Abstract
Objective
The main objective of this study was to determine the effect of different diets for early-weaned (EW) calves on rumen development, and how this affects fat deposition in the longissimus dorsi of adult Korean Hanwoo beef cattle.
Methods
Three EW groups were established (each n = 12) in which two- week-old Hanwoo calves were fed for ten weeks with milk replacer+concentrate (T1), milk replacer+concentrate+ roughage (T2), or milk replacer+concentrate+30% starch (T3); a control group (n = 12) was weaned as normal. At six months, 5 calves of each group were slaughtered and their organs were assessed and rumen papillae growth rates were measured. The remaining calves (n = 7 in each group) were raised to 20 months for further analysis.
Results
Twenty-month-old EW calves had a higher body weight (BW), backfat thickness (BF), longissimus dorsi muscle area (LMA) and intramuscular fat (IMF) than the control (p<0.05). Organ growth, rumen histology, and gene expression patterns in the 6-month-old calves were positively related to the development of marbling in the loin, as assessed by ultrasound analysis (p<0.05). In the group fed the starch-enriched diet (T3), higher BW, BF, LMA, and IMF were present. The IMF beef quality score of 20-month-old cattle was 1+ for the T2 and T3 diets and 1 for the T1 diet (p<0.05).
Conclusion
Papillae development was significantly greater in calves fed on high-concentrate diets and this may have resulted in the improved beef quality in the EW dietary groups compared to the control.
Keywords: Calf, Early Weaning, Starch, Rumen Development, Ultrasound
INTRODUCTION
Early weaning of calves using a high energy diet can induce early postnatal metabolic imprinting and offers a possible means for improving carcass yield and beef quality. Lucas [1] defined metabolic imprinting as an epigenetic reaction to a nutritional challenge during early life that permanently changes physiological outcomes in later life. Generally, early weaning strategies use high energy concentrate diets for calves during the post-weaning feeding period [2]. Schoonmaker et al [3] reported that an ad libitum high-concentrate feed improved intramuscular fat (IMF) deposition in early-weaned (EW) steers during the growing stage; however, fat deposition rates were lower when they were fed the same diet in the finishing period. The EW bulls and steers deposit IMF, which enables fast and efficient growth, although considerable amounts of energy may be diverted to forming subcutaneous fat [4].
The utilization of starter diets for young calves at an early age is the best approach for improv ing both rumen papillae development and subsequent performance to attain a favorable growth rate [5]. Appropriate starter diets containing high levels of milk protein are the most suitable for rumen development. It is essential to acclimatize ruminal microorganisms to the increase in freely fermentable carbohydrates that may occur due to a sudden change from ad libitum access of a forage-related diet to a cereal-based diet; failure to do so can induce a host of metabolic disorders [6] that may have long term detrimental effects or may be lethal [7]. The National Research Council [8] announced that calf starter should be comparatively high in readily fermentable carbohydrates to support the fermentation required for suitable ruminal tissue growth. High energy concentrate feeds provide a greater capacity for calves to obtain the maximum dry matter intake (DMI), achieve a high average daily gain (ADG), and produce sufficient volatile fatty acids (VFAs) [9]. In calves fed high energy concentrate, the size of the rumen papillae increases due to the influence of starch, which is converted by microorganisms to VFA butyrate and alters the rumen to a slightly acidic state [10]. Generally rumen microorganisms multiply, proliferate, and produce energy in the form of VFA acetate, propionate and butyrate. VFAs, particularly butyrate, can stimulate papilla growth, and accelerate rumen motility and muscle growth [11]. Thus the length and width of the papillae and mucosa and the thickness of the rumen are excellent parameters to assess the effects of different diets on the development of the rumen.
An alternative strategy to a high energy diet is to wean the calves early and to feed them a high concentrate diet for a defined period to stimulate rumen development with rich VFAs before calves are switched to growing stage diets. The main objectives of this study was to measure the effect on beef calves of a short period diet that had high energy concentrates and was starch rich; various nutritional combinations were tested. The study sought to estimate the effects on Korean Hanwoo steers of a post-early weaning calf management system on growth performance and carcass characteristics.
MATERIALS AND METHODS
Ethics statement
Hanwoo cattle care and all experimental procedures were examined and permitted by the Institutional Animal Care and Use Committee of the National Institute of Animal Science (No. 2013-162).
Animals, diets, and experimental design
A total of 48 male Hanwoo (Korean native cattle, Bos taurus coreanae) calves were used in the experiment. The calves were purchased from commercial farms to obtain a uniform group of animals of the same age (15±4 days). Before weaning, all calves were fed milk by their mothers. The calves were randomly allotted to four treatments (each group n = 12; three pens per treatment, four calves per pen). Among four treatment groups, one group was separated, this control group was treated using the conventional feeding strategy of mother’s milk+roughage (Timothy hay). The control group calves stayed with their dams and were fed on roughage until the weaning period was finished. The remaining three metabolic imprinting treatment groups were fed with the following dietary treatments for 10 weeks: milk replacer (MR) +concentrate (T1); MR+concentrate+roughage (T2); and MR+ concentrate+30% starch (T3). Environmental temperature in the steady was maintained at least at 15°C. Calves were vaccinated for protection against various infections and animal health conditions were monitored daily by the animal caretakers. The ingredients and nutrient compositions of the high-concentrate diets are shown in Table 1. The composition of the concentrates was designed to meet the mineral and vitamin requirements for beef cattle [8]. The concentrates were offered from day 20 to 90 (10 weeks), starting at 1.5% of body weight (BW) and then regularly increased to provide ad libitum consumption for all metabolic imprinting treatment groups. For convenience, the weaning period was divided into 8 phases, each of 10 days. The quantity of formulated concentrate feed and MR was gradually increased from one phase to the next based on the calf average BW (Supplementary Table S1). Land O’ Lakes Product (US) MR was reconstituted with water in the weaning phase and was fed manually by bottle to the calves for the first few days and then using a bucket as soon as the calves were able to drink. The MR was supplied daily in two equally sized portions at 0700 and 1600 h. The solid feed (concentrate diet) was supplied directly after each milk meal using the same bucket. Timothy grass was provided ad libitum for T2 dietary treatment calves, from 31 days of the EW period. The MR and high concentrate diets were provided to all dietary groups at the same time. After providing 2 times per day, feed refusals were recorded daily for each calf. Water was provided ad libitum for all animals. After the EW period, the control and different dietary groups were combined (3 calves per pen) and fed the identical feed with ad libitum access to water in the cattle barn. Entire the experimental period, calfs BW and body condition score was measured every two weeks in the morning before feeding by using the 5-ping scale explained by Lowman et al [12]. Average daily feed intake (ADFI) was estimated by dividing the total feed intake by the days of experiment and the number of calves in each treatment group and this figure is expressed as dry mass (DM) basis. The ADG was concluded by dividing BW gain by the number of days on dietary feed.
Table 1.
Dry matter composition of diets. Ingredients and chemical composition of high concentrate diets provided to calves during the early weaning period
| Items | Concentrate1) | Starch2) 30% |
|---|---|---|
| Corn (%) | 13 | 30.4 |
| Wheat (%) | 10 | 10 |
| Beet pulp pellet (%) | 5 | 5 |
| Coconut oil meal (%) | 2.5 | - |
| Gluten feed (%) | 6 | 3 |
| Rice hull (%) | 1 | 1 |
| Wheat bran (%) | 32 | 14 |
| Wheat fl off (%) | 5 | 5 |
| Almond shell (%) | 3 | 2.89 |
| Soy hull (%) | - | 3.74 |
| Rapeseed meal (%) | 2 | 2 |
| Soybean meal (%) | 7.09 | 11.3 |
| Distiller dried grains (%) | 3 | 3 |
| Limestone (%) | 3 | 1.97 |
| Molasses cane (%) | 3 | 3 |
| Salt (%) | 0.6 | 0.6 |
| Mineral pyroxene (%) | 0.2 | 0.2 |
| Vitamin pyroxene (%) | 0.12 | 0.12 |
| Others (%) | 3.49 | 2.78 |
| SUM | 100 | 100 |
| Nutrient composition (%) | ||
| Dry matter | 87.88 | 87.49 |
| Crude protein | 15 | 15 |
| Ether extract | 2.81 | 2.71 |
| Crude fiber | 8.54 | 8.03 |
| Crude ash | 8.83 | 6.84 |
| Calcium | 1.36 | 1 |
| Phosphorus | 0.56 | 0.44 |
| Nitrogen free extract | 52.72 | 54.96 |
| Non fiber carbohydrate | 32.33 | 37.77 |
| Acid detergent fiber | 10.58 | 10 |
| Neutral detergent fiber | 28.91 | 25.18 |
| Total digestible nutrients | 67.84 | 72.41 |
Concentrate diet fed to all metabolic imprinting dietary treatment calves, such as T1 (milk replacer+concentrate), T2 (milk replacer+concentrate+roughages) and T3 (milk replacer+concentrate+30% starch). Weaning period at 20±4 days of age to 90±4 d of age.
Starch diet fed only imprinting calves of T3 (milk replacer+concentrate+30% starch) dietary treatment from weaning at 20±4 days of age to 90±4 d of age.
Assessment of the anatomical development
At six months, five calves were randomly selected from the control and each dietary treatment, and euthanized using captive-blot stunning and exsanguination. The remaining calves (n = 7) from each treatment were raised for further investigations. Internal organs such as liver, spleen, kidney, and heart were collected from all the treatments euthanized calves and washed with water and removed excess water with blotting papers then weighed. For the morphometric assessment of the digestive tract, the abdominal cavity was opened and removed separately for the four compartments of rumen-reticulum, omasum, and abomasum. The contents of the each compartment was emptied then rinsed repeatedly with water until clean, drained of excess water and weighed. The rumen surface area was measured with the help of a scanner (Scanjet 4C, Hewlett Packard, Palo Alto, CA, USA). Ruminal dissection was performed according to the procedure of McGavin and Morrill [13]. Four pieces of ruminal wall from the following nine pieces (~1.5 cm×1.5 cm) were collected from nine different areas based on Lesmeister et al [14]; these areas are right side (RB) and left side (LB) caudal dorsal sac; right side (RC) and left side (LC) cranial dorsal sac; right side (RD) and left side (LD) cranial ventral sac; caudal portion (A) of the caudal ventral blind sac; and right side (RE) and left side (LE) ventral portion of the caudal ventral blind sac. All samples were washed with double distilled water and placed in a falcon tube containing 10% buffered neutral formalin until analysis.
Ruminal histology
The histological measurement and consequent microscopic assessment of rumen mucosa and sites of sampling of the rumen wall for further morphometric examination were carried out as explained by Suarez et al [15]. Briefly, the ruminal samples were removed from the formalin, trimmed, and dehydrated thorough a graded ethanol series, immersed in xylol, and embedded in paraffin wax. Sections (5 μm) were cut from all tissues and the slides were stained with hematoxylin and eosin and periodic-acid Schiff. Rumen papillary length, width, and thickness of the muscle layers were measured on the stained sections using an optical microscope, magnification 12.5×. Twenty intact and well-oriented papillae were measured from each tissue sample. The measurements for each slide contain; the ratio of mucosa length to serosa length (RMSL) as a measure of the total absorptive surface, which was concluded as the length of the mucosal surface within a slide divided by the length of the corresponding serosa. Mucosa and rumen thickness was measured from at least three randomly selected sites per slide on each sample; the data from three sections were averaged for each location.
Analysis of gene expression by quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to measure mRNA transcript levels of three genes related to ruminal developmental. Total RNA was isolated from rumen epithelium tissue according to the kit manufacturer’s instructions (Qiagen, Hilden, Germany). One microgram of total RNA was added to a 20 μL reaction mixture for cDNA synthesis using the iNtRON maxime RT pre-Mix kit (Sungnam, South Korea). qRT-PCR was performed using an Applied Biosystems SYBR Green PCR Master Mix (Warrington, UK) on a Rotor-Gene 6000 Real-Time PCR system. Amplification was carried out with the following schedule: 1 cycle at 95°C for 10 min; 40 cycles at 95°C for 30 s, annealing at 55°C for 30 s, and 72°C for 30 s; 1 cycle of 72°C for 10 min. The primer sequences for 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2), aldo-keto reductase family 1, member C1-like (AKR1C1), and fatty acid binding protein 3 (FABP3) were obtained from a previous study [13]. mRNA levels were normalized to an endogenous control 18S rRNA gene. The 2−ΔΔCt method was used to determine the relative mRNA abundance.
Ultrasound data and image analysis
For measurement of the longissimus dorsi muscle area (LMA), backfat thickness (BF), and IMF in the remaining cattle of each group, ultrasound images of the right side of each animal were obtained. These images included the spinal column and perpendicular to the 12th and 13th ribs, and transversely over the LMA. They were taken from 12 to 20 months at 2 monthly intervals by a trained technician. The IMF score at the 13th rib can represent the fat content of the whole loin. Hair was removed and the skin surface was cleaned before ultrasound measurements and image capture. An Aloka SSD-500V ultrasound system equipped with a 12.5-cm 3.5-MHz transducer (Aloka Co. Ltd., Wallingford, CT, USA) was used; soybean oil was used as an aid to sound wave focusing medium. Ultrasound images of LMA, BF, and IMF were captured and measured using Cattle Performance Enhancement Company ultrasound image software (Image pro+, Media Cybernetics, Rockville, MD, USA). Ultrasound measurements of BF and LMA started at 12 months of age and IMF score was assessed from 18 months at 2 monthly intervals. The BF was not identified at 12 or 14 months of age but could be measured from 16 months, demonstrating that this metric is more appropriate for Hanwoo cattle aged at least 16 months old.
Statistical analysis
Growth performance parameters and ruminal parameters are presented as means±standard error. Different group means were compared using one-way analysis of variance followed by Duncan’s multiple range test, to identify differences among groups. Statistical significance was set as p<0.05.
RESULTS
Feed intake and growth performance
The ADFI of the treatment groups are presented in Table 2. At 2 weeks of age, the mean BW of calves did not show any significant differences among the control, T1, T2, and T3 dietary treatments. From 4 to 10 weeks, the T3 group calves had higher BW than control, T1 and T2 dietary treatments (Table 2). The ADG rates of the treatment groups over the experimental period are also presented in Table 2. Over the entire experimental period, the average daily BW gain was higher in control, followed by T3, both were higher than T1 and T2 treatment groups (p<0.05).
Table 2.
Average body weight, daily gain, and daily feed intake during the metabolic imprinting period, and the ruminal surface area of six-month-old calves from the control and dietary treatment group
| Items | Dietary treatments1) | |||
|---|---|---|---|---|
|
| ||||
| Control | T1 | T2 | T3 | |
| Body weight2) (kg) | ||||
| at 2 wk | 38.97±3.79 | 40.12±2.92 | 38.43±2.37 | 41.13±2.21 |
| at 4 wk | 49.63±4.02b | 40.32±3.02c | 40.02±2.37c | 50.86±1.92a |
| at 6 wk | 53.56±3.53b | 49.24±3.46c | 52.12±4.33b | 55.96±3.39a |
| at 8 wk | 60.68±3.62a | 54.21±3.31c | 57.41±3.44b | 61.21±2.99a |
| at 10 wk | 68.98±3.82a | 61.64±3.16b | 63.87±3.80c | 69.57±2.58a |
| ADG (kg/d) | 0.77±0.06a | 0.66±0.23c | 0.65±0.17c | 0.72±0.13b |
| ADFI (g/d) | NA | 1.03±0.12 | 1.00±0.13 | 0.99±0.04 |
| RSA (cm2) | 1,358±93.39c | 2,276±81.32a | 1,829±74.17b | 1,884±60.75b |
ADG, average daily gain; ADFI, average daily feed intake; NA, not applicable; RSA, rumen surface area.
Different dietary treatment groups.
Calves weight during the experimental period at different dietary treatment groups.
Values are mean with standard errors.
Within horizontal rows, means with different superscript letters are significantly different (p<0.05).
Internal organ development
Organ development was assessed in six-month old calves. The weights of the rumen-reticulum, omasum, and abomasum in the different treatment groups are shown in Table 3. The combined rumen-reticulum, omasum, and abomasum weights of the T1, T2, and T3 groups were greater than in the control group (p<0.05). Abomasum weight was higher in the T3 group than the T1 and T2 groups, which were similar to the control, while omasum showed large differences among EW dietary treatments than the control. We have also shown the weights of the duodenum, small and large intestines, liver, spleen, kidney, and heart in the Table 3. The duodenum, small and large intestine (except in the T2 group), and liver organ weights were higher (p<0.05) in the treatment groups than the control; the weights of these organs increased from the control to the T3 group. The remaining organs did not show significant variation between control and dietary treatment groups.
Table 3.
Average weight of various internal organs in six-month-old calves from the control and metabolic imprinted dietary groups
| Organ name1) | Dietary treatments2) | |||
|---|---|---|---|---|
|
| ||||
| Control | T1 | T2 | T3 | |
| Rumen and reticulum (kg) | 1.22±0.10c | 2.76±0.26a | 2.08±0.06b | 2.92±0.09a |
| Omasum (kg) | 0.17±0.02c | 0.53±0.06a | 0.45±0.04b | 0.50±0.07a |
| Abomasum (kg) | 0.45±0.01 | 0.45±0.01 | 0.45±0.04 | 0.50±0.04 |
| Duodenum (kg) | 0.10±0.00 | 0.10±0.01 | 0.12±0.01 | 0.14±0.04 |
| Small and large intestine (kg) | 2.77±0.24c | 2.66±0.14c | 2.93±0.12b | 3.26±0.22a |
| Liver (kg) | 1.32±0.04c | 1.52±0.10b | 1.56±0.12b | 1.62±0.09a |
| Spleen (kg) | 0.30±0.02 | 0.24±0.01 | 0.26±0.04 | 0.27±0.03 |
| Kidney (kg) | 0.37±0.03b | 0.38±0.02b | 0.33±0.06c | 0.43±0.02a |
| Heart (kg) | 0.53±0.03 | 0.49±0.05 | 0.56±0.04 | 0.57±0.03 |
Various internal organs of control and early weaned dietary treatment calves.
Different dietary treatments.
Values are mean with standard errors.
Within horizontal rows, means with different superscript letters are significantly different (p<0.05).
Rumen development
The EW treatment groups had a higher ruminal surface area than the control group (p<0.05) (Table 2). The T1 group had a larger surface area than the T2 and T3 groups. Papillae lengths, widths, mucosal thickness, and ruminal thickness in the nine sampled locations of the rumen are shown in Table 4. Papillae length and width were significantly greater (p<0.05) in most of sampled ruminal locations in the T1, T2, and T3 dietary groups when compared with the control group. Mucosal thickness and ruminal thickness were also significantly greater in most of locations in the T1, T2, and T3 dietary groups than in the control group. Papillae widths in five locations (LB, LC, LE, RB, and RC) and ruminal thickness in four locations (LB, LD, RE, and RD) did not show significant differences. Most of the ruminal parameters in the T1 and T3 groups were significantly higher than the control and T2 groups. Dorsal sac papillae from calves of all dietary groups are shown in Figure 1. The appearance of the papillae, in terms of length and width, changed from simple to complex in the control to T3 group. The frequency of clumped ruminal papillae at slaughter was larger in the EW treatment groups compared with the control group; the frequency of papillae fell from the T1 to T3 groups.
Table 4.
Mean dimensions of distinct physical regions in the rumen papillae tissues of six-month-old calves from the control and different metabolic imprinted groups
| Rumen area1) | Treatment2) | Papillae length (μm) | Papillae width (μm) | Mucosa thickness (μm) | Ruminal thickness (μm) |
|---|---|---|---|---|---|
| LB | Control | 793.17±135.05d | 265.65±21.04 | 1,311.19±160.95d | 3,578.51±254.80 |
| T1 | 1,942.52±145.60a | 350.73±18.26 | 3,924.99±297.32a | 6,815.86±501.48 | |
| T2 | 1,351.13±211.28b | 340.97±23.09 | 1,794.69±254.49b | 4,414.41±180.02 | |
| T3 | 981.41±95.53c | 346.70±10.42 | 1,561.43±185.54c | 4,623.24±74.94 | |
| LC | Control | 681.37±118.44d | 279.69±23.69 | 1,186.10±107.14d | 3,790.05±274.72d |
| T1 | 2,128.06±196.66a | 365.49±18.61 | 3,428.86±164.60b | 5,566.48±339.28b | |
| T2 | 1,495.35±42.11b | 356.52±18.36 | 1,734.16±60.75c | 4,789.86±266.27c | |
| T3 | 1,070.46±40.37c | 359.90±14.20 | 4,294.89±199.40a | 6,973.71±241.66a | |
| LD | Control | 1,735.91±190.43b | 304.42±10.29b | 2,421.37±223.46c | 5,402.20±443.31 |
| T1 | 2,147.65±275.63a | 324.98±25.12b | 3,488.07±301.45b | 6,043.09±345.24 | |
| T2 | 2,261.17±137.98a | 313.26±6.18b | 3,373.55±204.36b | 5,344.71±43.29 | |
| T3 | 1,734.77±130.48b | 433.73±17.76a | 3,716.89±181.38a | 6,617.41±213.43 | |
| A | Control | 547.33±61.86d | 226.46±7.26c | 1,047.49±109.68d | 3,301.74±134.44c |
| T1 | 1,377.72±129.14a | 337.03±14.48a | 3,230.90±160.28b | 6,581.31±249.79a | |
| T2 | 935.55±73.69c | 299.27±5.85b | 1,692.83±106.58c | 4,081.07±181.01b | |
| T3 | 1,118.72±22.25b | 361.87±5.62a | 4,014.20±280.76a | 6,542.01±220.41a | |
| LE | Control | 969.25±186.55c | 298.16±5.02 | 1,738.28±296.59b | 3,714.08±171.31 |
| T1 | 1,778.34±175.11a | 319.78±6.36 | 3,066.91±363.97a | 6,198.75±501.91 | |
| T2 | 808.25±23.59c | 285.27±8.15 | 1,503.75±91.14c | 4,158.11±117.5 | |
| T3 | 1,127.60±97.53b | 341.73±13.59 | 2,919.24±381.83a | 5,437.26±480.12 | |
| RE | Control | 980.76±103.70c | 309.04±18.11c | 1,501.10±148.13c | 4,711.04±118.83 |
| T1 | 2,121.90±255.52a | 351.77±18.71b | 2,957.39±292.09a | 6,223.50±419.17 | |
| T2 | 1,571.37±160.14b | 417.78±20.97a | 3,042.75±200.98a | 5,978.92±300.52 | |
| T3 | 1,070.07±59.65c | 416.73±25.85a | 2,723.22±288.91b | 6,402.19±400.53 | |
| RB | Control | 836.45±71.13c | 303.79±6.70 | 1,528.65±108.95c | 3,574.47±202.21c |
| T1 | 1,807.21±83.52a | 341.20±6.45 | 3,399.51±163.77a | 5,583.05±144.86a | |
| T2 | 1,439.37±93.65b | 324.05±4.21 | 2,276.74±147.12b | 5,165.37±293.05b | |
| T3 | 1,524.04±107.59b | 386.18±12.32 | 3,299.25±216.63a | 5,770.03±282.62a | |
| RC | Control | 417.65±18.24c | 307.79±16.44 | 1,114.80±52.22c | 3,506.54±146.41c |
| T1 | 942.84±102.14a | 366.51±4.78 | 2,166.25±235.27a | 4,655.20±213.14a | |
| T2 | 505.68±44.51b | 345.53±15.68 | 1,305.56±82.32b | 4,062.25±55.80b | |
| T3 | 428.87±19.25c | 309.93±3.75 | 1,247.87±126.81b | 3,520.89±163.40c | |
| RD | Control | 947.08±103.93d | 269.89±8.12c | 1,607.20±204.84d | 4,559.68±394.26 |
| T1 | 1,891.67±152.53a | 319.37±5.45b | 2,265.41±181.79c | 5,395.82±320.76 | |
| T2 | 1,638.51±88.54b | 282.96±11.33c | 2,947.93±139.56a | 6,038.54±29.53 | |
| T3 | 1,243.29±107.81c | 354.49±3.23a | 2,762.36±166.53b | 6,692.93±115.07 |
Different rumen physical areas: LB, left side caudal dorsal sac; LC, left side cranial dorsal sac; LD, left side cranial ventral sac; A, caudal portion of the caudal ventral blind sac; LE, left side ventral portion of the caudal ventral blind sac; RE, right side ventral portion of the caudal ventral blind sac; RB, right side caudal dorsal sac; RC, right side cranial dorsal sac; RD, right side cranial ventral sac.
Different dietary treatments.
Values are mean with standard errors.
Within vertical rows, means with different superscript letters are significantly different (p<0.05).
Figure 1.
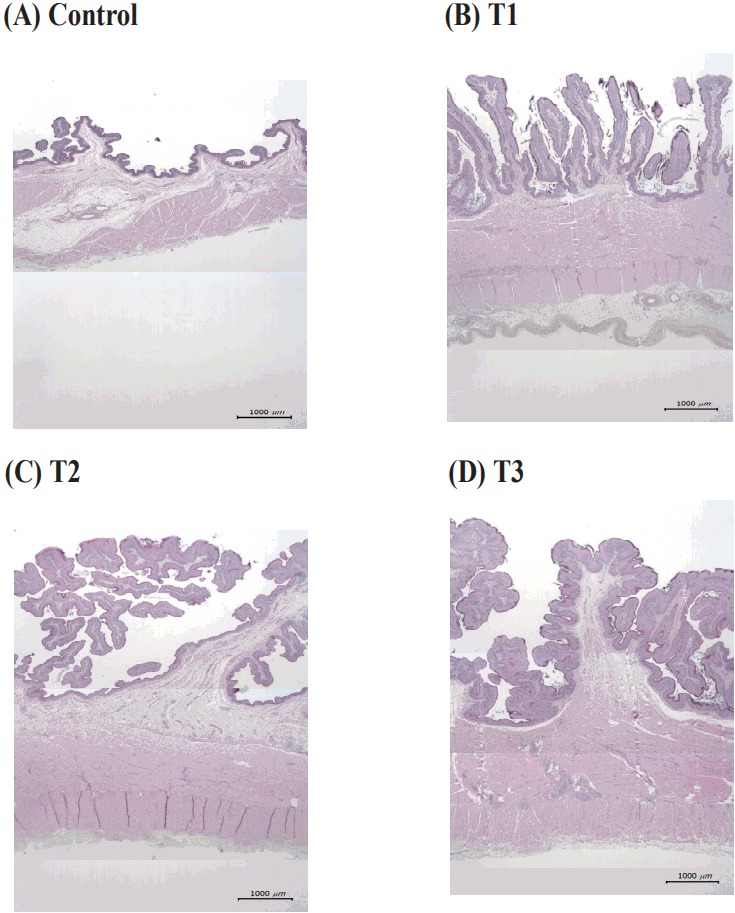
Images from representative rumen papillae in six-month-old calves from different dietary treatment groups. (A) Control = maternal milk+Timothy hay, (B) T1 = milk replacer+concentrate, (C) T2 = milk replacer+concentrate+Timothy hay, (D) milk replacer+concentrate+30% starch. All sections of the ruminal papillae were stained with hematoxylin and eosin; ×12.5 magnification; bar = 1,000 μm.
Analysis of rumen development gene expression studies by quantitative RT-PCR
In the present study, HMGCS2 expression gradually increased from the T1 to T3 group, when compared with the control group. Expression of HMGCS2 was ~3.5 fold higher in T1 and T2 groups than the control and ~5 fold higher in the T3 group (p = 0.05) (Figure 2). AKR1C1 was also up-regulated in T1 and T3 groups, but down-regulated in the T2 group, compared with the control group (p = 0.05). Expression of AKR1C1 was up-regulated ~1.5 fold in the T1 group and ~3 fold change in the T3 group compared to the control (p = 0.05). FABP3 expression was slightly higher in T1 and T3 groups and slightly lower in the T2 group compared with the control (p = 0.05). According to Kato et al [16], the levels of expression HMGCS2, AKR1C1, and FABP3 genes have been suggested as indicators of rumen development.
Figure 2.
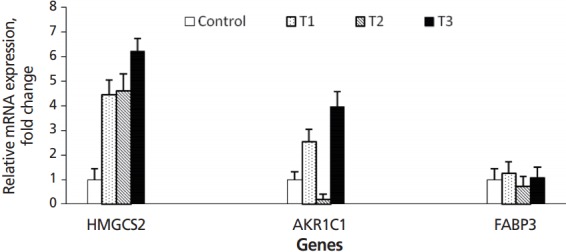
The expression levels of HMGCS2, AKR1C1, and FABP3 genes in rumen reticulum tissues of Hanwoo cattle. Quantitative real-time polymerase chain reaction analysis was performed on total RNA extracted from six months rumen epithelium tissues in control, T1, T2, and T3 dietary treatment calves. 18S rRNA was used as the internal control. All data were normalized using 18S rRNA gene and expressed as fold change over the value obtained from the control group calves. Values are mean±standard errors (represented by vertical bars). HMGCS2, 3-hydroxy-3-methylglutaryl-CoA synthase 2; AKR1C1, aldo-keto reductase family 1, member C1-like; FABP3, fatty acid binding protein 3.
Carcass measurement using ultrasound scanning and image analysis
The effects of the four treatments on BF, LMA, IMF in growing and fattening cattle are shown in Table 5. At 16, 18, and 20 months of age, BF was higher (p<0.05) in the T3 group cattle than the control, T1, or T2 groups (Table 5). The LMA growth rate increased from 12 to 20 months of age in all dietary and control groups (p<0.05). In particular, LMA growth rate was higher in the T3 group compared to the control and other dietary groups. At 18 and 20 months of age, the LMA growth rate was much higher than in previous months. The LMA growth rate in T1 and T2 groups was slightly higher than in the controls at any of the sampling intervals (p<0.05). The IMF score also steadily increased in dietary treatment groups compared with the control group. The increase in IMF score was high from 18 to 20 months of age in all treatment groups, particularly the T3 group. We compared our results with the Korean beef quality grading system based on the IMF score. The beef quality of 20 month old growing and fattening cattle in the T2 and T3 dietary treatments IMF was evaluated as grade 1+ and the T1 group was evaluated as grade 1 (p< 0.05). The BW of all growing stage cattle were higher in the EW treatments than the control group (p<0.05). From the outset of the experiment, BW gradually increased in all cattle groups (Table 5). At 16 to 20 months of age, BW differences increased between the control and high energy dietary groups.
Table 5.
Ultrasound data and body weights of control and different metabolic imprinted dietary groups at different growing stages
| Parameters2) | Cattle age1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 12 months | 14 months | 16 months | 18 months | 20 months | |||||||
|
|
|
|
|
|
|||||||
| LMA (cm2) | LMA (cm2) | BF (mm) | LMA (cm2) | BF (mm) | LMA (cm2) | IMF(%) | BF (mm) | LMA (cm2) | IMF(%) | ||
| Treatment name3) | Control | 35.8±1.59b | 37.16±1.70c | 2.1±0.78 | 44.9±2.50b | 3.2±1.19b | 49.6±3.62b | 9.92±1.07c | 4.56±1.23b | 53.33±4.61b | 15.0±3.51c |
| T1 | 36.03±1.63b | 38.2±2.35b | 2.02±0.76 | 45.1±7.36b | 2.63±0.62c | 47.25±1.94c | 11.28±0.28b | 3.74±0.71c | 53.91±5.89b | 14.71±1.92c | |
| T2 | 36.56±1.79b | 39.02±1.66b | 2.36±0.16 | 45.33±1.15b | 3.12±0.28b | 48.08±1.64c | 12.42±1.42a | 3.84±1.26c | 53.57±5.47b | 17.28±3.04b | |
| T3 | 39.31±3.08a | 43.16±2.30a | 2.74±0.49 | 47.78±2.30a | 4.32±0.69a | 52.16±3.15a | 12.83±1.64a | 5.46±0.97a | 60.38±9.20a | 20.2±5.35a | |
| Body weight (kg) | Control | 236.2±11.66b | 273.5±13.65b | 325.8±16.69c | 371.0±18.47c | 449.8±24.33c | |||||
| T1 | 238.5±12.10b | 268.0±11.88b | 338.9±13.43b | 387.3±15.89b | 471.0±20.72b | ||||||
| T2 | 248.1±19.75a | 284.8±19.05a | 344.3±19.05b | 397.2±18.49b | 468.6±24.35b | ||||||
| T3 | 249.6±12.99a | 288.2±10.89a | 355.1±10.89a | 409.0±16.60a | 493.0±12.68a | ||||||
LMA, longismuss muscle area; BF, Backfat; IMF, intramuscular fat.
Different growing and fattening stages of Hanwoo.
Different growing parameter analysis of Hanwoo.
Different dietary treatments: control = mother milk+roughages; T1 = milk replacer+concentrate; T2 = milk replacer+concentrate+roughages; T3 = milk replacer+concentrate+30% starch.
Values are mean with standard errors.
Within vertical rows, means with different superscript letters are significantly different (p<0.05).
DISCUSSION
In this study, we compared the growth and fattening performances of cattle that had been weaned early and fed different high energy concentrate feeds for 10 weeks with cattle that have been conventionally raised. The DMI did not vary significantly among the three high energy treatments during the EW period. This result is similar to that of Moya et al [17] who did not find any significant differences in DMI in heifers fed with grain barley and corn silage. Sarwar et al [18] suggested that DMI is inversely related to digestibility. We observed here that ADG was higher in the control and T3 dietary groups than the T1 and T2 groups. The reduced ADG for T1 and T2 may be due to differences in digestibility between solid feed and MR [19], and perhaps by the energy needed for rumen development. Generally, initiation of solid feed intake and subsequent ruminal fermentation results in an increase in rumen tissue mass and papillae growth [9]. According to Brown et al [20], ADG over both the first 28 d and the whole feeding stage was reduced by 8% after limiting intake of the final diet during adaptation compared with changing diet composition. We assume that some of our treatment groups show; gradual weaning of higher concentration and MR fed calves can enhance solid feed consumption, trigger rumen development and help increase energy intakes to support better BW gain during and after EW, as was also shown previously [21,22].
We also necessary to see if the rumen development is correlated with the BW gain of the different dietary treatment calf. But the rumen surface area and histology of the papillae, and also the length and width of the papillae per square centimeter were not correlated with the weight gain in some of the dietary treatment calf. So there were calves with higher rumen surface and histology; and good papillae length and width, containing rumen but still a low BW gain and also calves with a high BW gain, but with a rumen surface, histology, and papillae which were not well developed. It means that in this study, some of the weight gain of dietary calf could not be used to predict the development of rumen and with that the calf capacity to uptake high concentrate energy and protein from its diet. These results are not what we estimated that the better the rumen was developed the better the calf would grow because calf could absorb more energy.
According to Johnson et al [23], the size of visceral organs varies with the level of dietary intake. Oxygen utilization or energy spending in these organs rise after feeding and change in accordance with the level of feed intake [24]. In our experiment, the weights of the duodenum, small and large intestine, and liver increased gradually in control, T1, T2, and T3 dietary treatments, in that order. According to Ortigues and Doreau [25], all of these organs have a great influence on total oxygen utilization due to their high metabolic activity; modifications in the energy metabolism of these organs might have a profound effect on the effectiveness of energy consumption of the whole animal. Weight fluctuations in these organs appear to be directly proportional to the type of dietary intake [23].
In the current study, the weight of the rumen-reticulum was increased in the high energy treatments compared with the control group, in the order T3, T1, and T2. These outcomes may be due to better development of rumen muscle in response to dietary stimulation [25]. The higher rumen weights in T1, T2, and T3 calves compared to the control group support the interpretation that high energy and starch rich feeds can provide the necessary chemical stimuli to increase rumen-reticulum weight, physical capacity, and size in calves [26]. The similar rumen length, width, and wall thickness values in EW calves might be attributed to the use of MR, concentrates, Timothy hay, and starch. VFAs produced by fermentation of digested concentrate feed solids can stimulate increases in ruminal papillae length, width, and thickness in young calves [26]. Starch based (grains) starter diets can promote production of VFAs, particularly butyrate, and the associated low rumen pH is believed to activate papillae growth in the calf rumen wall [14]. Likewise, in this study, analysis of different location within the rumen showed differences among the treatment groups for papillae length, width, mucosa and ruminal thickness in the MR+concentrate feed (T1), MR+concentrate+roughage (T2), and MR+concentrate +30% starch (T3) dietary treatment groups compared to the control group (Table 4). Experimental evidence indicates that intake of roughage stimulates the development of the reticulo-rumen as well as increasing the weight, size, and thickness of tissues and the growth of normal papillae [27]. However, feed concentrates that are rich in starch have a greater effect in stimulating papillae than roughage in early life.
Direct visual examination of the ruminal papillae showed no clear differences in shape, length, or width between different diets. However, microscopic analysis showed that dorsal sac papillae from calves fed with mother’s milk (control) were uniform, flattened, and tongue-shaped (Figure 1A), while those of calves from the T1, T2, and T3 groups were irregular, thicker, and starting to branch (Figure 1B, C, D). Increased branching and papillae thickness were present after the high starch treatment (T3) compared to the other groups. In a previous report, Bartle and Preston [28] found that steers on a high concentrate feed had a higher incidence of clumped ruminal papillae at slaughter compared to animals on low concentrate feeds. The development of branches may be an adaptation to increase surface area and overcome decreased absorption due to parakeratosis. Our findings here are similar to those of McGavin and Morrill [13], who reported that papillae in the cranial region were very sensitive to the type of diet, and that dietary feed particles might float and form a hay mat, which might give more physical stimulation in the dorsal sac than in the ventral or cranial sacs.
The AKR1C1, HMGCS2, and FABP3 genes are associated with regulation of rumen development during early weaning. Here, we found that expression of HMGCS2 was significantly higher in the rumen in the T1, T2, and T3 groups than in the control group. This gene also plays a significant role in ketogenesis in the sheep rumen epithelium during development, and is up-regulated in the rumen epithelium of EW Holstein calves and Japanese Black male calves [29,16] Augmented production of VFAs influenced by the intake of solid feed during weaning may encourage ketogenesis in cattle rumen epithelial cells by the activation of HMGCS2 and peroxisome proliferator-activated receptor alpha to encourage papillary growth [29]. Our results indicate that HMGCS2 regulates efficient energy spending in response to enhanced levels of VFAs and long-chain fatty acids as a consequence of the introduction of a starch rich concentrate feed into the rumen. When compared with the control, AKR1C1 expression was up-regulated in T1 and T3 dietary treatments and down-regulated in the T2 group. AKR1C1 is a member of aldo-keto reductase superfamily and catalyzes the breakdown of aldehydes, ketones, monosaccharides, ketosteroids, and prostaglandins. In general, solid concentrate feed encourages an increase in VFA production, elevates the rate of transformation of ketone bodies, and reduces the pH in the rumen. Therefore, we hypothesize that AKR1C1 performs an important function in the regulation of intra-ruminal pH levels by decreasing the formation of ketone bodies [16]. The FABP3 gene did not show any obvious change in the T1 and T3 groups, but was down-regulated in the T2 group compared to the control group. According to Wang et al [30], FABP3 inhibits cell growth and propagation by suppressing the cell cycle and down-regulates cell growth in human bone marrow. From our results, the decreased FABP3 expression after weaning in the roughage (T2) group might activate proliferation of rumen epithelial cells.
Analyses of the final carcass using ultrasound showed that BF thickness, LMA, and IMF scores were higher in the T1, T2, and T3 treatments than in the control group, especially in the starch rich dietary treatment (T3). The T2 and T3 groups had a beef IMF of 1+ while the T1 group had a grade 1 quality. Similarly, accelerated finishing systems for EW bulls and steers have produced carcasses with consistently high marbling scores [31]. According to Smith and Crouse [32], glucose supplies 50% to 75% of the acetyl units for IMF deposition, and increased serum insulin would probably lead to improved uptake of glucose by peripheral tissues. Consequently, starch fermentation may result in increased blood glucose and insulin levels and may be a crucial component in activating intramuscular adipocyte growth in young calves fed a high-starch diet. However, feeding EW calves a high-grain diet might accelerate physiological maturity and, in consequence, produce extremely fat or lightweight carcasses [31]. A previous study revealed that steers fed a high concentrate diet ad libitum and a high-forage diet ad libitum have the largest LMA at 218 d, whereas limit-fed steers showed little LMA [3]. That study also showed that the marbling score of EW cattle at slaughter was not much affected by feeding treatment during the growing phase. Physiological maturity was accelerated in cattle fed a high-concentrate diet throughout the trial owing to their elevated growth rate. Our results here showed some difference to earlier studies; these differences might be a consequence of variations in the time of slaughter (BW, age, LMA, BF, and IMF score), calf age, and breed, or in the EW diet composition (e.g., MR, high concentration), or interaction among these factors.
The MR plays a vital role in EW treatment for calves, and con tributes to the development of BF, LMA, and IMF at growing and fattening stages of cattle. Recently, researchers have shown that enhancements in growth and feed efficiency can be acquired by feeding greater quantities of MR or augmented concentration of nutrients in MR [33]. High concentrates and corn-related diets have been revealed to improve BF and IMF and resulting marbling score compared with fiber related diets when used in accelerated finishing systems as part of an EW program [4]. We hypothesize that feeding an increased energy diet containing high concentrate and starch content will lead to an increase in rumen digestibility, cause ruminal fermentation to favor larger propionate production and higher absorption, and result in higher growing and fattening performances. We predict this increase in ruminal propionate concentration in calves at early stages corresponds to the increased BF, LMA, and IMF score in the body, and also influences BW gain in the EW-high energy concentrate feed groups in later growing and fattening stages. Thus, EW in combination with providing a concentrate with high starch to calves at an early stage is an essential dietary regimen to improve BF, LMA, IMF score, and final carcass quality.
CONCLUSION
This experiment found that metabolic imprinting in response to nutritional stimulation during early life through application of readily digestible high energy diets for EW calves, altered meat quality parameters in later life, in particular BF, LMA, and IMF scores. Growth performance, organ development, and ruminal data in calves slaughtered at six months supported the results of the BF, LMA, and IMF analyses. In the future, we shall perform transcriptome studies for IMF, subcutaneous, and omental fats; and we shall also analyze the carcass quality and quantity at final slaughter. Our initial results may indicate that EW with a high energy rich in starch might be feasible for production of high quality marbling beef.
Supplementary Data
ACKNOWLEDGMENTS
This work was carried out with the support of “Research Program for Agriculture Science & Technology Development (Project No. PJ01203101)” National Livestock Research Institute, Rural Development Administration, Republic of Korea.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Lucas A. Programming by early nutrition a man. Ciba Found Symp. 1991;156:38–50. [PubMed] [Google Scholar]
- 2.Arnett AM, Dikeman ME, Daniel MJ, et al. Effects ofvitamin A supplementation and weaning age on serum and liver retinol concentrations, carcass traits, and lipid composition in market beef cattle. Meat Sci. 2009;81:596–606. doi: 10.1016/j.meatsci.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Schoonmarker JP, Cecava MJ, Faulkner FL, et al. Effect of source of energy and rate of growth on performance, carcass characteristics, ruminal fermentation, and serum glucose and insulin of early-weaned steers. J Anim Sci. 2003;81:843–55. doi: 10.2527/2003.814843x. [DOI] [PubMed] [Google Scholar]
- 4.Myers SE, Faulkner DB, Ireland FA, Berger LL, Parrett DF. Production systems comparing early weaning to normal weaning with or without creep feeding for beef steers. J Anim Sci. 1999;77:300–10. doi: 10.2527/1999.772300x. [DOI] [PubMed] [Google Scholar]
- 5.Bach AA, Gimenez J, Juaristi L, Ahedo J. Effects of physical from of a starter for dairy replacement calves on feed intake and performance. J Dairy Sci. 2007;90:3028–33. doi: 10.3168/jds.2006-761. [DOI] [PubMed] [Google Scholar]
- 6.Cheng KJ, McAllister TA, Popp JD, et al. A review of bloat in feedlot cattle. J Anim Sci. 1998;76:299–308. doi: 10.2527/1998.761299x. [DOI] [PubMed] [Google Scholar]
- 7.Nagaraja TG, Chengappa MM. Liver abscess in feedlot cattle: A review. J Anim Sci. 1998;76:287–98. doi: 10.2527/1998.761287x. [DOI] [PubMed] [Google Scholar]
- 8.National Research Council (NRC) Nutrient requirements of beef cattle. 7th rev. ed. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 9.Suarez BJ, Van Reenen CG, Stockhofe N, Dijkstra J, Gerrits WJJ. Effect of roughage source and roughage to concentrate ratio on animal performance and rumen development in veal calves. J Dairy Sci. 2007;90:2390–403. doi: 10.3168/jds.2006-524. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Hidaka K, Mishima T, et al. Effect of sugar supplementation on rumen protozoa profile and papillae development in retarded growth calves. J Vet Med Sci. 2010;72:1471–4. doi: 10.1292/jvms.09-0399. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen NB, Sehested J, Jensen SK, Vestergaard M. Effect of milk allowance on concentrate intake, ruminal environment, and ruminal development in milk-fed holstein calves. J Dairy Sci. 2007;90:4346–55. doi: 10.3168/jds.2006-885. [DOI] [PubMed] [Google Scholar]
- 12.Lowman BG, Scott NA, Somerville SH. Condition scoring for cattle. Edinburgh, UK: East of Scotland College of Agriculture; 1976. (Tech. Bull. No. 6). [Google Scholar]
- 13.McGavin MD, Morrill JL. Scannig electron microscopy of ruminal papillae in calves fed various amounts and forms of roughage. Am J Vet Res. 1976;37:497–508. [PubMed] [Google Scholar]
- 14.Lesmeister KE, Heinrichs AJ. Effects of corn processing on growth characteristics, rumen development and rumen parameters in neonatal dairy calves. J Dairy Sci. 2004;87:3439–50. doi: 10.3168/jds.S0022-0302(04)73479-7. [DOI] [PubMed] [Google Scholar]
- 15.Suárez BJ, Van Reenen CG, Gerrits WJJ, et al. Effects of supplementing concentrates differing in carbohydrate composition in veal calf diets: II. Rumen development. J Dairy Sci. 2006;89:4376–86. doi: 10.3168/jds.S0022-0302(06)72484-5. [DOI] [PubMed] [Google Scholar]
- 16.Kato D, Suzuki Y, Haga S, et al. Utilization of digital differential display to identify differentially expressed genes related to rumen development. Anim Sci J. 2016;87:584–90. doi: 10.1111/asj.12448. [DOI] [PubMed] [Google Scholar]
- 17.Moya D, Mazzenga A, Holtshausen L, et al. Feeding behavior and ruminal acidosis in beef cattle offered a total mixed ration or dietary components separately. J Anim Sci. 2001;89:520–30. doi: 10.2527/jas.2010-3045. [DOI] [PubMed] [Google Scholar]
- 18.Sarwar M, Firkins JL, Eastridge ML. Effect of replacing neutral detergent fibre of forage with soy hulls and corn gluten feed for dairy heifers. J Dairy Sci. 1991;74:1006–17. [Google Scholar]
- 19.Labussiere E, Dubois S, van Milgen J, Bertrand G, Noblet J. Effect of solid feed on energy and protein utilization in milkfed veal calves. J Anim Sci. 2009;87:1106–19. doi: 10.2527/jas.008-1318. [DOI] [PubMed] [Google Scholar]
- 20.Brown MS, Ponce CH, Pulikanti R. Adaptation of beef cattle to high-concentrate diets: Perfrormance and ruminal metabolism. J Anim Sci. 2006;84:E25–E33. doi: 10.2527/2006.8413_supple25x. [DOI] [PubMed] [Google Scholar]
- 21.Roth BA, Keil NM, Gygax L, Hillmann E. Influence of weaning method on health status and rumen development in dairy calves. J Dairy Sci. 2009;92:645–56. doi: 10.3168/jds.2008-1153. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney BC, Rushen JP, Weary DM, de Passille AMB. Duration of weaning, starter intake, and weight gain of dairy calves fed large amounts of milk. J Dairy Sci. 2010;93:148–52. doi: 10.3168/jds.2009-2427. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DE, Johnson KA, Baldwin RL. Changes in liver and gastrointestinal tract energy demands in response to physiological workload in ruminants. J Nutr. 1990;120:649–55. doi: 10.1093/jn/120.6.649. [DOI] [PubMed] [Google Scholar]
- 24.Seal CJ, Reynolds CK. Nutritional implications of gastrointestinal and liver metabolism in ruminants. Nutr Res Rev. 1993;6:185–208. doi: 10.1079/NRR19930012. [DOI] [PubMed] [Google Scholar]
- 25.Ortigues I, Doreau M. Responses of the splanchnic tissues of ruminants to changes in intake: absorption of digestion end products, tissue mass, metabolic activity and implications to whole animal energy metabolism. Ann Zootech. 1995;44:321–46. [Google Scholar]
- 26.Khan MA, Lee HJ, Lee WS, et al. Starch source evaluation in calf starter: II. Ruminal parameters, rumen development, nutrient digestibilities, and nitrogen utilization in Holstein calves. J Dairy Sci. 2008;91:1140–49. doi: 10.3168/jds.2007-0337. [DOI] [PubMed] [Google Scholar]
- 27.Warner RG, Flatt WP. Physiology of digestion in the ruminant. London UK: Butterworths Pub. Co; 1965. [Google Scholar]
- 28.Bartle SJ, Preston RL. Roughage level and limited maximum intake regimens for feedlot steers. J Anim Sci. 1992;70:3293–303. doi: 10.2527/1992.70113293x. [DOI] [PubMed] [Google Scholar]
- 29.Connor EE, Baldwin RL, Li CJ, Li RW, Chung H. Gene expression in bovine rumen epithelium during weaning identifies molecular regulators of rumen development and growth. Funct Integr Genomics. 2013;13:133–42. doi: 10.1007/s10142-012-0308-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Zhou Y, Andreyev O, et al. Overexpression of FABP3 inhibits human bone marrow derived mesenchymal stem cell proliferation but enhances their survival in hypoxia. Exp Cell Res. 2014;323:56–65. doi: 10.1016/j.yexcr.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoonmaker JP, Loerch SC, Fluharty FL, Zerby HN, Turner TB. Effect of age at feedlot entry on performance and carcass characteristics of bulls and steers. J Anim Sci. 2002;80:2247–54. doi: 10.2527/2002.8092247x. [DOI] [PubMed] [Google Scholar]
- 32.Smith SB, Crouse JD. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J Nutr. 1984;114:792–800. doi: 10.1093/jn/114.4.792. [DOI] [PubMed] [Google Scholar]
- 33.Brown EG, Vandehaar MJ, Daniels KM, et al. Effects of increasing energy and protein intake on body growth and carcass composition of heifer calves. J Dairy Sci. 2005;88:585–94. doi: 10.3168/jds.S0022-0302(05)72722-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


