Abstract
Objective
The effects of vaccinating 18-day-old chicken embryos with the combination of recombinant Eimeria profilin plus Clostridium perfringens (C. perfringens) NetB proteins mixed in the Montanide IMS adjuvant on the chicken immune response to necrotic enteritis (NE) were investigated using an Eimeria maxima (E. maxima)/C. perfringens co-infection NE disease model that we previously developed.
Methods
Eighteen-day-old broiler embryos were injected with 100 μL of phosphate-buffered saline, profilin, profilin plus necrotic enteritis B-like (NetB), profilin plus NetB/Montanide adjuvant (IMS 106), and profilin plus Net-B/Montanide adjuvant (IMS 101). After post-hatch birds were challenged with our NE experimental disease model, body weights, intestinal lesions, serum antibody levels to NetB, and proinflammatory cytokine and chemokine mRNA levels in intestinal intraepithelial lymphocytes were measured.
Results
Chickens in ovo vaccinated with recombinant profilin plus NetB proteins/IMS106 and recombinant profilin plus NetB proteins/IMS101 showed significantly increased body weight gains and reduced gut damages compared with the profilin-only group, respectively. Greater antibody response to NetB toxin were observed in the profilin plus NetB/IMS 106, and profilin plus NetB/IMS 101 groups compared with the other three vaccine/adjuvant groups. Finally, diminished levels of transcripts encoding for proinflammatory cytokines such as lipopolysaccharide-induced tumor necrosis factor-α factor, tumor necrosis factor superfamily 15, and interleukin-8 were observed in the intestinal lymphocytes of chickens in ovo injected with profilin plus NetB toxin in combination with IMS 106, and profilin plus NetB toxin in combination with IMS 101 compared with profilin protein alone bird.
Conclusion
These results suggest that the Montanide IMS adjuvants potentiate host immunity to experimentally-induced avian NE when administered in ovo in conjunction with the profilin and NetB proteins, and may reduce disease pathology by attenuating the expression of proinflammatory cytokines and chemokines implicated in disease pathogenesis.
Keywords: Eimeria, Profilin, Clostridium perfringens, Necrotic Enteritis, Immunity, In ovo Vaccination
INTRODUCTION
Necrotic enteritis (NE) is one of the most important enteric infectious diseases affecting global poultry production with an estimated annual economic loss of more than $2 billion, largely attributable to increased costs associated with medical treatments and impaired growth performance [1,2]. Host-pathogen interaction in NE is complex and the nature of host and pathogen genetic factors implicated in NE pathogenesis is still unknown [3,4]. NE is caused by infection with toxin-producing, virulent strains of Clostridium perfringens (C. perfringens) particularly in association with predisposing factors, such as a high protein diet and intestinal damage following co-infection with Salmonella bacteria [5] or Eimeria protozoa [6–8]. C. perfringens α-toxin is a multifunctional phospholipase ubiquitously produced by all five bacterial types, and until recently, was considered as the major virulence factor in chickens [9,10]. More recently, the necrotic enteritis B-like (NetB) toxin, a β-pore-forming toxin of the α-hemolysin family [11], was identified in disease-causing C. perfringens isolates [10, 12] and has been evaluated as a vaccine candidate in small-scale vaccination trials [13].
Control of NE in commercial broiler production has been relatively well-managed by the use of in-feed antibiotic growth promoters (e.g. bacitracin, lincomycin, and virginiamycin). However, due to increasing worldwide restrictions on the use of antibiotic growth promoters, there is an increasing need for alternative strategies to reduce the incidence and severity of NE in commercial flocks [13,14]. Identification of alternative management practices to control disease has been hindered by the difficulty of experimentally reproducing NE by C. perfringens infection alone [6]. An Eimeria/C. perfringens co-infection model system replicates many of the clinical features of field NE, including body weight loss and the development of intestinal lesions directly by the invading pathogens, as well as indirectly through a proinflammatory cytokine/chemokine storm elicited in response to the microorganisms [1,6,15]. This experimental model, and those described by others [1,6,8], have led to the development and evaluation of novel strategies that may be of benefit to reduce field infections. Among these new approaches is immunization with subunit protein vaccines derived from Eimeria and C. perfringens in the presence of adjuvants to stimulate adaptive and protective immune responses [13]. In particular, the Montanide ISA and IMS adjuvants are aqueous-based microemulsions with demonstrated efficacy for enhancing the immunogenicity of a variety of animal vaccines, including those for avian coccidiosis [13,16].
In ovo vaccination has been successfully used to protect against poultry infectious diseases since the initial observations by Sharma and Witter [17] that 18-day-old embryos develop post-hatch immunity against the immunizing agents. Subsequent research indicated that vaccination of late-stage chicken embryos was safe and induced immunity earlier compared with post-hatch immunization [18]. Compared with other routes of immunization, in ovo vaccination also offers the advantages of reducing physiologic stress associated with post-hatch immunization, more precise and uniform vaccine dosing, multiple-agent vaccination, ease of handling, and reduced labor costs. Our previous studies showed that immunization of broilers at day 18 of embryogenesis with the Eimeria recombinant profilin protein induced protection against post-hatch challenge infection with live parasites [19,20]. The current study was undertaken to assess the ability of the novel Montanide IMS adjuvants, IMS 106 and IMS 101 which are specifically designed for in ovo vaccination, to enhance protective immunity to avian NE when co-administered with the profilin and NetB proteins at 18 days of embryo development.
MATERIALS AND METHODS
Recombinant profilin and NetB proteins
E. acervulina recombinant profilin protein was expressed in Escherichia coli (E. coli) as a maltose-binding protein (MBP) fusion protein, purified on an amylase affinity column, and treated with factor Xa (New England Biolabs, Ipswich, MA, USA) to separate MBP from profilin as described [21]. The C. perfringens NetB toxin gene was cloned by polymerase chain reaction (PCR) from the DNA of a virulent bacterial strain isolated from an outbreak of NE as described [22]. Recombinant NetB protein was expressed in E. coli with a 6xHis epitope tag and purified on a Ni-NTA agarose affinity column as described [22]. Both proteins migrated as single bands on Coomassie blue-stained SDS-polyacrylamide gels and reacted with profilin- or NetB-specific antibodies on Western blots [22]. Lipopolysaccharide (LPS) was removed from the purified recombinant proteins by using the Endotoxin Removal Solution (Sigma, St. Louis, MO, USA) and the level of endotoxin was checked by the PierceLAL Chromogenic Endotoxin Quantitation Kit (Rockford, IL, USA). Recombinant proteins with less than 0.1 EU/mL LPS (data not shown) was used in this paper.
The Montanide IMS adjuvants
The Montanide IMS 106 and IMS 101 adjuvants (Seppic, Paris, France) are a dispersion of liquid nanoparticles in an aqueous phase containing a proprietary immunostimulating component and compatible with all kinds of buffered antigens. Recombinant purified profilin and NetB proteins were mixed with IMS adjuvants at a 50:50 ratio (wt:wt) as recommended by the manufacturer.
In ovo immunization
Embryonated eggs of commercial broiler chickens (Moyer’s Chicks, Inc., Quakertown, PA, USA) were candled to select well-developed embryos and randomly distributed into 4 groups (20 eggs/group) as follows (Table 1): i) unimmunized and uninfected control group, ii) unimmunized and Eimeria maxima (E. maxima) plus C. perfringens co-infected group, iii) profilin plus NetB-immunized and co-infected group, and iv) profilin/NetB plus IMS adjuvant-immunized and co-infected group. At 18 days of embryonic development, the eggs were injected using the Intelliject system (Avitech, Easton, MD, USA) with 100 μL of phosphate-buffered saline (PBS), pH 7.4 (unimmunized controls), 50 μg of profilin plus 50 μg of NetB, or 50 μg of profilin plus 50 μg of NetB in combination with 50 μL of IMS adjuvant as described [19,20].
Table 1.
Experimental design
| Treatment group | Number of birds | Protein (100 μg/bird) | Adjuvant | Challenge infection (EM+CP1)) |
|---|---|---|---|---|
| 1 | 20 | - | - | - |
| 2 | 20 | - | - | + |
| 3 | 20 | Profilin | - | + |
| 4 | 20 | Profilin+NetB | - | + |
| 5 | 20 | Profilin+NetB | IMS 1142106 | + |
| 6 | 20 | Profilin+NetB | IMS 1142101 | + |
Chickens were orally infected with 1.0×104 oocysts/bird of E. maxima (EM) at day 14 post-hatch and with 1.0×109 CFU/bird of C. perfringens (CP) at day 18.
Experimental NE disease model
Experimental NE was induced by co-infection with E. maxima and C. perfringens as described [6,13]. Briefly, chickens were infected by oral gavage on day 14 post-hatch with 1.0×104 oocysts/bird of E. maxima Beltsville strain 41A. At day 18, birds were infected by oral gavage with 1.0×109 colony forming units/bird of C. perfringens strain Del-1. Chickens were fed an antibiotic-free, certified organic starter diet containing 18% crude protein (dry matter basis) between days 1 and 14 followed by a standard grower diet containing 24% (dry matter basis) crude protein between days 15 and 28. All animal protocols were approved by the USDA Beltsville Area Institutional Animal Care and Use Committee.
Determination of NE-induced clinical parameters
Body weight gains and gut lesion scores were used to assess NE clinical signs as described [13]. Body weights were measured on all chickens between days 14 and 20 (days 0–6 post-infection with E. maxima) and expressed as g/bird. For intestinal lesions, 5 chickens from each group were randomly selected at day 2 post-infection with C. perfringens (day 6 post-infection with E. maxima), intestines were removed, and lesions were scored on a numerical scale from 0 (none) to 4 (high) in a blinded fashion by 3 independent observers as described [6,13].
Serum anti-NetB antibody levels
Peripheral blood was collected by cardiac puncture from 3 birds/group immediately following euthanasia on day 7 following infection with C. perfringens. Sera were obtained by low speed centrifugation, pooled, and used in an enzyme-linked immunosorbent assay (ELISA) to measure anti-NetB antibody levels as described [13]. Briefly, 96-well microtiter plates were coated overnight with 1.0 μg/well of purified recombinant NetB protein. The plates were washed with PBS containing 0.05% Tween (PBS-T) and blocked with PBS containing 1% BSA. Sera (100 μL/well) were incubated for 2 h at room temperature with gentle agitation, the plates were washed with PBS-T, and bound antibody was detected with peroxidase-conjugated rabbit anti-chicken immunoglobulin G secondary antibody (Sigma, USA) and peroxidase-specific substrate. Optical density at 450 nm (OD450) was measured with an automated microplate reader (Bio-Rad, Richmond, CA, USA). The final antibody concentrations were calculated as ng/mL using our laboratory standard graph. Standard graph was obtained by conducting ELISA as described above using purified NetB antigen as the coating antigen and purified chicken immnoglobulin which detects NetB as the detecting antibody. Serially diluted antibodies of known protein concentrations were used to develop a standard curve that covers the detection ranges of 0 to 1,000 ng/mL antibodies. Fresh egg yolks containing high titer antibodies to C. perfringens NetB antigen were used to purify chicken antibodies using PierceChicken IgY Purification Kit (Pierce Biotechnology, Rockford, lL, USA).
Quantitation of cytokine and chemokine levels in intestinal intraepithelial lymphocytes
Intestinal tissues between the jejunum and the ileac region were harvested from 3 birds/group on day 2 following infection with C. perfringens, cut open longitudinally, washed gently with ice-cold Hank’s balanced salt solution (Sigma, USA), and intra epithelial lymphocytes (IELs) were isolated by density gradient centrifugation as described [6,13,19]. Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) and 5.0 μg of total RNA were treated with 1.0 U of DNase I and 1.0 μL of 10X reaction buffer (Sigma, USA), incubated for 15 min at room temperature, 1.0 μL of stop solution was added to inactivate DNase I, and the mixture was heated for 10 min at 70°C. RNA was reverse-transcribed using the StrataScript first-strand synthesis system (Stratagene, La Jolla, CA, USA) according to the manufacturer’s protocol. Quantitative real time polymerase chain reaction (qRT-PCR) oligonucleotide primers for chicken lipopolysaccharide-induced tumor necrosis factor-α factor (LITAF), tumor necrosis factor superfamily 15 (TNFSF15), interleukin-8 (IL-8), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) internal control are listed in Table 2. Amplification and detection were carried out using equivalent amounts of total RNA with the Mx3000P system and Brilliant SYBR Green qPCR master mix (Stratagene, USA). Standard curves were generated using log10 diluted standard RNAs and the levels of individual transcripts were normalized to those of GAPDH by the Q-gene program [6,23]. Each sample was analyzed in triplicate. To normalize individual replicates, the logarithmic-scaled threshold cycle (Ct) values were transformed to linear units of normalized expression prior to calculating mean and standard deviation values for the references and individual targets, followed by the determination of mean normalized expression using the Q-gene program.
Table 2.
Oligonucleotide primers used for quantitative RT-PCR
| RNA target | Primer sequences | PCR product size (base pair) | GenBank accession no. |
|---|---|---|---|
| TNFSF15 | |||
| Forward | 5′-CCTGAGTATTCCAGCAACGCA-3′ | 292 | NM_01024578 |
| Reverse | 5′-ATCCACCAGCTTGATGTCACTAAC-3′ | ||
| IL-8 | |||
| Forward | 5′-GGCTTGCTAGGGGAAATGA-3′ | 200 | NM_205498.1 |
| Reverse | 5′-AGCTGACTCTGACTAGGAAACTGT-3′ | ||
| GAPDH | |||
| Forward | 5′-GGTGGTGCTAAGCGTGTTAT-3′ | 264 | NM_204305.1 |
| Reverse | 5′-ACCTCTGTCATCTCTCCACA-3′ | ||
qRT-PCR, quantitative real time polymerase chain reaction; TNFSF15, tumor necrosis factor superfamily 15; IL-8, interleukin-8; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
All data were subjected to one-way analysis of variance using SPSS 15.0 for Windows (SPSS, Inc., Chicago, IL, USA). Mean values were compared using the Duncan’s multiple range test and differences were considered statistically significant at p<0.05.
RESULTS
Effects of vaccination with NetB proteins plus IMS adjuvants on body weight gains and gut lesions
Animals in ovo immunized with profilin+NetB/IMS 106 or profilin+NetB/IMS 101 exhibited significantly increased body weight gains between days 0 and 6 post-infection with E. maxima compared with the PBS or antigens- only group (Figure 1). Chickens in ovo immunized with profilin+NetB/IMS 106 or profilin+NetB/IMS 101 showed significantly reduced lesion scores at 2 days post-infection with C. perfringens compare with birds given the PBS control or profilin alone group (Figure 2). However, no differences in lesion scores were seen between the adjuvant-only group and the PBS control groups.
Figure 1.
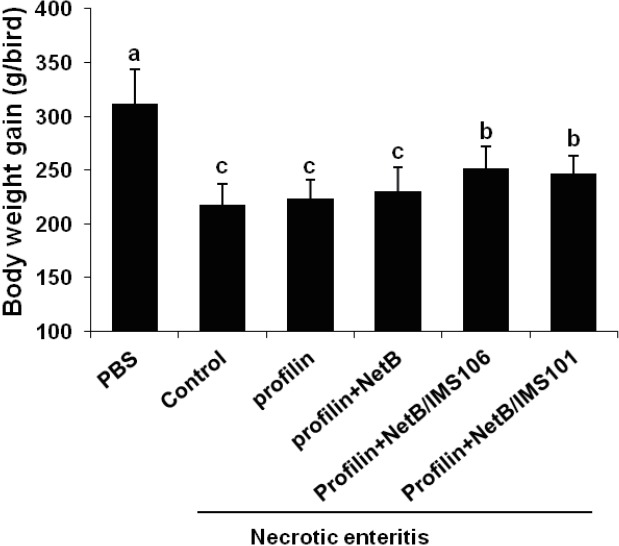
Effects of in ovo immunization with recombinant profilin plus necrotic enteritis B-like proteins plus Montanide IMS adjuvants on body weight gains. Chicken were immunized and were uninfected or co-infected with Eimeria maxima and Clostridium perfringens as described in Table 1. Body weight gains were measured between days 14 and 20. Each bar represents the mean±standard deviation value (n = 20). Bars not sharing the indicated letters are significantly different according to the Duncan’s multiple range test (p<0.05).
Figure 2.
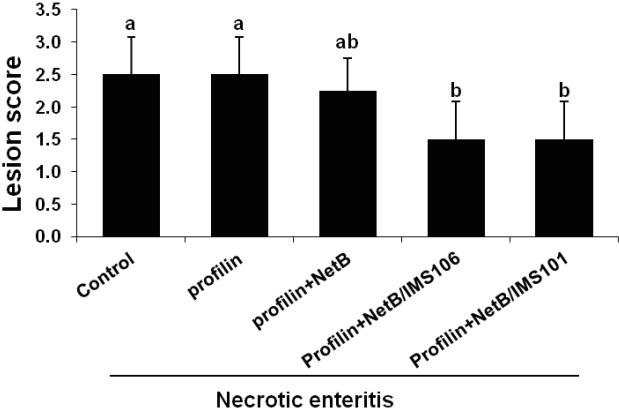
Effects of in ovo immunization with recombinant profilin plus necrotic enteritis B-like (NetB) proteins plus Montanide IMS adjuvants on gut lesions. Chicken were in ovo injected with PBS (NE control) or profilin plus NetB plus IMS 106 or IMS 101 with the indicated protein/adjuvant combinations. The birds were co-infected with Eimeria maxima (EM) on day 14 and Clostridium perfringens (CP) on day 18, and lesion scores were assessed on day 20 using a scale from 0 (none) to 4 (high). Each bar represents the mean±standard deviation. value of the combined observations on 5 chicken by three independent observers (n = 15). Bars not sharing the indicated letters are significantly different according to the Duncan’s multiple range test (p<0.05).
Effect of vaccination with NetB proteins plus IMS adjuvants on serum antibody responses
Chickens in ovo immunized with profilin+NetB/IMS 106 and co-infected with E. maxima/C. perfringens had significantly greater levels of NetB-reactive serum antibodies at day 7 C. perfringens post infection compared with infected animals given PBS or profilin alone (Figure 3). Similarly, co-infected birds immunized with profilin+NetB/IMS 101 had significantly greater concentrations of NetB-reactive antibodies compared with infected animals immunized with the negative PBS control group (Figure 3).
Figure 3.
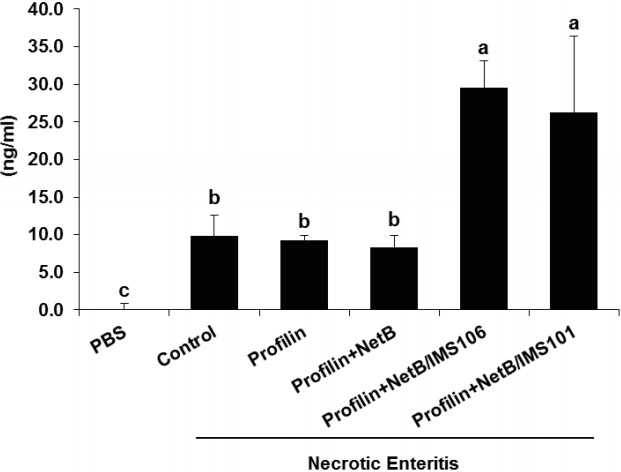
Effects of in ovo immunization with recombinant profilin plus NetB proteins in combination with Montanide adjuvants on necrotic enteritis B-like (NetB) antibody levels after necrotic enteritis (NE) infection. Chicken were in ovo immunized and were uninfected or co-infected with Eimeria maxima (EM) and Clostridium perfringens (CP) as described in Table 1. The concentration of serum antibodies against NetB toxin were measured by enzyme-linked immunosorbent assay at the indicated days post-hatch. Each bar represents the average antibody concentration in ng/mL (n = 3). The letters (a, b) indicate significantly increased NetB antibody concentrations comparing the profilin+NetB/IMS 106 and profilin+NetB/IMS 101 groups with profilin alone or NE groups (p<0.05). Numerical values shown below the figure are average serum antibody concentrations.
Effect of vaccination with NetB proteins plus IMS adjuvants on IEL chemokine/cytokine mRNA levels
Compared with the PBS and antigen only groups, chickens in ovo immunized with profilin+NetB/IMS 106 or profilin+NetB/ IMS 101 and co-infected with E. maxima/C. prefringens had significantly reduced levels of IEL transcripts encoding IL-8 (Figure 4). Similarly, TNFSF15 transcript levels were diminished in the profilin+NetB/IMS 106 or profilin+NetB/IMS 101 group (Figure 4), compared with the other respective experimental and control groups.
Figure 4.
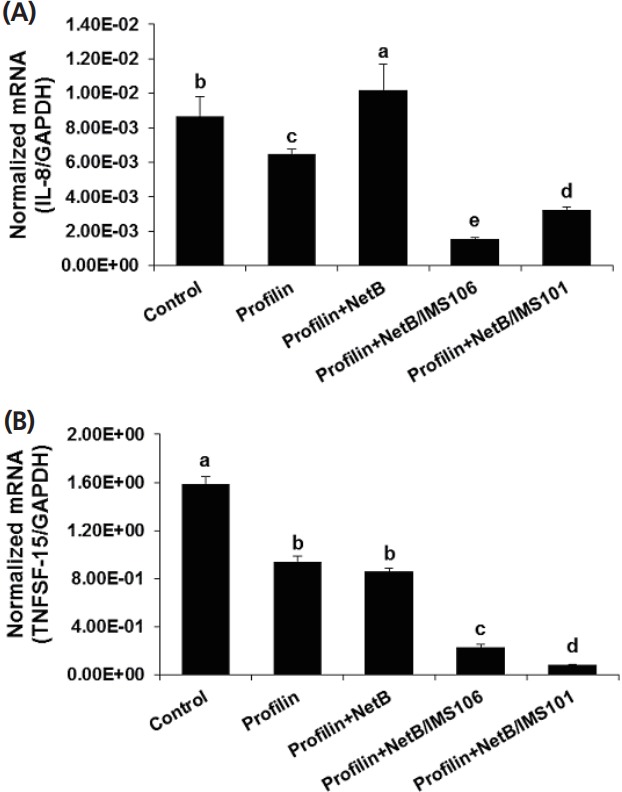
Effects of in ovo vaccination with profilin plus necrotic enteritis B-like (NetB) proteins plus IMS 101 or IMS 106 on IL-8, and TNFSF15 transcript levels in intestinal IELs. Chicken were in ovo immunized and co-infected with Eimeria maxima and Clostridium perfringens as described in Table 1. Intestinal IELs were isolated at day 20, and the levels of (A) IL-8 and (B) TNFSF15 mRNAs were measured by quantitative RT-PCR at day 20 and normalized to GAPDH transcript levels. Each bar represents the mean±SD. value (n = 4). Within each graph, bars not sharing the indicated letters are significantly different according to the Duncan’s multiple range test (p<0.05). Numerical values below the figures show the averages normalized RT-PCR measurements. IL-8, interleukin-8; TNFSF15, tumor necrosis factor superfamily 15; IELs, intraepithelial lymphocytes; qRT-PCR, quantitative real time polymerase chain reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
DISCUSSION
This study was conducted to evaluate the effect of in ovo immunization with recombinant profilin on combination with Montanide adjuvants IMS 106 or IMS 101 as a vaccine/adjuvant complex candidate, local and systemic immune responses against experimental NE disease model in commercial broilers. The major findings are: i) chickens immunized with profilin+NetB/IMS 106 or profilin+NetB/IMS 101 and co-infected with C. perfringens and E. maxima had increased body weight gains compared with the antigens only without adjuvant, and PBS groups; ii) greater NetB antibody levels were apparent in the profilin+NetB/IMS 106 and profilin+NetB/IMS 101 groups compared with PBS or antigens only (profilin+NetB) group; and iii) decreased levels of IEL IL-8, and TNFSF15 gene transcripts were observed in chickens in ovo immunized with profilin+NetB/ IMS 106 or IMS 101 compared with the antigen alone control.
The increasing number of legislative restrictions and the volun tary withdrawal of antibiotic growth promoters worldwide will continue to impact poultry production and health. The rising incidence of Clostridium infections and development of NE in commercial chickens has been associated with the withdrawal of antibiotics during poultry production [1,2]. Therefore, a better understanding of host- and environmentally-related factors on C. perfringens infections will be necessary for the development of effective sustainable strategies aimed to reduce the negative consequences of NE. The limited progress in understanding the complexity of host-pathogen interactions in NE underscores the urgent need for more fundamental research in host immunity against Clostridium pathogens in order to develop effective control strategies against NE [6]. Etiology of NE is complex, but coccidiosis is a well-known factor that predisposes birds to NE [2] and has been identified to have a synergistic relationship with C. perfringens during the development of experimental NE [6]. Coccidia multiplication stages in the intestinal epithelium initiate gut mucosal damage and C. perfringens colonize the damaged intestinal epithelium further destructing the enterocytes [2]. Thus, ability to reduce coccidiosis-afflicted gut damage will reduce NE induced by C. perfringens. Although the etiology of NE is not clearly known, coccidiosis is considered to be a major risk factor [19]. In this study, we used the NE disease model using co-infection of E. maxima and C. perfringens as previously described [6]. In this model, different strains of E. maxima and C. perfringens were screened to select the combination that induced the typical NE lesion in the gut [6].
Previous investigations of avian NE vaccines have included C. perfringens recombinant proteins, attenuated bacteria, and live expression vectors, or naked DNAs containing bacterial genes [7, 13,14,24]. While many NE vaccine development efforts in poultry have focused on α-toxin, these toxin-based vaccines have not provided the same level of efficacy compared with clostridial vaccines in other animals, presumably because α-toxin is not an essential virulence factor for C. perfringens in chickens [9]. On the other hand, Lovland et al [25] reported that a C. perfringens toxoid A vaccine administered intramuscularly with aluminum hydroxide adjuvant reduced subclinical NE compared with non-immunized controls. The commercial Netvax vaccine, incorporating a C. perfringens type A toxoid and formulated as an oil emulsion, has shown promise in controlling NE in commercial poultry flocks following intramuscular administration [26]. In a previous study, we demonstrated the efficacy of the Montanide ISA 71 water-in-oil adjuvant for increasing protective immunity against experimental NE following immunization with recombinant NetB toxin [13]. More specifically, subcutaneous vaccination of broilers with purified NetB plus ISA 71 prior to co-infection with E. maxima and C. perfringens increased body weight gains, reduced gut lesion scores, and enhanced serum anti-NetB antibody levels compared with controls. To the best of our knowledge, however, no studies have been reported demonstrating the feasibility of in ovo vaccination for protection against avian NE in a laboratory-based disease model or under field conditions.
In the last few years, in-ovo vaccination technology has been extended for other vaccines, including live and recombinant vaccines, and efforts to extend it for other viral, bacterial and coccidiosis vaccines are in progress. A few reports have now been published describing new strategies for immunizing embryos and showed effective stimulation and the induction of protective immunity [18–20]. Many different types of chemical compounds and formulations have since then shown to be effective in increasing humoral and/or cell-mediated immune responses in animals [27]. The Montanide IMS series of adjuvants comprise oil-free, ready-to-use aqueous mixtures of liquid nanoparticles ranging from 50 nm to 500 nm in size, together with a generally recognized as safe, proprietary, immunostimulating compound. IMS adjuvants are easily manufactured, well-tolerated in animals, and possess excellent storage stability, and have been used together with piscine, porcine, equine, and avian vaccines [28,29]. IMS 1313 in combination with recombinant profilin protein augmented protective immunity in chickens against infection by multiple species of Eimeria compared with vaccination with profilin alone [29].
Løvland et al [25] reported that the C. perfringens toxoid A vaccine administered with aluminum hydroxide adjuvant reduced subclinical NE compared with non-immunized controls. In previous study, we demonstrated the efficacy of the ISA 71 VG water-in-oil adjuvant in potentiating protective immunity against experimental NE disease following immunization with a recombinant clostridial protein in combination with ISA 71 adjuvant [13]. More specifically, vaccination of broilers with clostridial NetB or PFO proteins plus ISA 71 VG prior to co-infection with E. maxima and C. perfringens increased body weight gains, reduced gut lesion score, and enhanced NetB serum antibody levels compared with vaccination with adjuvant alone. Although C. perfringens occurs naturally in the gut in healthy chickens, its potential to produce overt NE is dependent upon a number of factors, including a high protein feed mix, and the presence of other enteric pathogens, Salmonella and coccidian parasites [2,5]. Eimeria profilin has been considered as a potential vaccine candidate for controlling coccidiosis, malaria, and toxoplasmosis because of their capacity to polymerize actin for host invasion [21,30]. In the current study, we therefore asked whether the recombinant profilin plus clostridial NetB protein in combination with Montanide IMS was a more effective adjuvant compared with NetB plus adjuvant using in ovo immunization. In our previous embryo vaccination studies, we demonstrated that circulating antibodies were induced following in ovo injection with a DNA or recombinant protein vaccine [19,20,29,30]. Therefore, in this study, we measured serum antibodies against NetB to investigate if IMS 106, or IMS 101 adjuvant augments serum antibodies that could be beneficial to NE protection.
Body weight gains and gross gut lesions are commonly used to assess the severity of experimental NE [1,5]. In the present study, there was significant difference in the body weight of birds in ovo immunized with profilin+NetB/IMS 106, or profilin+NetB/IMS 101 and prior to infection with E. maxima and C. perfringens. The profilin+NetB/IMS106 group showed the highest body weight among groups, showing 10% higher body weight than control PBS group. Our results showed that the immunoenhancing effects of in ovo vaccination with profilin+NetB/IMS 101 or profilin+ NetBF/IMS 106 on body weight and intestinal gut lesion scores implies that the mode of action of these vaccine/adjuvant complex may involve anti-bacterial effector mechanisms influencing intestinal structure and/or function. These results are consistent with our previous report [13].
In previous studies, we have shown that although cell-mediated immunity is of importance in poultry immunity against these parasites, serum antibody titres highly correlated with survivability following C. perfringens infection. The present study indicates that IMS 106 or IMS 101 adjuvants can modulate humoral antibody response in broiler chickens as seen in this study and described earlier [13]. Serum antibody levels against clostridial antigen Net-B protein were measured as parameters of humoral immunity in vaccinated chickens. To confirm and extend our previously study, this experiment was done using in ovo immunization method. In birds in ovo immunized with NetB+profilin/IMS 106 and NetB+profilin/IMS 101, the levels of serum antibodies reactive with Net-B antigen were significantly increased compared with profilin or profilin plus NetB group. Higher NetB toxin antibody titers were observed in those groups, of E. maxima/C. perfringens co-infected chickens, each compared with the other vaccine/adjuvant groups, as well as with untreated and infected controls and infected chickens that received antigen (profilin+ NetB) alone without adjuvant. The results indicated that in ovo immunization with a recombinant profilin plus NetB protein in combination with Montanide IMS 106 or IMS 101 adjuvant enhanced the antibody levels of chickens and the antibody response might play a role in protection of chickens against NE disease. Furthermore, IMS 106 and IMS 101 did not show any toxicity for developing embryos based on the hatchability data (Results not shown).
Even though there has been significant progress in under standing the molecular mechanisms for the pathogenesis of C. perfringens infection in chickens, the immunology relating to C. perfringens infection, including immune recognition of the pathogen, is still poorly understood [1,3]. Park et al [6] demonstrated that intestinal IELs from an NE model system using chickens co-infected with C. perfringens and E. maxima revealed that gene transcripts encoding interferon (IFN)-α, IFN-γ, IL-1β, and IL-10 were significantly increased in NE-infected birds compared with uninfected controls. In addition, Collier et al [15] observed that chickens with induced NE exhibited significantly increased levels of IFN-γ, IL-4, and IL-10 in the intestine compared with healthy controls. In this study, they observed that the expression patterns of 2 genes, IFN-γ, and IL-10, were increased in NE-infected birds compared with healthy controls. Generally, IFN-γ regulates adaptive immunity by activating lymphocytes and enhancing the expression of MHC class II antigens. In a previous study, co-administration of the ISA 71 adjuvant with recombinant NetB toxin increased protection against experimental avian NE while concomitantly decreasing the levels of intestinal IEL transcripts encoding LITAF, TNFSF15, and IL-8 [13]. In the present study, the level of transcripts encoding the cytokine IL-8 production was significantly decreased in profilin+NetB/IMS 106, and profilin+NetB/IMS 101 groups compared to antigens alone group without adjuvant. In addition, TNFSF15 mRNA levels were also significantly decreased in the birds that immunized with profilin+NetB/IMS 106 and profilin+NetB/IMS 106 groups. These finding indicated that the cellular response was activated and may appear that in ovo vaccination with profilin plus NetB clostridial proteins in combination with Montanide IMS 106 or IMS 101 adjuvants decreased the levels of these immune mediators, particularly IL-8 and TNFSF15, while simultaneously increasing outcomes of protection against NE. These results are consistent with our previous reports as well as other report [7,13] and suggest that profilin+NetB/IMS 106 or IMS 101 used as a vaccine complex regulates cellular immunity by altering the expression levels of these proinflammatory or Th1 cytokine genes in the intestine of infected animals with E. maxima/C. perfringens.
Avian coccidiosis is one of the most widespread infectious diseases of chickens [30]. The etiologic agent of avian coccidiosis is Eimeria, a genus of eukaryotic obligate intracellular parasites belonging to the phylum Apicomplexa, along with the genera Plasmodium, Cryptosporidium, and Toxoplasma, among others. These parasites infect the intestinal tract and clinical manifestations of infection include damage to the intestinal epithelium, decreased nutrient absorption, inefficient feed utilization, and impaired growth rate, which, in severe cases, may lead to mortality [30]. Eimeria parasites induce leakage of plasma proteins by killing epithelial cells as a consequence of the intracellular stages of their lifecycle. Moreover, according to Collier et al [15], a coccidial infection enhances mucus production in the intestine. Recombinant NetB protein in combination with Montanide adjuvants are capable of enhancing protective immunity to a degree greater than that achieved by use of the vaccine alone. In a previous study, we showed that subcutaneous vaccination with the NetB protein in combination with Montanide ISA 71 adjuvant significantly protected chickens against body weight loss induced by necrotic enteritis [13]. In addition, parasite replication was significantly reduced in chickens given the profilin–pcDNA vaccine along with the IL-1, IL-8, IL-15, IFN-c, transforming growth factor–β4, or lymphotactin genes compared with profilin alone [30]. In conclusion, this study provides evidence that immunization of chicken embryos with Eimeria profilin and C. perfringens NetB proteins, in combination with the IMS adjuvant, increases protection against post-hatch experimental NE. Future studies will be required to elucidate the molecular and cellular mechanisms through which this adjuvant enhance immunity to avian NE. A better understanding of host-pathogen interactions in NE and the identification of the nature of host immune responses that are critical for protection against C. perfringens infection will contribute to the development of logical intervention strategies to reduce the negative consequences of NE.
ACKNOWLEDGMENTS
This project was supported, in part, by a Trust agreement established between ARS, USDA and Seppic, Inc. (Puteaux, France). The authors thank Margie Nichols for her significant contribution to this research.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Lee KW, Lillehoj HS, Jeong W, Jeoung HY, An DJ. Avian necrotic enteritis: Experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult Sci. 2011;90:1381–90. doi: 10.3382/ps.2010-01319. [DOI] [PubMed] [Google Scholar]
- 2.Williams R. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–80. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- 3.Oh ST, Lillehoj HS. The role of host genetic factors and host immunity in necrotic enteritis. Avian Pathol. 2016;45:313–6. doi: 10.1080/03079457.2016.1154503. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu T, Ohtani K, Hirakawa H, et al. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci. 2002;99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shivaramaiah S, Wolfenden R, Barta J, et al. The role of an early Salmonella typhimurium infection as a predisposing factor for necrotic enteritis in a laboratory challenge model. Avian Dis. 2011;55:319–23. doi: 10.1637/9604-112910-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 6.Park SS, Lillehoj HS, Allen PC, et al. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008;52:14–22. doi: 10.1637/7997-041707-Reg. [DOI] [PubMed] [Google Scholar]
- 7.Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–34. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–7. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- 9.Keyburn AL, Sheedy SA, Ford ME, et al. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun. 2006;74:6496–500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keyburn AL, Bannam TL, Moore RJ, Rood JI. NetB, a pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins. 2010;2:1913–27. doi: 10.3390/toxins2071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rood JI, Keyburn AL, Moore RJ. NetB and necrotic enteritis: the hole movable story. Avian Pathol. 2016;45:295–301. doi: 10.1080/03079457.2016.1158781. [DOI] [PubMed] [Google Scholar]
- 12.Keyburn AL, Boyce JD, Vaz P, et al. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4:e26. doi: 10.1371/journal.ppat.0040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang SI, Lillehoj HS, Lee S-H, et al. Vaccination with Clostridium perfringens recombinant proteins in combination with MontanideTM ISA 71 VG adjuvant increases protection against experimental necrotic enteritis in commercial broiler chickens. Vaccine. 2012;30:5401–6. doi: 10.1016/j.vaccine.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni RR, Parreira VR, Sharif S, Prescott JF. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin Vaccine Immunol. 2007;14:1070–7. doi: 10.1128/CVI.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collier CT, Hofacre CL, Payne AM, et al. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet Immunol Immunopathol. 2008;122:104–15. doi: 10.1016/j.vetimm.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Aucouturier J, Ascarateil S, Dupuis L. The use of oil adjuvants in therapeutic vaccines. Vaccine. 2006;24:S44–S5. doi: 10.1016/j.vaccine.2005.01.116. [DOI] [PubMed] [Google Scholar]
- 17.Sharma J, Witter R. Embryo vaccination against Marek’s disease with serotypes 1, 2 and 3 vaccines administered singly or in combination. Avian Dis. 1983:453–63. [PubMed] [Google Scholar]
- 18.Ricks C, Avakian A, Bryan T, et al. In ovo vaccination technology. Adv Vet Med. 1999;41:495–515. [PubMed] [Google Scholar]
- 19.Ding XC, Lillehoj HS, Quiroz MA, Bevensee E, Lillehoj EP. Protective immunity against Eimeria acervulina following in ovo immunization with a recombinant subunit vaccine and cytokine genes. Inf Immun. 2004;72:6939–44. doi: 10.1128/IAI.72.12.6939-6944.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, Lillehoj HS, Jang SI, et al. Embryo vaccination of chickens using a novel adjuvant formulation stimulates protective immunity against Eimeria maxima infection. Vaccine. 2010;28:7774–8. doi: 10.1016/j.vaccine.2010.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lillehoj HS, Choi KD, Jenkins MC, et al. A recombinant Eimeria protein inducing interferon-γ production: comparison of different gene expression systems and immunization strategies for vaccination against coccidiosis. Avian Dis. 2000:379–89. [PubMed] [Google Scholar]
- 22.Lee K, Lillehoj HS, Li GX, et al. Identification and cloning of two immunogenic Clostridium perfringens proteins, elongation factor Tu (EF-Tu) and pyruvate:ferredoxin oxidoreductase (PFO) of C. perfringens. Res Vet Sci. 2011;91:E80–E6. doi: 10.1016/j.rvsc.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Short technical report processing of gene expression data generated by quantitative Real-time RT-PCR. Biotechniques. 2002;32:1372–9. [PubMed] [Google Scholar]
- 24.Prescott J, Sivendra R, Barnum D. The use of bacitracin in the prevention and treatment of experimentally-induced necrotic enteritis in the chicken. The Canadian Vet J. 1978;19:181. [PMC free article] [PubMed] [Google Scholar]
- 25.Lovland A, Kaldhusdal M, Redhead K, Skjerve E, Lillehaug A. Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Pathol. 2004;33:83–92. doi: 10.1080/0379450310001636255. [DOI] [PubMed] [Google Scholar]
- 26.Crouch CF, Withanage GSK, de Haas V, Etore F, Francis MJ. Safety and efficacy of a maternal vaccine for the passive protection of broiler chicks against necrotic enteritis. Avian Pathol. 2010;39:489–97. doi: 10.1080/03079457.2010.517513. [DOI] [PubMed] [Google Scholar]
- 27.Bowersock TL, Martin S. Vaccine delivery to animals. Adv Drug Deliv Rev. 1999;38:167–94. doi: 10.1016/s0169-409x(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 28.Deville S, de Pooter A, Aucouturier K, et al. Influence of adjuvant formulation on the induced protection of mice immunized with total soluble antigen of Trichinella spiralis. Vet Parasitol. 2005;132:75–80. doi: 10.1016/j.vetpar.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Jang SI, Lillehoj HS, Lee SH, et al. MontanideTM IMS 1313 N VG PR nanoparticle adjuvant enhances antigen-specific immune responses to profilin following mucosal vaccination against Eimeria acervulina. Vet Parasitol. 2011;182:163–70. doi: 10.1016/j.vetpar.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Lillehoj H, Jang S, Lee S, Lillehoj E. Intestinal health: Key to maximise growth performance in livestock. Wageningen Academic Publishers; 2015. Chapter 4: Avian coccidiosis as a prototype intestinal disease—host protective immunity and novel disease control strategies; p. 79. [Google Scholar]


