Abstract
Proteins of the α/β-hydrolase fold family share a common structural fold, but perform a diverse set of functions. We have been studying natural mutations occurring in association with congenital disorders in the α/β-hydrolase fold domain of neuroligin (NLGN), butyrylcholinesterase (BChE), acetylcholinesterase (AChE). Starting from the autism-related R451C mutation in the α/β-hydrolase fold domain of NLGN3, we had previously shown that the Arg to Cys substitution is responsible for endoplasmic reticulum (ER) retention of the mutant protein and that a similar trafficking defect is observed when the mutation is inserted at the homologous positions in AChE and BChE. Herein we show further characterization of the R451C mutation in NLGN3 when expressed in HEK-293, and by protease digestion sensitivity, we reveal that the phenotype results from protein misfolding. However, the presence of an extra Cys doesn’t interfere with the formation of disulfide bonds as shown by reaction with PEG-maleimide and estimation of the molecular mass changes. These findings highlight the role of proper protein folding in protein processing and localization.
Keywords: protein folding, disulfide bonds, protein processing
INTRODUCTION
The α/β-hydrolase fold superfamily comprises a group of proteins with a common structural motif referred to as the α/β-hydrolase fold domain, or the cholinesterase homologous (or like) domain. Although all the members of the family share common structural features, they show great diversity in protein function. The cholinesterase subfamily presents catalytic hydrolytic functions, thyroglobulin is involved in precursor secretion and thyroid hormone production and the neuroligins (NLGNs) are heterophilic cell adhesion proteins. Other non-enzymatic adhesion proteins of the α/β-hydrolase fold superfamily include: glutactin, neurotactin, gliotactin [1] [2]. The NLGNs (1 to 4) are a family of multi-domain post-synaptic proteins involved in extracellular heterophilic adhesion interactions within the synapse [3]. Their function is critical for formation and maintenance of synaptic selectivity and function through the association with the pre-synaptic partner proteins, the neurexins (1 to 3) (NRXNs). NLGN-NRXN trans-synaptic association is essential for maturation and function of inhibitory and excitatory synapses [4]. The α/β-hydrolase domain of the NLGNs is involved in the heterophilic interaction with the NRXNs, therefore the structural organization of this domain is crucial for the recognition properties of the family members. Structural homology of the NLGN extracellular domain with other proteins in the α/β-hydrolase fold superfamily, such as the cholinesterases and thyroglobulin, suggests possible common mechanisms of protein folding. We have been interested in disease-related mutations naturally occurring in the α/β-hydrolase fold domain. In the case of the NLGNs, genetic alterations (point mutations, exon deletions, and premature truncations) have been reported in the genes encoding for NLGN3 and NLGN4 in patients with autism spectrum disorders (ASD) [5]. Interestingly, mutations affecting members of the NRXNs protein families have also been associated with ASD, indicating that improper localization and function of both partnering proteins at the synapse may contribute to an imbalance in the excitatory/inhibitory networks, leading to impaired neuronal signalling [6].
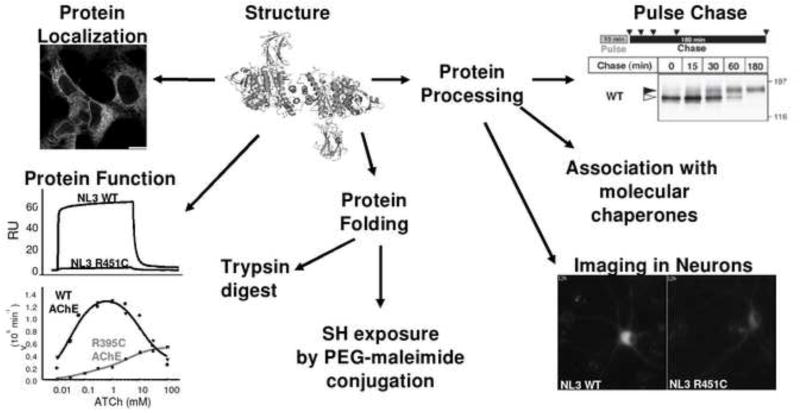
We have characterized a mutation found in NLGN3 in select cases of autism [7] as well as in BChE, in cases of post-succinylcholine apnea [8]. We found that the R451C mutation in NLGN3, when introduced in AChE and BChE, causes comparable protein trafficking impairments with the mutant protein arrested in the ER. Despite severe intracellular retention, R395C AChE was still active, although its catalytic properties were altered [9]. In order to study mutations mapping in the α/β-hydrolase fold domain of NLGN, BChE, and AChE, we propose an approach employing NLGN3 as a prototype protein to study if the ER retention of R451C NLGN3 is a consequence of a protein folding defect and if the mutation is responsible for altered processing and trafficking of NLGN3 (figure2).
Figure 2. Schematic representation of an experimental approach to study a mutation in the α/β-hydrolase fold proteins.
Determination of mutant protein location with immunofluorescence staining; protein function studies by surface plasmon resonance (NLGNs) or determination of enzyme catalytic constants (ChEs); protein folding by proteolytic digestion with trypsin, and exposure of free cysteine by treatment with PEG-maleimide. Possible alteration of glycosylation processing is studied by sensitivity to glycosidases, pulse-chase metabolic labelling and differential association of wild type and mutant proteins with molecular chaperones during protein biosynthesis. Protein trafficking in the cellular context of the nervous system can be approached using imaging techniques.
EXPERIMENTAL METHODS
Proteolytic Digests
Full length flag-tagged NLGN3 proteins from HEK293 cells stably transfected with wild type or mutant constructs were immunoprecipitated with the anti-FLAG M2 monoclonal antibody (Sigma, St. Louis, MO) and treated with trypsin (10,000 units/mg protein, Sigma-Aldrich, St. Louis, MO, USA cat. No. T6567) in 50mM Tris HCl, pH 7.4. Immunopure immobilized protein G (Thermo Fisher Scientific, Inc, Rockford, Il, USA) containing the immunoprecipitated NLGN protein was incubated at room temperature for 5 minutes with the indicated increasing trypsin concentrations (from 0 to 10μg/ml). The reaction was stopped by heating the samples at 90°C after the addition of an equal volume of 2-fold concentrated SDS-PAGE loading buffer (100 mM Tris-HCl, pH 6.8, 4% SDS, 2% β-mercaptoethanol, 0.2% bromophenol blue and 20% glycerol). Protein degradation was analyzed by SDS-PAGE and immunoblotting using the commercial anti-NLGN antibody diluted 1:1000 (clone 4F9, cat.No. 129 011 Synaptic Systems, Goettingen, Germany).
PEG-Maleimide Treatment
Immunoprecipitated full length wild type and mutant NLGN3 proteins were incubated in 2.5mM methoxy polyethylene glycol maleimide, (JenKem Technology, Allen, TX, USA), dissolved in 50mM Tris HCl, pH 7.4, in the presence of 2% SDS, for 1 hour at 30°C. Sample buffer containing 2% β-mercaptoethanol was added to elute the protein from the immobilized protein G. Samples were boiled and band shifts were analyzed by SDS-PAGE and immunoblotting using the anti-NLGN commercial antibody (see above).
RESULTS
Folding defects of R451C NLGN3
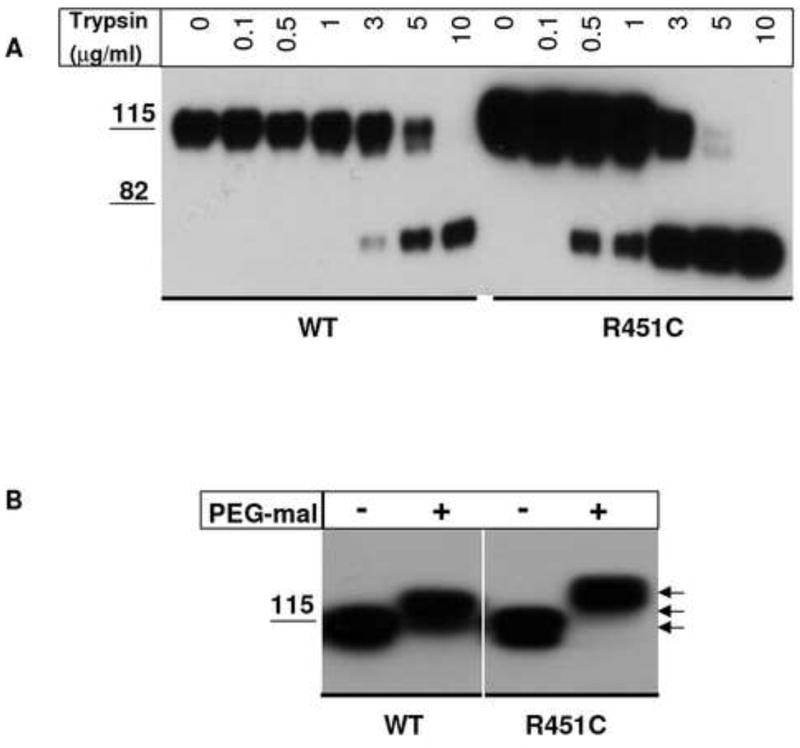
To ascertain whether the R451C mutation affects folding of the α/β-hydrolase fold domain, we used NLGN3 as a template to perform trypsin digestion experiments. If the Cys substitution caused a significant difference in folding between wild type and mutant protein, exposure of trypsin-sensitive sites would be different, leading to variations in trypsin sensitivity. As shown in figure 1A, the mature form of wild type NLGN3 was quite resistant to trypsin requiring up to 10μg/ml for complete digestion. In contrast the mutant protein with incomplete glycosylation processing shows higher sensitivity to proteolytic digestion at lower trypsin concentrations, starting from 3μg/ml, even in the presence of higher NLGN concentrations. This result suggests that the core-glycosylated immature form of R451C NLGN3 is more loosely folded than the fully processed-glycosylated form of wild type NLGN3 with its more complex oligosaccharide chains.
Figure 1. Trypsin sensitivity and PEG-maleimide reaction of NLGN3 wild type and R451C mutant protein.
A) Immunoprecipitated NLGN3 wild type and R451C were treated for 5 min with trypsin at the indicated concentrations and analyzed by SDS PAGE in reducing conditions followed by immunoblotting with an anti-NLGN antibody. Mutant protein is more sensitive to the digestion with trypsin compared to wild type. B) PEG-mal conjugation with free cysteines in NLGN3 wild type and R451C proteins. NLGN3 immunoprecipitated proteins are treated with 1mM PEG5,000-maleimide in denaturing conditions and band shifts are observed on a SDS PAGE followed by immunoblotting with an anti-NLGN antibody. R451C NLGN3 shows a band shift likely corresponding to the alkylation of three cysteine thiols (arrows).
Disulfide bond formation in wild type and R451C NLGN3
PEG5,000-maleimide (PEG-mal) was used to study whether the R451C mutation is affecting formation of disulfide bonds in NLGN3. The native protein presents three disulfide bonds and 2 free cysteines: one buried in the core of the α/β–hydrolase domain (position 293 of the protein sequence) and one in the intracellular domain (position 775 of the protein sequence) [10]. After treatment with PEG-maleimide, the alkylated cysteines should add an additional mass to the protein (5kDa to each reactive cysteine thiol) that will appear as a band shift on a 10% SDS-PAGE gel when compared to the untreated protein. Wild type NLGN3 reacts with PEG-mal with a band shift that likely result from the conjugation of both unpaired cysteines (C293 and C775) (Figure 2B). When the R451C mutation is inserted, PEG-maleimide conjugation of mutant NLGN3 shows a further band shift likely corresponding to the alkylation of three cysteine thiols. This indicates that C451 is not affecting the formation of pre-existing disulfide bonds in the wild type protein and does not disulfide bond with the free C293.
DISCUSSION
In order to study mutations arising naturally in the cholinesterase-like domain of proteins of the α/β-hydrolase domain superfamily, we have employed a model template of an autism-linked mutation, R451C, located in the α/β-hydrolase domain of NLGN3. In figure 2 we show how the study of a mutation in the α/β-hydrolase protein family can be approached experimentally to reveal mutational effects on protein folding, processing and trafficking in transfected HEK-293 cells by a biochemical approach and by applying imaging techniques in neurons to follow the protein from synthesis to translocations into the dendrites (Figure 2). Our group has been studying, collaboratively with other groups [9] [11], the R451C NLGN3 mutation, since it was reported to be associated with the autism spectrum disorders [7]. It has been shown that, when the R451C mutation is present in NLGN3, the mutant protein is significantly retained in the ER, where its adhesive functions are also altered [9] [11]. From the NLGN4 crystal structure the mutation appears in a surface location, but far from the binding site of β–neurexin [12] [13] [14]. At the same time, no data are thus far available on processing of the NLGNs and protein trafficking in neurons. To further study the alteration provoked by the R451C NLGN3 mutation, we used proteolytic digestion to show that when the mutation is present, R451C NLGN3 folding is altered, when compared to the wild type protein. Nonetheless, the extra Cys does not seem to interfere with the disulfide bond formation since the introduced sulfhydryl appears free to be alkylated by PEG-maleimide. Studying the processing of mutant NLGN3 and its trafficking in neurons (De Jaco et al., submitted) will clarify the extent of the folding deficiency and may allow for designing therapeutic interventions for certain congenital disorders with mutations in the α/β-hydrolase fold domain.
Acknowledgments
Funded by the USPHS Grant R37 GM-18360 to P.T, Autism Speaks #2617 to D.C., and Compagnia San Paolo Bando Programma in Neuroscienze 2008 to ADJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5(3):197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 2.Carr PD, Ollis DL. Alpha/beta hydrolase fold: an update. Protein Pept Lett. 2009;16(10):1137–1148. doi: 10.2174/092986609789071298. [DOI] [PubMed] [Google Scholar]
- 3.Ichtchenko K, Nguyen T, Sudhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271(5):2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- 4.Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54(6):919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455(7215):903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisé MF, El-Husseini A. The neuroligin and neurexin families: from structure to function at the synapse. Cellular and Molecular Life Sciences. 2006;63(16):1833–1849. doi: 10.1007/s00018-006-6061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Frahm J, Fischer J, Bourgeron T, Ehrenreich H, Brose N. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105(5):1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yen T, Nightingale BN, Burns JC, Sullivan DR, Stewart PM. Butyrylcholinesterase (BCHE) genotyping for post-succinylcholine apnea in an Australian population. Clin Chem. 2003;49(8):1297–1308. doi: 10.1373/49.8.1297. [DOI] [PubMed] [Google Scholar]
- 9.De Jaco A, Comoletti D, Kovarik Z, Gaietta G, Radic Z, Lockridge O, Ellisman MH, Taylor P. A mutation linked with autism reveals a common mechanism of endoplasmic reticulum retention for the alpha,beta-hydrolase fold protein family. J Biol Chem. 2006;281(14):9667–9676. doi: 10.1074/jbc.M510262200. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman RC, Jennings LL, Tsigelny I, Comoletti D, Flynn RE, Sudhof TC, Taylor P. Structural characterization of recombinant soluble rat neuroligin 1: mapping of secondary structure and glycosylation by mass spectrometry. Biochemistry. 2004;43(6):1496–1506. doi: 10.1021/bi035278t. [DOI] [PubMed] [Google Scholar]
- 11.Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P. The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci. 2004;24(20):4889–4893. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, Bourne Y, Marchot P. Structural analysis of the synaptic protein neuroligin and its beta-neurexin complex: determinants for folding and cell adhesion. Neuron. 2007;56(6):979–991. doi: 10.1016/j.neuron.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Liu H, Shim AH, Focia PJ, He X. Structural basis for synaptic adhesion mediated by neuroligin-neurexin interactions. Nat Struct Mol Biol. 2008;15(1):50–56. doi: 10.1038/nsmb1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arac D, Boucard AA, Ozkan E, Strop P, Newell E, Sudhof TC, Brunger AT. Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron. 2007;56(6):992–1003. doi: 10.1016/j.neuron.2007.12.002. [DOI] [PubMed] [Google Scholar]