total of 1.5 × 106 live cells were plated in 100-mm dish and cultured for 30 days at 37°C without passaging. Media was changed every 2 days. The stability of transgene expression was analyzed 30 days after plating by assessing the intensity and frequency of GFP expression by florescence microscopy and compared to cells after 1 day of high-density plating.
RESULTS
MSC transduction with adenoviral vector
To investigate adenovirus transduction efficiency, MSCs were transduced with Ad-GFP at various MOIs for 2 h and then examined at 48 h post-infection by fluorescence microscopy and flow cytometry. Results demonstrate a linear increase in the frequency of GFP-positive cells with increasing MOI with no GFP signal detected at 0 MOI and about 80% GFP-positive cells at 200 MOI. The histogram (Fig. 1A) and bar diagram (Fig. 1C) show the frequency of GFP-positive cells at the indicated transduction conditions. Merged fluorescence and phase contrast images of MSCs transduced at 200 MOI are shown in Fig. 1B. To enhance adenoviral transduction efficiency, MSCs were incubated with CAR Receptor Booster for 2 h. Analysis of GFP expression frequency and intensity at day 2 post-transduction showed a significant increase in transduction efficiency in the CAR booster-treated group (Supplementary Fig. S1).
Fig. 1.
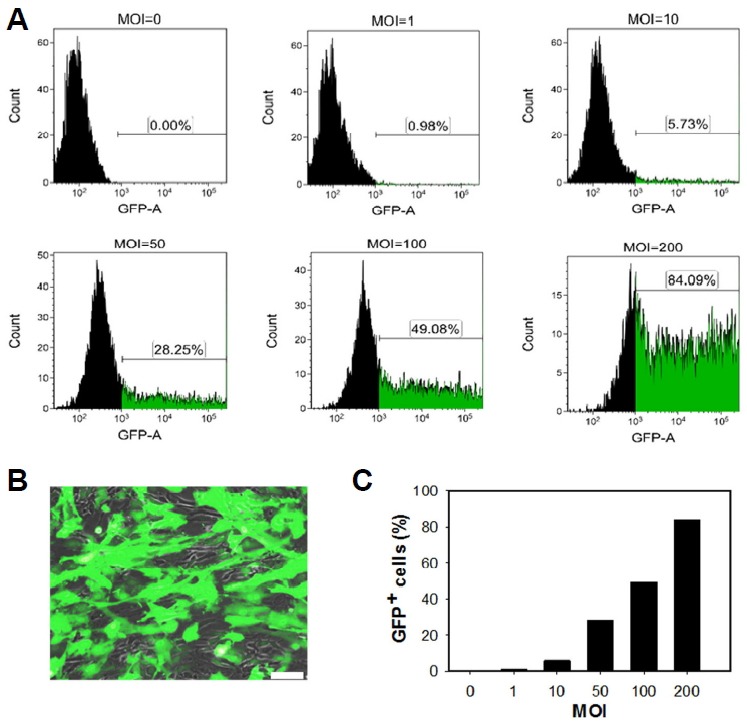
Adenoviral gene transduction of mesenchymal stem cells (MSCs).
(A) Fluorescence-activated cell-sorting analysis of MSCs transduced with GFP-expressing adenovirus (1–200 multiplicity of infection [MOI]) and untransduced controls. (B) Microscopy images of GFP expression in MSCs transduced at 200 MOI. Scale bar = 50 μm. (C) The percentage of GFP positive cells with increasing MOI.
Transgene expression and growth kinetics of Ad-GFP-transduced MSCs
To assess the effect of adenoviral transduction on the proliferation of cells, Ad-GFP-transduced MSCs were cultured under standard conditions for 2 passages. Cell counting studies revealed the linear decrease in the total cell numbers with increasing MOI after 12 days in culture (Figs. 2A and 2B). The number of GFP-positive cells and mean fluorescence intensity were also significantly decreased in subsequent passage (Figs. 2C and 2D). Normal MSCs exhibit fibroblastic morphology in adherent monolayer cultures. Consistently, MSCs retained a normal fibroblastic morphology at 2 days post-transduction that gradually changed to a flattened, oval, and enlarged shape, mostly in GFP-positive cells (arrows in Fig. 2A). Senescence-associated beta-galactosidase (SA-beta-gal) staining confirmed that these morphological changes were the result of cell senescence (Fig. 2F).
Fig. 2.
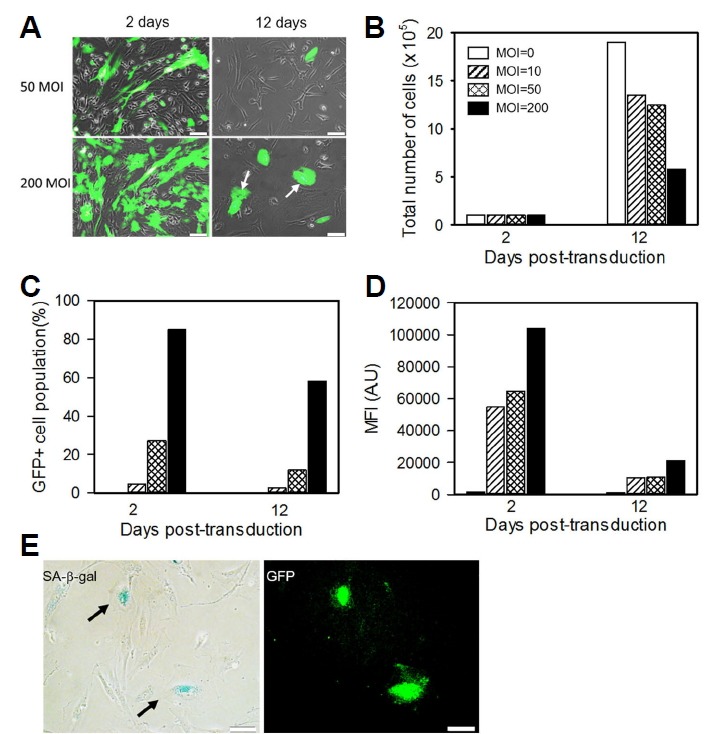
Transgene expression in Ad-GFP MSCs.
(A) Representative fluorescence images of transduced cells at 2 and 12 days post-transduction. GFP-positive cells display a larger cell size with flattened morphology (arrows). Scale bars = 50 μm (B-D) The total number of cells, frequency of GFP-positive cells, and mean GFP intensity were measured using flow cytometry. (E) Senescence-associated β-galactosidase activity was examined in transduced MSCs (MOI 50) and cultured for one passage. Arrows indicated positive X-gal staining. Scale bars = 50 μm.
Cell surface marker expression and multi-lineage differentiation
To evaluate the effects of adenoviral transduction on the MSC phenotype, cell surface marker expression and multilineage differentiation potential were examined. As shown in Fig. 3, GFP-positive cells and GFP-negative cells displayed similar phenotype profiles. Ad-GFP transduced MSCs were positive for CD105, CD29, CD73 and CD90 while being negative for HLA-DR, CD34. The population of surface marker and GFP double positive was similar to transduction efficiency of MSCs by Ad-GFP at 50 MOI.
Fig. 3.
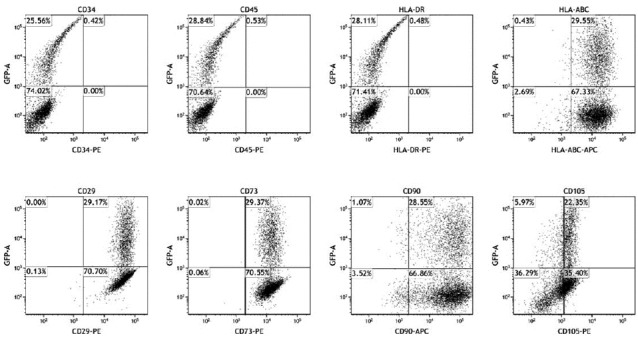
Surface antigen expression profiles of Ad-GFP MSCs.
MSCs transduced with GFP-expressing adenovirus (MOI at 50) were examined for MSC surface marker expression at 2 days post-transduction. Isotype antibodies served as negative control.
We also assessed the effect of adenoviral transduction on MSC differentiation into osteocytes, adipocytes, and chondrocytes. As expected, osteocytic cultures exhibited mineralization nodules over the MSC monolayer that stained positively with Alizarin S, confirming successful osteocyte differentiation (Figs. 4A and 4B). Similarly, GFP immunohistochemistry and Alcian blue staining revealed metachromatic extracellular matrix deposition in the GFP-positive chondrogenic MSC cultures (Figs. 4C and 4D). The chondrogenic spheroids in both the MSCs and Ad-GFP MSCs group were similarly sized and produced extracellular matrix to a similar extent (Supplementary Figs. S2A and S2B), suggesting that Ad-GFP MSCs and control MSCs could still differentiate into chondrogenic- and osteogenic-lineages. In contrast, the majority of GFP-positive cells lacked the capacity for adipogenic differentiation as shown by the absence of lipid vacuoles, which were readily apparent in GFP-negative and untransduced counterparts (Figs. 4E–4H and Supplementary Fig. S2C). Collectively, these data confirmed that Ad-GFP MSCs efficiently differentiated into chondrogenic and osteogenic lineages, whereas their ability for form adipocytes were markedly reduced.
Fig. 4.
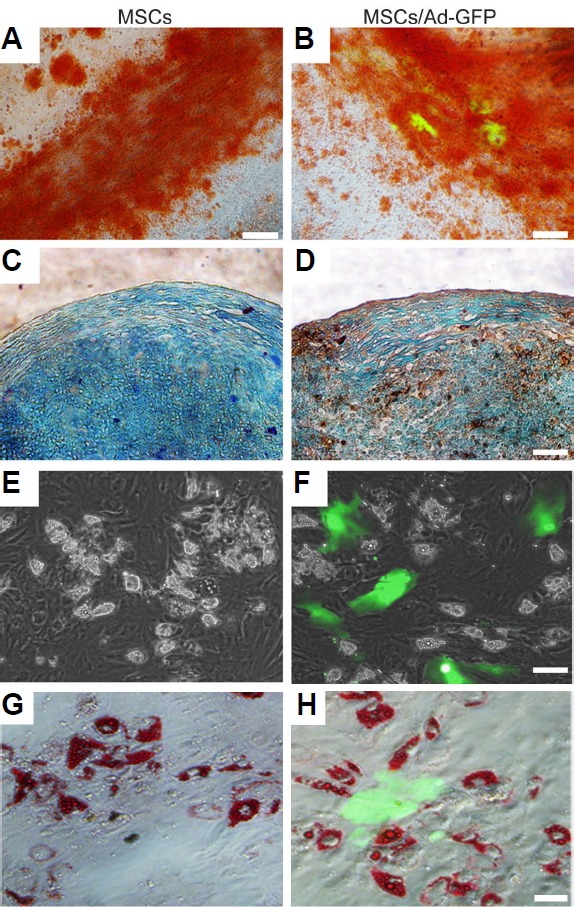
Mesodermal differentiation potential of Ad-GFP MSCs.
(A, B) Osteogenic differentiation was assessed by alizarin red S staining to visualize calcium deposits. (C, D) Chondrogenic differentiation was assessed by Alcian blue and immunohistochemical staining to detect glycosaminoglycans and GFP expression, respectively. Dark brown colored cells in (D) indicate GFP-positive cells. (E, F) Adipogenesis was monitored for intracellular lipid accumulation by microscopy and (G, H) oil-red O staining. All photomicrographs are the merges of GFP and bright field images. Scale bars = 50 μm.
Long-term transgene expression under growth-restricted conditions
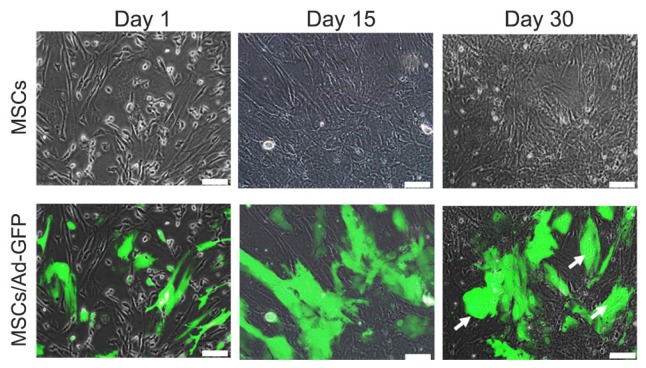
To examine long-term GFP expression stability, transduced MSCs were cultured for 30 days without passaging. Contrary to proliferating cultures, Ad-GFP MSCs subjected to growth-restrictive conditions showed stable GFP expression for at least 1 month in culture (Fig. 5). However, long-term cultured MSCs acquired a senescent morphology with enlarged cell size (Fig. 5, arrows).
Fig. 5.
Transgene stability in Ad-GFP MSCs under non-dividing culture conditions.
Long-term transgene stability was monitored after 30 days of culturing under conditions unfavorable to cell division (i.e, high-density plating with limited passaging). GFP expression was then assessed by fluorescence microscopy. Arrows indicate representative cells.
DISCUSSION
The present study demonstrates the dose-dependent transduction efficiency of GFP adenovirus. Most significantly, we found that transduced MSCs expressed typical MSC surface markers and effectively differentiated into osteocytes and chondrocytes, but showed attenuated proliferation and an impaired adipogenic differentiation capacity.
It is known that human primary MSCs are relatively resistant to wild-type adenoviral infection because of their low expression level of the adenoviral receptor CAR (Suzuki et al., 2014). In the present study, we found that MSCs could be transduced by adenovirus in a dose-dependent manner. Pretreatment of CAR Receptor Booster increased receptor density on the cell surface, which resulted in a significant increase in GFP- positive cell frequency and fluorescence intensity as compared to non-treated cells; this suggests that low CAR expression is a major limitation to adenoviral transduction in MSCs. Although MSCs exhibit low CAR expression, they can be transduced by increasing the dose of adenovirus, suggesting that additional receptors are present on the surface of MSCs. Adenovirus infection is mediated by CAR molecules via the knob domain of the fiber proteins and the major histocompatibility complex (MHC) class Ia-2 domain on the host cell surface (Breyer et al., 2001; Crystal, 1999; Gerard and Collen, 1997). Our MSCs expressed MHC class I (HLA-ABC) at high levels on their surface, which suggests that MHC class I may contribute to adenoviral transduction.
Recombinant adenoviruses are a widely used gene delivery system for ex vivo therapy (Lou et al., 1999; Musgrave et al., 1999; Peterson et al., 2005). Adenoviral vectors are known to be safe because they are episomal and therefore do not integrate into host chromosome. Hence, we observed that the number of GFP-positive cells and mean fluorescence intensity diminished with continued culturing, with < 1% GFP-positive cells present after 2 passages (data not shown). The decline in the number of GFP-positive cells over time can be explained by the replication deficiency of the adenoviral vector and by the fact that adenoviral vector does not integrate in the host cellular chromosome.
Surface antigenicity is an important criterion in transplantation therapy using stem cells because altered surface antigen expression in stem cells resulting from ex vivo manipulation can cause immune rejection of even autografted stem cells. Particularly, HLA-DR is an MHC class II cell surface receptor encoded by the human leukocyte antigen complex. The complex of HLA-DR and its ligand may lead to immune responses. Previously, it has been shown that the HLA-DR expression may be induced depending on the cultivation conditions of MSCs (Romieu-Mourez et al., 2007). Adenovirally-tranduced MSCs retain the classical MSC specific surface markers, i.e., CD29, CD73, CD90, and CD105, and are negative for CD34, CD45, and HLA-DR. Thus, it is noteworthy that transduction with adenoviral vectors does not trigger expression of HLA-DR and thus warrants a potential use of MSCs in ex-vivo therapy.
Most MSCs with high GFP fluorescence showed attenuated proliferation and flat cell morphology with some inclusion bodies, but remained GFP-positive in subsequent passages. These cells stained positive for SA-β-gal, indicative of cell senescence. It is possible that strong GFP protein disturbed the senescence mechanism because it is known that GFP has cellular cytotoxicity (Ansari et al., 2016). However, we found that the senescence-like flattened morphology also appeared in cells transduced with beta-galactosidase expressing adenovirus (data not shown); therefore, higher transduction of adenoviral vectors can effect the proliferation capability of MSCs.
Preserving the differentiation potential of MSCs after adenoviral transduction is important for ex vivo MSC therapy aimed at regeneration of mesenchymal tissues. Consistent with the results of previous studies, GFP-positive cells readily differentiated into osteocytes and chondrocytes (Bosch et al., 2006; Conget and Minguell, 2000); however, GFP-positive cells rarely differentiated into adipocytes in the presence of adipogenic stimuli, although other study reported that the adenovirus-transduced MSCs could still undergo adipogenic differentiation (Hung et al., 2004). Hung et al. (2004) suggested that transduced MSCs preserved adipogenic differentiation potential, but population of transduced cells in differentiated cells were decreased through the period of adipogenic induction. They couldn’t explain why the population of transduced cells decreased through adipogenic differentiation. In our study, very few GFP-positive cells were positive for oil red O staining (Supplementary Fig. S2D), supporting a diminished adipogenic differentiation capacity as compared to normal, non-transduced MSCs. We were unable to determine whether this difference was a direct result of adenovirus transduction or the transduced subpopulations had a restrictive differentiation potential.
Our findings suggest that adenovirus modification of MSCs could serve as reliable method to transiently express beneficial factors in MSCs without risking potential adverse effects of long-term transgene expression. Nevertheless, the development of proper transduction conditions should be determined before adenovirus-modified MSCs are transitioned for use in clinical settings.
Supplementary Information
ACKNOWLEDGMENTS
This research was supported by a grant (14172MFDS974 to S-SK and HS-K) from Ministry of Food and Drug Safety in 2016.
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Ansari AM, Ahmed AK, Matsangos AE, Lay F, Born LJ, Marti G, Harmon JW, Sun Z. Cellular GFP toxicity and immunogenicity: potential confounders in in vivo cell tracking experiments. Stem Cell Rev. 2016;12:553–559. doi: 10.1007/s12015-016-9670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boregowda SV, Phinney DG. Therapeutic applications of mesenchymal stem cells: current outlook. BioDrugs. 2012;26:201–208. doi: 10.1007/BF03261879. [DOI] [PubMed] [Google Scholar]
- Bosch P, Fouletier-Dilling C, Olmsted-Davis EA, Davis AR, Stice SL. Efficient adenoviral-mediated gene delivery into porcine mesenchymal stem cells. Mol Reprod Dev. 2006;73:1393–1403. doi: 10.1002/mrd.20593. [DOI] [PubMed] [Google Scholar]
- Breyer B, Jiang W, Cheng H, Zhou L, Paul R, Feng T, He TC. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1:149–162. doi: 10.2174/1566523013348689. [DOI] [PubMed] [Google Scholar]
- Byun HM, Suh D, Jeong Y, Wee HS, Kim JM, Kim WK, Ko JJ, Kim JS, Lee YB, Oh YK. Plasmid vectors harboring cellular promoters can induce prolonged gene expression in hematopoietic and mesenchymal progenitor cells. Biochem Biophys Res Commun. 2005;332:518–523. doi: 10.1016/j.bbrc.2005.04.155. [DOI] [PubMed] [Google Scholar]
- Chan-Il C, Young-Don L, Heejaung K, Kim SH, Suh-Kim H, Kim SS. Neural induction with neurogenin 1 enhances the therapeutic potential of mesenchymal stem cells in an amyotrophic lateral sclerosis mouse model. Cell Transplant. 2013;22:855–870. doi: 10.3727/096368912X637019. [DOI] [PubMed] [Google Scholar]
- Chang DY, Yoo SW, Hong Y, Kim S, Kim SJ, Yoon SH, Cho KG, Paek SH, Lee YD, Kim SS, et al. The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. Int J Cancer. 2010;127:1975–1983. doi: 10.1002/ijc.25383. [DOI] [PubMed] [Google Scholar]
- Chen RF, Lee CY. Adenoviruses types, cell receptors and local innate cytokines in adenovirus infection. Int Rev Immunol. 2014;33:45–53. doi: 10.3109/08830185.2013.823420. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol. 2008;40:815–820. doi: 10.1016/j.biocel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp Hematol. 2000;28:382–390. doi: 10.1016/s0301-472x(00)00134-x. [DOI] [PubMed] [Google Scholar]
- Crystal RG. In vivo and ex vivo gene therapy strategies to treat tumors using adenovirus gene transfer vectors. Cancer Chemother Pharmacol. 1999;43(Suppl):S90–99. doi: 10.1007/s002800051105. [DOI] [PubMed] [Google Scholar]
- Deng Y, Yang Z, Terry T, Pan S, Woodside DG, Wang J, Ruan K, Willerson JT, Dixon RA, Liu Q. Prostacyclin-producing human mesenchymal cells target H19 lncRNA to augment endogenous progenitor function in hindlimb ischaemia. Nat Commun. 2016;7:11276. doi: 10.1038/ncomms11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH, Eschenhagen T, Stein W, Wu JC, Robbins RC, et al. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009;120:S247–254. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- Garza-Veloz I, Romero-Diaz VJ, Martinez-Fierro ML, Marino-Martinez IA, Gonzalez-Rodriguez M, Martinez-Rodriguez HG, Espinoza-Juarez MA, Bernal-Garza DA, Ortiz-Lopez R, Rojas-Martinez A. Analyses of chondrogenic induction of adipose mesenchymal stem cells by combined co-stimulation mediated by adenoviral gene transfer. Arthritis Res Ther. 2013;15:R80. doi: 10.1186/ar4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard RD, Collen D. Adenovirus gene therapy for hypercholesterolemia, thrombosis and restenosis. Cardiovasc Res. 1997;35:451–458. doi: 10.1016/s0008-6363(97)00134-x. [DOI] [PubMed] [Google Scholar]
- Haleem-Smith H, Derfoul A, Okafor C, Tuli R, Olsen D, Hall DJ, Tuan RS. Optimization of high-efficiency transfection of adult human mesenchymal stem cells in vitro. Mol Biotechnol. 2005;30:9–20. doi: 10.1385/MB:30:1:009. [DOI] [PubMed] [Google Scholar]
- Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213–2219. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SC, Lu CY, Shyue SK, Liu HC, Ho LL. Lineage differentiation-associated loss of adenoviral susceptibility and Coxsackie-adenovirus receptor expression in human mesenchymal stem cells. Stem Cells. 2004;22:1321–1329. doi: 10.1634/stemcells.2003-0176. [DOI] [PubMed] [Google Scholar]
- Ju XD, Lou SQ, Wang WG, Peng JQ, Tian H. Effect of hydroxyurea and etoposide on transduction of human bone marrow mesenchymal stem and progenitor cell by adeno-associated virus vectors. Acta Pharmacol Sin. 2004;25:196–202. [PubMed] [Google Scholar]
- Kim MD, Kim SS, Cha HY, Jang SH, Chang DY, Kim W, Suh-Kim H, Lee JH. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp Mol Med. 2014;46:e110. doi: 10.1038/emm.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SS, Choi JM, Kim JW, Ham DS, Ghil SH, Kim MK, Kim-Kwon Y, Hong SY, Ahn SC, Kim SU, et al. cAMP induces neuronal differentiation of mesenchymal stem cells via activation of extracellular signal-regulated kinase/MAPK. Neuroreport. 2005;16:1357–1361. doi: 10.1097/01.wnr.0000175243.12966.f5. [DOI] [PubMed] [Google Scholar]
- Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, Chang DY, Cho KG, Kim SU, Huh Y, et al. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008;26:2217–2228. doi: 10.1634/stemcells.2008-0108. [DOI] [PubMed] [Google Scholar]
- Kumar S, Mahendra G, Nagy TR, Ponnazhagan S. Osteogenic differentiation of recombinant adeno-associated virus 2-transduced murine mesenchymal stem cells and development of an immunocompetent mouse model for ex vivo osteoporosis gene therapy. Hum Gene Ther. 2004;15:1197–1206. doi: 10.1089/hum.2004.15.1197. [DOI] [PubMed] [Google Scholar]
- Larsen S, Lewis ID. Potential therapeutic applications of mesenchymal stromal cells. Pathology. 2011;43:592–604. doi: 10.1097/PAT.0b013e32834ab72d. [DOI] [PubMed] [Google Scholar]
- Lee CI, Kohn DB, Ekert JE, Tarantal AF. Morphological analysis and lentiviral transduction of fetal monkey bone marrow-derived mesenchymal stem cells. Mol Ther. 2004;9:112–123. doi: 10.1016/j.ymthe.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Lou J, Xu F, Merkel K, Manske P. Gene therapy: adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 1999;17:43–50. doi: 10.1002/jor.1100170108. [DOI] [PubMed] [Google Scholar]
- Lu L, Zhao C, Liu Y, Sun X, Duan C, Ji M, Zhao H, Xu Q, Yang H. Therapeutic benefit of TH-engineered mesenchymal stem cells for Parkinson’s disease. Brain Res Brain Res Protoc. 2005;15:46–51. doi: 10.1016/j.brainresprot.2005.03.002. [DOI] [PubMed] [Google Scholar]
- McMahon JM, Conroy S, Lyons M, Greiser U, O’Shea C, Strappe P, Howard L, Murphy M, Barry F, O’Brien T. Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Dev. 2006;15:87–96. doi: 10.1089/scd.2006.15.87. [DOI] [PubMed] [Google Scholar]
- da Meirelles LS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Moon HH, Joo MK, Mok H, Lee M, Hwang KC, Kim SW, Jeong JH, Choi D, Kim SH. MSC-based VEGF gene therapy in rat myocardial infarction model using facial amphipathic bile acid-conjugated polyethyleneimine. Biomaterials. 2014;35:1744–1754. doi: 10.1016/j.biomaterials.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, Kelley P, Stumm N, Mi S, Muller R, et al. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther. 2001;3:449–461. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- Musgrave DS, Bosch P, Ghivizzani S, Robbins PD, Evans CH, Huard J. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone. 1999;24:541–547. doi: 10.1016/s8756-3282(99)00086-1. [DOI] [PubMed] [Google Scholar]
- Park JS, Suryaprakash S, Lao YH, Leong KW. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods. 2015;84:3–16. doi: 10.1016/j.ymeth.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, Lieberman JR. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–129. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu-Mourez R, François M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007;179:1549–58. doi: 10.4049/jimmunol.179.3.1549. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kawamura K, Li Q, Okamoto S, Tada Y, Tatsumi K, Shimada H, Hiroshima K, Yamaguchi N, Tagawa M. Mesenchymal stem cells are efficiently transduced with adenoviruses bearing type 35-derived fibers and the transduced cells with the IL-28A gene produces cytotoxicity to lung carcinoma cells co-cultured. BMC Cancer. 2014;14:713. doi: 10.1186/1471-2407-14-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsugawa T, Kobayashi N, Okitsu T, Noguchi H, Watanabe T, Matsumura T, Maruyama M, Fujiwara T, Sakaguchi M, Tanaka N. Lentiviral transfer of the LacZ gene into human endothelial cells and human bone marrow mesenchymal stem cells. Cell Transplant. 2002;11:481–488. [PubMed] [Google Scholar]
- Tsuda H, Wada T, Ito Y, Uchida H, Dehari H, Nakamura K, Sasaki K, Kobune M, Yamashita T, Hamada H. Efficient BMP2 gene transfer and bone formation of mesenchymal stem cells by a fiber-mutant adenoviral vector. Mol Ther. 2003;7:354–365. doi: 10.1016/s1525-0016(02)00062-x. [DOI] [PubMed] [Google Scholar]
- Vorburger SA, Hunt KK. Adenoviral gene therapy. Oncologist. 2002;7:46–59. doi: 10.1634/theoncologist.7-1-46. [DOI] [PubMed] [Google Scholar]
- Wang WQ, Dong K, Zhou L, Jiao GH, Zhu CZ, Li WW, Yu G, Wu WT, Chen S, Sun ZN, et al. IL-37b gene transfer enhances the therapeutic efficacy of mesenchumal stromal cells in DSS-induced colitis mice. Acta Pharmacol Sin. 2015;36:1377–1387. doi: 10.1038/aps.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The United States National Institutes of Health Clinical Trial Database 2017 www.clinicaltrials.gov.
- Zhang XY, La Russa VF, Reiser J. Transduction of bone-marrow-derived mesenchymal stem cells by using lentivirus vectors pseudotyped with modified RD114 envelope glycoproteins. J Virol. 2004;78:1219–1229. doi: 10.1128/JVI.78.3.1219-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bergelson JM. Adenovirus receptors. J Virol. 2005;79:12125–12131. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.