Abstract
Aims
Pragmatic randomized clinical trials (pRCTs) collect data that have the potential to improve medical care significantly. However, these trials may be undermined by the requirement to obtain written informed consent, which can decrease accrual and increase selection bias. Recent data suggest that the majority of the US public endorses written consent for low‐risk pRCTs. The present study was designed to assess whether this view is specific to the US.
Methods
The study took the form of a cross‐sectional, probability‐based survey, with a 2 × 2 factorial design, assessing support for written informed consent vs. verbal consent or general notification for two low‐risk pRCTs in hypertension, one comparing two drugs with similar risk/benefit profiles and the other comparing the same drug being taken in the morning or at night. The primary outcome measures were respondents' personal preference and hypothetical recommendation to a research ethics committee regarding the use of written informed consent vs. the alternatives.
Results
A total of 2008 adults sampled from a probability‐based online panel responded to the web‐based survey conducted in May 2016 (response rate: 61%). Overall, 77% of respondents endorsed written consent. In both scenarios, the alternative of general notification received significantly more support (28.7–37.1%) than the alternative of verbal consent (12.7–14.0%) (P = 0.001). Forty per cent of respondents preferred and/or recommended general notification rather than written consent.
Conclusions
The results suggested that, rather than attempting to waive written consent, current pRCTs should focus on developing ways to implement written consent that provide sufficient information without undermining recruitment or increasing selection bias. The finding that around 40% of respondents endorsed general notification over written consent raises the possibility that, with educational efforts, the majority of Spaniards might accept general notification for low‐risk pRCTs.
Keywords: clinical trial regulation, general notification, low‐risk, pragmatic trials, verbal informed consent, written informed consent
What is Already Known about this Subject
To facilitate recruitment and reduce selection bias, commentators have proposed the use of brief verbal consent or general notification in place of in‐depth written consent for low‐risk pragmatic randomized controlled trials (pRCTs).
However, a survey from the US found that the majority of the public endorses written consent for low‐risk pRCTs. It is unknown whether this support for written consent is specific to the US or applies in the EU as well.
What this Study Adds
The present study found that the majority of the Spanish population endorses written consent for low‐risk pRCTs.
This finding suggests that support for written consent is not restricted to the US, and pRCTs in the EU should focus on developing approaches to written consent that provide sufficient information without undermining recruitment or increasing selection bias.
The finding that around 40% of respondents endorse general notification over written consent suggests that, with educational efforts, it might be feasible in the future to conduct low‐risk pRCTs in Spain, and perhaps in other EU countries, using general notification rather than study‐specific written consent.
Introduction
Pragmatic randomized clinical trials (pRCTs) that assess standard‐of‐care interventions are important for improving clinical care. In particular, these studies can provide important data to help national health systems determine which interventions provide the best healthcare benefit for the money 1 Yet, EU and US regulations require written consent for almost all clinical trials, no matter what level of risk they pose. This requirement, which leads to complex and long (15 pages or more) consent forms, which take a long time to read and are difficult to understand 2, has the potential to undermine the value of pRCTs. In response to these concerns, some commentators have argued that written informed consent is not ethically necessary for pRCTs 3, 4, 5, 6 that pose no or only minimal incremental risk compared with standard clinical care 7
While these theoretical arguments are valuable, it is important to assess what the public thinks of conducting low‐risk pRCTs without written consent. A study in the US found that the majority of respondents (70–82%) were willing to waive written consent for pRCTs, but only when it would make a study ‘too difficult to conduct’ 8. In addition, Nayak et al. 9 found that the majority of US respondents (63%) endorse written consent for low‐risk pRCTs, while 37% endorse the alternative of verbal consent or general notification 9. Recognizing that views on informed consent can vary by culture 10 we replicated the Nayak et al. 9 survey in Spain, to assess whether majority support for written consent for low‐risk pRCTs is specific to the US.
Methods
Survey characteristics and administration, and ethics review
A nationally representative online survey of Spanish citizens was conducted between 9 and 20 May 2016. The study protocol was reviewed by the research ethics committee (REC) of the Universidad Autónoma de Madrid (Madrid, Spain), which granted approval on 14 March 2016 (Ref. # CEI‐70‐1265).
The survey was administered to participants belonging to the Netquest (GfK group) panel, Spain. This is a probability‐based online closed panel to which potential members are invited to join, with the goal of ensuring that it is representative of the non‐institutionalized civilian Spanish population [with the exception of the oldest (≥75 years) age group]. Netquest comprises more than 198 000 adult members and has the ISO 26362, specific for free‐access panels. Panellists receive nonsurvey‐specific incentives through a point‐based reward programme; points can be exchanged for more than 1200 different items.
The survey was developed as described elsewhere 9. In short, two pretesting sessions and two pilot surveys were used to ensure comprehension of the US survey and obtain open‐ended feedback. The survey was then revised following feedback from two anonymous peer reviewers for Time‐sharing Experiments for the Social Sciences 9. The US version of the survey was translated into Spanish and adapted to the Spanish health system environment. The final Spanish version was identical to the US version, except for a few sentences in the introduction. We omitted references to ‘The Learning Healthcare System’ 11 – a concept unknown in Spain – and substituted a short description of conducting research in the context of providing healthcare (Supporting Information Data S1). The composition of the REC (as opposed to that of the US Institutional Review Board) was also adapted to the Spanish regulation.
To assess comprehension of the Spanish version, we conducted a test on 100 individuals and found that most of them (80–100%) correctly answered seven true/false questions regarding the survey, such as on the need to have clinical trials approved by an REC and whether the patient's treatment could be changed during the trial (Supporting Information Data S2).
The survey assessed respondents' views on consent for low‐risk pRCTs. Key elements of the survey are shown in Box 1. All respondents received a common introduction regarding the possibility of conducting research in the context of providing healthcare and the need for clinical trials to be approved by a REC. It was also explained that hypertension is a condition that affect millions of Spaniards and that, without treatment, it can lead to serious health complications. Immediately after the introduction, respondents were randomly assigned to receive one of two pRCT scenarios.
pRCT scenarios (see Box 1)
Scenario 1. A pRCT comparing two widely used antihypertensive drugs that have been approved by the Spanish health authorities. Respondents were informed that both drugs had a low‐risk profile and were effective antihypertensive agents. The examples were based on two commonly used diuretics, chlorthalidone and hydrochlorothiazide. However, in the survey, they were called CTD and TRT, to prevent response bias among respondents who might be familiar with the drugs.
Scenario 2. A pRCT assessing a once‐daily antihypertensive drug taken in the morning vs. at night. Respondents were told that prescribers usually do not inform patients when to take these drugs.
Box 1
Experimental design of the survey (Modified from Nayak et al9).
| Research conducted at the time of providing healthcare |
Hospitals that integrate research as part of care provision Patients informed that studies are conducted through letters, posters and brochures All studies are reviewed and approved by a REC, which comprises researchers, clinicians, ethicists, patient representatives and community members |
|||
| High blood pressure |
Affects millions of persons in Spain Can lead to stroke, heart attack and/or kidney disease if untreated |
|||
| Pragmatic RCT scenario |
Scenario 1: Drug ‘CTD’ or ‘TRT’?
Two health authorities‐approved medicines Both effective in lowering high blood pressure; similar adverse effects Unknown which is more effective |
Scenario 2: Dose timing, ‘morning’ or ‘night’?
Patients told to take medicine at same time each day Unknown whether morning or night more effective |
||
| Trial proposal |
Random assignment to CTD or TRT Patient's medicine can be changed at any time by patient or physician |
Random assignment of whether told to take medicine at morning or night Patient's medicine can be changed at any time by patient or physician |
||
| Debate | REC is debating the best way to get consent for this study | |||
| Consent options |
Written consent
vs. verbal consent |
Written consent
vs. general notification |
Written consent
vs. verbal consent |
Written consent
vs. general notification |
| Written consent | • Some members argue that patients should give study‐specific written consent | |||
| • Consent form would include purpose, risks and benefits, alternatives, method of maintaining privacy, and contact information; participation would be voluntary | ||||
| • Written consent would require extra time and effort | ||||
| • In some cases, if written consent is required, studies may not be conducted | ||||
| Alternative option | General notification | |||
| • Other members argue that because the risks are low, general notification through posters, brochures and letters is enough | ||||
| • Eligible patients would be automatically enrolled without being informed | ||||
| Verbal consent | ||||
| • Other members argue that because the risks are low, verbal consent is enough | ||||
| • Patient's physician would briefly explain the study | ||||
Shows the 2 × 2 factorial design and information presented to respondents. Half received a drug RCT scenario comparing two first‐line drugs; the others received a dose‐timing RCT scenario comparing morning vs. night dosing. Half of participants in each group chose between written consent and general notification; the rest chose between written consent and verbal consent. CTD, chlorthalidone; RCT, randomized, controlled trial; REC, research ethics committee; TRT = hydrochlorothiazide.
Consent options (see Box 1)
Respondents in each pRCT scenario were randomized to a choice between written informed consent vs. brief verbal consent, or between written informed consent vs. general notification.
Written consent: To allow comparison with Nayak et al. 9, the Spanish version described the same eight elements of consent as required in the US. However, respondents were not told that the eight listed elements were based on US regulations. All respondents were informed that obtaining written informed consent requires extra time and effort from both the physician and patient, making it difficult to integrate research studies like the one described into routine healthcare practice.
Verbal consent: the survey informed respondents that, in this case, the physician briefly explains the main features of the trial, that the drug will be randomly assigned and will ask the patient if he/she would like to participate.
General notification: respondents were told that all patients are informed through letters, posters and brochures that the hospital conducts randomized trials and that eligible patients would be enrolled without study‐specific consent.
Survey administration
Panel members were randomly assigned to one of the two scenarios (the drug pRCT or dose‐timing pRCT) at the time they were invited to participate in the survey. Respondents were further randomly assigned to general notification or verbal consent as an alternative to written consent when they reached that part of the survey (see Box 1). The randomizations were computer generated and were concealed from respondents. Simple probability‐based assignment was used for randomization, and neither stratification nor an imbalanced allocation scheme was used. To minimize missing data, respondents received a prompt if the primary outcome measures were left blank. Before starting the analysis, we decided to exclude respondents who did not answer the questions on both primary outcome measures. Responses from all participants were recorded in the database in an anonymized fashion; participants could not be identified by study investigators.
Outcome and measurements
Respondents were informed that the two choice options (i.e. written consent vs. general notification; written consent vs. verbal consent) were both supported by some members of the REC and were asked to make a recommendation to the REC. Respondents were also asked which of the two options they preferred personally.
Respondents were asked to evaluate the trial by indicating whether they agreed, using a seven‐point scale (1 = strongly disagree, 7 = strongly agree), with the following three statements: (i) ‘It is valuable to study whether one treatment option is more effective than the other for treating high blood pressure’; (ii) ‘Patients who participate in the randomized trial face greater risks than patients who receive usual care’; and (iii) ‘Patients who participate in the randomized trial are more likely to improve (lower) their high blood pressure than patients who receive usual care’.
Statistical analysis
It was estimated that a sample size of 500 respondents in each group would provide 80% power to detect a 9% absolute difference for all baseline levels of support, assuming a two‐sided α level of 0.05. To evaluate the representativeness of the sample in each group and to ensure the absence of statistically significant differences between groups, we compared the sample distribution of socio‐demographics, and diagnosis and control of hypertension in the four groups with that of the general population in Spain.
To replicate the study by Nayak et al. 9, recommendations to the REC and personal preferences for written consent or the alternative approach were dichotomized. Logistic regression models were used to assess whether the pRCT scenario and alternative consent/notification option were associated with respondents' recommendations and personal preferences. The models included the main effects for the research scenario (drug pRCT; dose‐timing pRCT) and the alternative option (general notification; verbal consent), as well as the interaction of the two factors. Conditional logistic regression was used to assess the relationship between respondents' recommendations to the REC and their personal preferences. Ordered logistic regression was used to assess the effect of the research scenario on respondents' perceptions of the study's value, risk and benefit. To evaluate the association between respondents' perceptions of the study's risk and support for the alternative option, the Pearson chi‐square test of independence corrected for bootstrap was used.
All analyses were conducted in IBM SPSS statistics, version 21 (Armonk, NY, US). According to final sample distribution, poststratification weights were not used. Statistical significance was defined as a P value less than 0.05, and all tests were two sided.
Results
Sample characteristics
The survey was presented to 3298 panel members and started by 2243, of which 45 dropped out before they were randomized to one of the two pRCT scenarios. After randomization, 179 were excluded for nonresponses to one of the two alternative options (written consent vs. general notification or written consent vs. verbal consent). Finally, 11 individuals were excluded for not responding to both primary outcomes (recommendation to the REC and personal preference), yielding 2008 panellist completers (response rate: 60.9%) (Figure 1).
Figure 1.
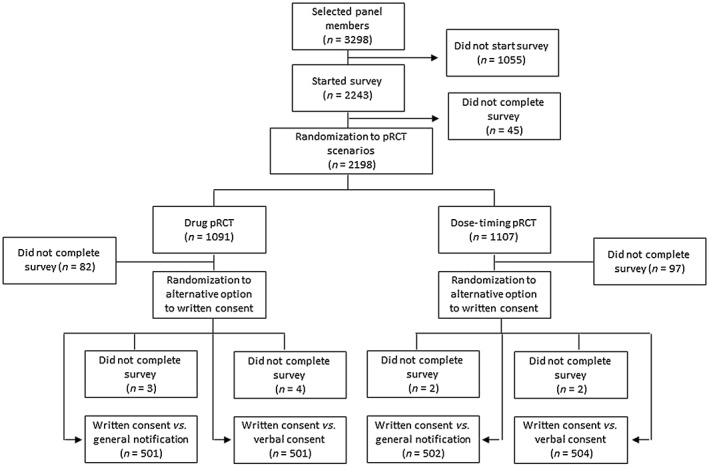
Study flow diagram. pRCT, pragmatic randomized controlled trial
The sample did not show statistically significant differences with the Spanish adult population in any of the variables shown in Table 1, except for age. In Spain, 11.4% 12 of the adult population is ≥75 years of age, whereas in the study sample only 1.9% of subjects fell within this age group. For this reason, poststratification weights were not used. 27.5% (552/2008) of respondents indicated that they had been diagnosed with hypertension and 16.8% (338/2008) reported being treated with prescription medications. This latter figure was similar to the 15.6% of known hypertensive people in Spain who receive treatment 13. The characteristics of the four groups are given in Table 1; they were similar across the four groups and resembled those of the Spanish population in almost all variables.
Table 1.
Characteristics of survey respondents
| Overall | Drug pRCT, % | Dose‐timing pRCT, % | ||||
|---|---|---|---|---|---|---|
| (n = 2008) n (%) | General notification (n = 501) | Verbal consent (n = 501) | General notification (n = 502) | Verbal consent (n = 504) | ||
| Age (years) | ||||||
| 18‐24 | 244 | (12.2) | 12.2 | 12.2 | 12.2 | 12.1 |
| 25‐34 | 349 | (17.4) | 17.4 | 17.4 | 17.3 | 17.5 |
| 35‐44 | 452 | (22.5) | 22.6 | 22.4 | 22.5 | 22.6 |
| 45‐54 | 411 | (20.5) | 20.4 | 20.6 | 20.5 | 20.4 |
| 55‐64 | 302 | (15.0) | 15.2 | 15.4 | 13.9 | 15.7 |
| 65‐74 | 211 | (10.5) | 10.2 | 10.0 | 11.8 | 10.1 |
| ≧75 | 39 | (1.9) | 2.2 | 2.2 | 1.8 | 1.6 |
| Gender | ||||||
| Male | 997 | (49.7) | 52.7 | 49.9 | 49.8 | 46.2 |
| Female | 1011 | (50.3) | 47.3 | 50.1 | 50.2 | 53.8 |
| Geographical area | ||||||
| North | 309 | (15.4) | 15.0 | 15.6 | 16.2 | 15.0 |
| North‐east | 381 | (19.0) | 20.6 | 17.6 | 18.5 | 19.3 |
| East | 285 | (14.2) | 12.8 | 15.0 | 13.8 | 15.3 |
| Central‐West | 526 | (26.2) | 27.0 | 26.3 | 27.3 | 24.3 |
| South | 361 | (18.0) | 16.9 | 17.9 | 17.8 | 18.9 |
| Islands | 146 | (7.2) | 7.8 | 7.6 | 6.4 | 7.4 |
| Marital status | ||||||
| Never married | 498 | (224.8) | 26.1 | 23.2 | 25.9 | 24.0 |
| Married | 974 | (48.5) | 45.3 | 51.5 | 51.2 | 46.0 |
| Divorced | 105 | (5.2) | 7.2 | 4.8 | 4.4 | 4.6 |
| Separated | 37 | (1.8) | 1.6 | 2.4 | 2.0 | 1.4 |
| Widowed | 45 | (2.2) | 2.2 | 2.4 | 1.6 | 2.8 |
| Living with partner | 316 | (15.7) | 15.2 | 15.2 | 13.5 | 19.0 |
| No answer | 33 | (1.6) | 2.4 | 0.6 | 1.4 | 2.2 |
| Annual household income (€) | ||||||
| <12 600 | 276 | (13.7) | 14 | 12.8 | 11.6 | 16.7 |
| 12 600–25 000 | 609 | (30.3) | 28.8 | 30.3 | 32.6 | 29.6 |
| 25 001–38 000 | 308 | (15.3) | 14.2 | 15.4 | 16.3 | 15.5 |
| 38 001–50 000 | 164 | (8.2) | 8.8 | 7.4 | 8.8 | 7.7 |
| >50 000 | 90 | (4.4) | 8.8 | 7.4 | 8.8 | 7.7 |
| No income | 89 | (4.4) | 4.8 | 4.6 | 4.2 | 4.2 |
| No answer | 472 | (23.5) | 24.4 | 24.2 | 22.3 | 23.2 |
| Employment status | ||||||
| Employed | 957 | (47.7) | 46.9 | 50.5 | 47.2 | 46.0 |
| Unemployed or other | 591 | (29.4) | 29.3 | 26.5 | 30.1 | 31.7 |
| Retired | 262 | (13.0) | 13.2 | 13.2 | 12.7 | 13.1 |
| Student | 198 | (9.9) | 10.3 | 9.8 | 10.0 | 9.1 |
| Education | ||||||
| Primary school | 394 | (19.6) | 19.4 | 20.2 | 19.5 | 19.4 |
| Secondary education | 508 | (25.3) | 26.7 | 21.2 | 26.7 | 26.6 |
| High school | 682 | (34.0) | 32.7 | 36.3 | 34.9 | 31.9 |
| University degree and postgraduate | 424 | (21.1) | 21.2 | 22.4 | 18.9 | 22.0 |
| Religious attendance | ||||||
| Regularly | 241 | (12.0) | 11.4 | 13.2 | 11.2 | 12.3 |
| Rarely | 370 | (18.4) | 20.0 | 18.0 | 16.9 | 18.8 |
| Never | 1196 | (59.6) | 58.9 | 60.5 | 62.2 | 56.7 |
| No answer | 201 | (10.0) | 9.8 | 8.4 | 9.8 | 12.1 |
| Ideology | ||||||
| 1 Extreme left | 73 | (2.8) | 2.6 | 3.4 | 3.4 | 1.8 |
| 2 | 405 | (15.1) | 15.4 | 18.2 | 13.3 | 13.7 |
| 3 | 501 | (18.2) | 19.6 | 16.6 | 17.7 | 18.8 |
| 4 Moderate | 800 | (31.1) | 29.5 | 31.1 | 32.7 | 31.2 |
| 5 | 260 | (9.8) | 10.2 | 9.8 | 9.2 | 9.9 |
| 6 | 97 | (3.8) | 2.4 | 5.2 | 3.8 | 4.0 |
| 7 Extreme right | 36 | (1.4) | 1.2 | 0.8 | 2.4 | 1.2 |
| No answer | 445 | (17.8) | 19.2 | 15.0 | 17.5 | 19.4 |
| Diagnosed with hypertension | ||||||
| Yes | 552 | (27.5) | 28.7 | 26.9 | 27.3 | 27.0 |
| No | 1382 | (68.8) | 67.9 | 68.7 | 68.7 | 70.0 |
| I don't know | 61 | (3.0) | 2.8 | 4.0 | 3.2 | 2.2 |
| No answer | 13 | (0.6) | 0.6 | 0.4 | 0.8 | 0.8 |
| Prescription treatment for hypertension | ||||||
| Yes, currently | 338 | (61.2) | 59.0 | 63.0 | 62.0 | 62.0 |
| Yes, but not currently | 58 | (10.5) | 15.3 | 5.9 | 11.7 | 7.4 |
| No | 154 | (27.9) | 25.7 | 31.1 | 25.5 | 30.3 |
| No answer | 2 | (0.4) | 0.7 | 0.4 | ||
pRCT, pragmatic randomized controlled trial
Recommendations to the REC and personal preferences
Overall, 77.1% of respondents said that they would definitely or probably recommend use of written consent to the REC (Figure 2A). In the drug pRCT, 29.7% said that they would recommend general notification, whereas 14% would recommend verbal consent. In the dose‐timing pRCT, 35.1% said that they would recommend general notification, whereas 12.7% would recommend verbal consent instead of written consent.
Figure 2.
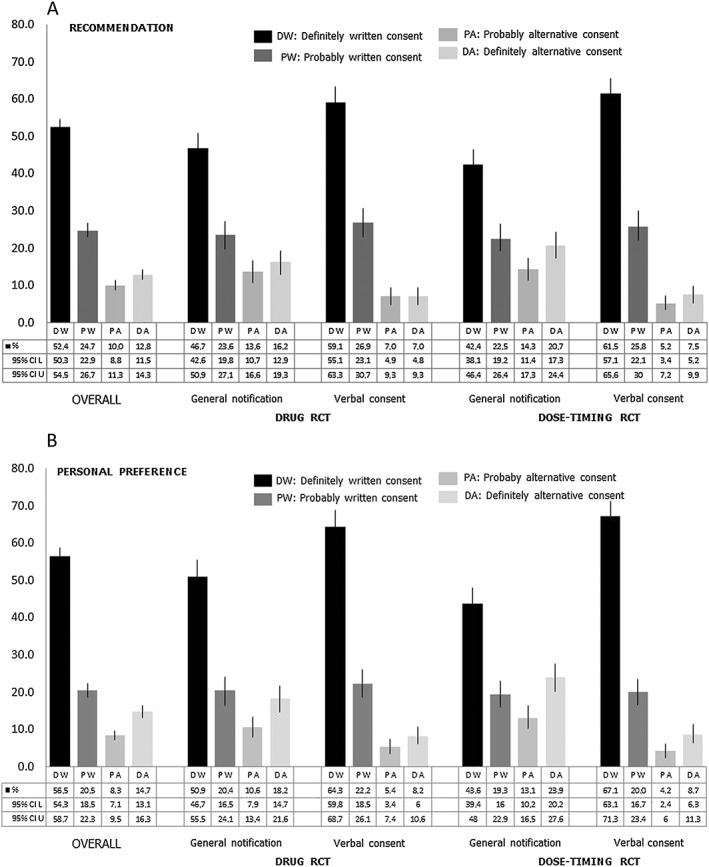
Recommendations to the research ethics committee (A) and personal preferences (B) for written consent and the alternative option. CI, confidence interval; L, lower limit; U, Upper limit; RCT, randomized controlled trial
Overall, 76.9% of respondents said that they would definitely or probably prefer the use of written consent (Figure 2B). In the drug pRCT, 28.7% preferred general notification, whereas 13.6% preferred verbal consent. In the dose‐timing pRCT, 37.1% preferred general notification, whereas 12.9% preferred verbal consent instead of written consent. Figure 3 shows respondents' recommendations to the REC and their personal preferences.
Figure 3.
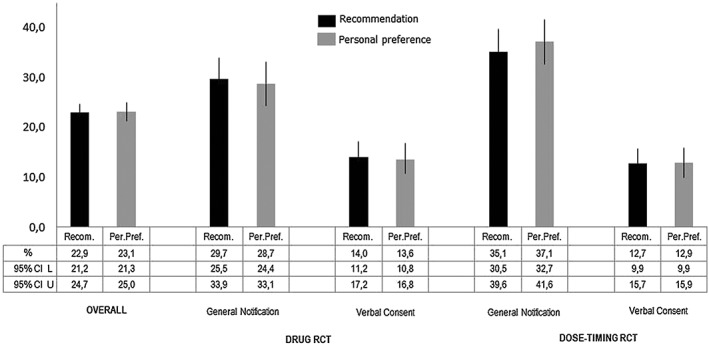
Support for alternative options to written consent. CI, confidence interval; L, lower limit; U, Upper limit; RCT, randomized controlled trial
Considering only the 1003 respondents who had to choose between written consent and general notification in both scenarios (drug pRCT and the dose‐timing pRCT), 39.8% preferred and/or recommended general notification. Similarly, among the 1005 respondents choosing between written consent and verbal consent, 16.7% preferred and/or recommended verbal consent.
Responses to the two items were consistent across groups, with most (85.6–93.4%) having the same recommendation and personal preference (Table 2). In both scenarios (drug pRCT and dose‐timing pRCT), consistency was statistically significantly greater (93.1% vs. 85.6%; P < 0.001) when verbal consent was the alternative option rather than general notification. The percentage of respondents who preferred the alternative option was not statistically significantly different in any of the four groups compared with the percentage of respondents who said that they would recommend the alternative option to the REC (Figure 3).
Table 2.
Cross‐tabulation of respondents' recommendation to the research ethics committee and personal preferences
| Variable | Overall, % (n = 2008) | Drug pRCT, % | Dose‐timing pRCT, % | ||
|---|---|---|---|---|---|
| General notification ( n = 501) | Verbal consent ( n = 501) | General notification ( n = 502) | Verbal consent ( n = 504) | ||
| Recommended written consent, preferred written consent | 71.8 | 63.7 | 82.6 | 56.8 | 83.9 |
| Recommended written consent, preferred alternative option | 5.4 | 6.6 | 3.4 | 8.2 | 3.4 |
| Recommended alternative option, preferred written consent | 5.2 | 7.6 | 3.8 | 6.2 | 3.2 |
| Recommended alternative option, preferred alternative option | 17.7 | 22.2 | 10.2 | 28.9 | 9.5 |
pRCT, pragmatic randomized controlled trial
A logistic regression model was used to test the effect of the experimental design of the survey on recommendations for using the alternative option over written consent. As mentioned above, the main effect was the alternative option presented; in both scenarios, drug pRCT and dose‐timing pRCT, a statistically significant higher percentage of respondents preferred the alternative option of general notification compared with those who preferred verbal consent (P = 0.001). Although recommendation of general notification was greater in the dose‐timing pRCT (35.1%) than in the drug pRCT (29.7%), this difference was not statistically significant (P = 0.078).
With respect to personal preferences, the effect of the alternative option presented reached statistical significance: more respondents preferred general notification than preferred brief verbal consent (P = 0.001). In this case, a statistically significant effect of the interaction ‘alternative option and pRCT scenario’ was observed. A higher percentage of respondents preferred general notification in the dose‐timing pRCT than in the drug pRCT (37.1% vs 28.7%; P = 0.003).
Views of pragmatic RCT scenarios
A large majority of respondents agreed that it is valuable to conduct the study, with no significant differences between the two scenarios: 87.8% in the drug pRCT and 88.7% in the dose‐timing pRCT (Table 3). Some 31% of respondents in both scenarios believed that trial participants would face greater risks than patients, and 43% in both scenarios believed that they were more likely to benefit (have a reduction in their blood pressure) than those receiving usual care.
Table 3.
Views of the public on statements about social value, risk and benefit of the pragmatic randomized controlled trial (pRCT) scenario
| Statement | Scenario | Response, % | P value | ||
|---|---|---|---|---|---|
| Disagree | Neutral | Agree | |||
| It is valuable to study whether one treatment option is more effective than the other for treating high blood pressure | Drug pRCTa | 4.5 | 7.7 | 87.8 | 0.586 |
| Dose‐timing pRCTb | 4.7 | 6.7 | 88.7 | ||
| Patients who participate in the randomized trial face greater risks than patients who receive usual care | Drug pRCTa | 34.7 | 31.6 | 33.6 | 0.053 |
| Dose‐timing pRCTb | 37.8 | 32.7 | 29.5 | ||
| Patients who participate in the randomized trial are more likely to improve (lower) their high blood pressure than patients who receive usual care. | Drug pRCTa | 22.3 | 34.7 | 43.0 | 0.650 |
| Dose‐timing pRCTb | 20.0 | 37.3 | 42.7 | ||
n = 1002
n = 1006
Relationship between risk perception and recommendation to the REC
Overall, respondents who believed that study participants face greater risks than patients receiving usual care were not more likely to recommend the alternative option to written consent compared with those who were neutral or disagreed. When general notification was the alternative option, there were no statistically significant differences in any of the scenarios. By contrast, and only in the dose‐timing pRCT scenario, respondents who believed that participants face greater risks than patients receiving usual care were significantly less likely (P < 0.001) to recommend verbal consent (6.6%) compared with respondents who were neutral (7.2%) or disagreed (21.5%) (Table 4).
Table 4.
Percentage of respondents recommending the alternative option on the basis of perception of the study's risk
| Variable | Perception of study's risk (95% CI), % a | All respondents (95% CI) | P value b | ||
|---|---|---|---|---|---|
| Disagreec | Neutrald | Agreee | |||
| Overall (n = 2008) | 25.3 (22.3–28.5) | 22.4 (19.2–26.0) | 20.5 (17.6–23.7) | 22.9 (21.2–24.7) | 0.107 |
| Drug pRCT | |||||
| General notification (n = 501) | 31.3 (24.0–39.0) | 27.7 (21.4–34.5) | 30.3 (23.1–37.2) | 29.7 (25.5–33.9) | 0.763 |
| Verbal consent (n = 501) | 17.8 (12.7–23.6) | 11.1 (6.6–16.2) | 12.2 (7.6–17.6) | 14.0 (11.3–17.2) | 0.155 |
| Dose‐timing pRCT | |||||
| General notification (n = 502) | 31.7 (24.5–38.7) | 39.5 (32.8–47.6) | 33.8 (26.3–41‐1) | 35.1 (30.5–39.5) | 0.275 |
| Verbal consent (n = 504) | 21.5 (15.6–27.2) | 7.2 (3.4–11.7) | 6.6 (2.9–11.3) | 12.7 (9.9–15.7) | <0.001 |
CI, confidence interval; pRCT, pragmatic randomized controlled trial
‘Patients who participate in the randomized trial face greater risks than patients who receive usual care’
Pearson's chi‐square test of independence corrected by bootstrap
1–3 on a seven‐point scale
4 on a seven‐point scale
5–7 on a seven‐point scale
Discussion
The main finding of the present study was that written informed consent is endorsed by the majority (77%) of the Spanish population for low‐risk pRCTs. This result is similar to that obtained in the US 9 and suggests that a preference for written informed consent in this setting is not restricted to the US. Hence, rather than attempting to waive the requirement for written consent, pRCTs in the EU should attempt to develop approaches to written consent that provide sufficient information without decreasing recruitment or increasing selection bias.
In the US survey, the alternative of verbal consent received more support than general notification 9. By contrast, in the present survey, the alternative of general notification received significantly more support (some 33%) than verbal consent (some 13%) (P = 0.001). This raises the possibility that, with additional educational efforts, the majority of the public in Spain, and perhaps other EU countries, might endorse general notification. This is important because the use of general notification has the potential to increase recruitment, reduce selection bias and make it more feasible to conduct pRCTs in real‐world settings.
There are some differences between the results obtained in Spain and in the US. 9. In particular, 72% and 88% of Americans and Spaniards, respectively, believed that it is valuable to conduct pRCTs; some 21% in the US and 31% in Spain believed that participants in pRCTs face additional risks. Finally, 18% in the US but 43% in Spain believed that participation in pRCTs offers additional benefits compared with standard care. This may reflect the fact that many respondents to the present survey did not understand that participation in the pRCT poses no additional risks and does not offer additional benefits compared with usual care. Greater experience with RCTs in the US compared with Spain, and the fact that the percentage of university graduates responding to the Spanish survey (21%) was lower than in the US study (29%) may help to explain these differences. Finally, in the present survey, significantly more respondents (P = 0.003) supported general notification in the dose‐timing pRCT than in the drug pRCT, something that was not observed in the US study. This result is consistent with the hypothesis that the dose‐timing pRCT might have be perceived among Spanish respondents as more innocuous than the drug pRCT 9.
Strengths and weaknesses of the study
To the best of our knowledge, this was the first study conducted in any EU member state assessing the opinion of the public with regard to written informed consent for low‐risk pRCTs. However, it had several important limitations. First, although the study was based on a probability‐based sample, it had a low percentage (1.9%) of ≥75‐year‐old participants. Second, the response rate was 61%. It was not possible to determine whether nonrespondents might have differed from respondents. Third, framing effects and the use of hypothetical scenarios might have influenced respondents' attitudes. In particular, the hypothetical scenarios, involving pRCTs conducted in clinical settings, were probably unfamiliar to many respondents. Lastly, the study design did not allow us to assess directly which alternative method (verbal consent or general notification) respondents would prefer or recommend.
Policy implications
The new EU regulation 14, to be implemented in 2018 15, distinguishes, for the first time, low‐intervention trials (phase IV trials comparing medicines used in accordance with the marketing authorization that pose minimal added risks) from all other trials. Nonetheless, this regulation 14 requires in‐depth written informed consent for all trials, except for cluster RCTs, which may enrol individuals who do not explicitly opt out.
The present findings suggest that it will be crucial to identify ways to implement written consent without undermining low‐risk pRCTs. For two low‐risk pRCTs, UK RECs have approved short consent forms that comply with EU regulations; the 15‐page consent forms for a typical explanatory RCT have been reduced to a two‐page document 16, 17. This approach – in which participants receive the minimum amount of information needed to make an informed decision – should be encouraged by EU RECs, especially for point‐of‐care pRCTs conducted using routinely collected data from electronic medical records 16, 17, 18. This short consent form could facilitate the recruitment of participants to pRCTs and could help to ensure that pRCTs are not terminated early owing to insufficient enrolment – a common feature within RCTs 19, 20. A further step to ease the consent process for low‐risk pRCTs, and hence facilitate recruitment even further, would be to accept the simplified consent the new EU regulation endorse for cluster RCTs. This is somewhat aligned with a qualitative study conducted in the UK, which found that the majority of 110 individuals supported the use of simplified ‘opt‐in’ models for low‐risk, point‐of‐care trials 21. This change would require an amendment of this regulation.
t the same time, it will be important to assess individuals from several EU countries regarding general notification vs. in‐depth written informed consent for low‐risk pRCTs. As information preferences may differ in response to hypothetical scenarios, it will be important to survey patients 22. If there is sufficient support, it might make sense to start educational efforts to inform the public about the possibility of conducting clinical trials at the same time as providing clinical care. If successful, general notification may increase recruitment and decrease selection bias, and thus significantly improve the evidence available to inform healthcare decision‐making. However, this will involve a change in the EU clinical trials regulation that will require a broad and in‐depth open discussion with interested stakeholders.
Competing Interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work (except for the financial support mentioned below); no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. R.D.R. and X.C. are trustees of the Victor Grifols i Lucas Foundation.
We are grateful to the 2008 anonymous respondents who participated in the survey. This work was supported in part by the Victor Grifols i Lucas Foundation (Barcelona, Spain: http://www.fundaciogrifols.org/en/web/fundacio/home ) – devoted to promote bioethics in the field of human health – but the Foundation had no role in the design of the study; analysis or interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication. The opinions expressed in this article are those of the authors and may not reflect the opinions of the organizations that they work for. D. W. is an NIH employee. The present work was funded in part by intramural research funds of the US NIH Clinical Center. However, the views expressed are the authors' own; they do not represent the position or policy of the NIH, the DHHS or the US government.
Supporting information
Data S1 Survey Questionnaire
Data S2 Test results
Data S3 Anonymous participant level data
Dal‐Ré, R. , Carcas, A. J. , Carné, X. , and Wendler, D. (2017) Public preferences on written informed consent for low‐risk pragmatic clinical trials in Spain. Br J Clin Pharmacol, 83: 1921–1931. doi: 10.1111/bcp.13305.
References
- 1. Garattini S, Jakobsen JC, Wetterslev J, Bertelé V, Banzi R, Rath A, et al. Evidence‐based clinical practice: overview of threats to the validity of evidence and how to minimise them. Eur J Intern Med 2016; 32: 13–21. [DOI] [PubMed] [Google Scholar]
- 2. Tam NT, Huy NT, Thoa le TB, Long NP, Trang NT, Hirayama K, et al. Participants' understanding of informed consent in clinical trials over three decades: systematic review and meta‐analysis. Bull World Health Organ 2015; 93: 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faden R, Kass N, Whicher D, Stewart W, Tunis S. Ethics and informed consent for comparative effectiveness research with prospective electronic clinical data. Med Care 2013; 51 (Suppl. 3): S53–S57. [DOI] [PubMed] [Google Scholar]
- 4. Faden RR, Beauchamp TL, Kass NE. Informed consent, comparative effectiveness, and learning health care. N Engl J Med 2014; 370: 766–768. [DOI] [PubMed] [Google Scholar]
- 5. Platt R, Kass NE, McGraw D. Ethics, regulation, and comparative effectiveness research: time for a change. JAMA 2014; 311: 1497–1498. [DOI] [PubMed] [Google Scholar]
- 6. Kim SY, Miller FG. Informed consent for pragmatic trials – the integrated consent model. N Engl J Med 2014; 370: 769–772. [DOI] [PubMed] [Google Scholar]
- 7. Lantos JD, Wendler D, Septimus E, Wahba S, Madigan R, Bliss G. Considerations in the evaluation and determination of minimal risk in pragmatic clinical trials. Clin Trials 2015; 12: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cho MK, Magnus D, Constantine M, Lee SS, Kelley M, Alessi S, et al. Attitudes toward risk and informed consent for research on medical practices: a cross‐sectional survey. Ann Intern Med 2015; 162: 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nayak RK, Wendler D, Miller FG, Kim SY. Pragmatic randomized trials without standard informed consent?: A national survey. Ann Intern Med 2015; 163: 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grady C. Enduring and emerging challenges of informed consent. N Engl J Med 2015; 372: 855–862. [DOI] [PubMed] [Google Scholar]
- 11. Institute of Medicine of the National Academies . The Learning Healthcare System: Workshop Summary, eds Olsen LA, Aisner D, Mc Ginnis JM. Washington, DC: The National Academy Press, 2007. [PubMed] [Google Scholar]
- 12. INE [(Spanish) Statistics National Institute] . Continuous municipal register statistics, 1 January 2016 [online] . Available at http://www.ine.es/jaxi/menu.do;jsessionid=F15BF31A490ECD361244D34CAC102326.jaxi01?type=pcaxis&path=%2Ft20%2Fe245&file=inebase&L=1 (last accessed 24 May 2017).
- 13. Banegas JR, Graciani A, de la Cruz‐Troca JJ, León‐Muñoz LM, Guallar‐Castillón P, Coca A, et al. Achievement of cardiometabolic goals in aware hypertensive patients in Spain: a nationwide population‐based study. Hypertension 2012; 60: 898–905. [DOI] [PubMed] [Google Scholar]
- 14. Regulation (EU) no. 536/2014 of the European Parliament and of the council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing directive 2001/20/EC. Official Journal of the European Union L 158/1–76. 27 May 2014 [online], Available at http://ec.europa.eu/health/files/eudralex/vol‐1/reg_2014_536/reg_2014_536_en.pdf (last accessed 24 May 2017).
- 15. European Medicines Agency . Clinical trial regulation [online]. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000629.jsp&mid=WC0b01ac05808768df (last accessed 24 May 2017).
- 16. Staa TP, Goldacre B, Gulliford M, Cassell J, Pirmohamed M, Taweel A, et al. Pragmatic randomised trials using routine electronic health records: putting them to the test. BMJ 2012; 344: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gale C, Hyde MJ, Modi N, WHEAT trial development group . Research ethics committee decision‐making in relation to an efficient neonatal trial. Arch Dis Child Fetal Neonatal Ed 2016. Sep 14. pii: fetalneonatal‐2016‐310935. https://doi.org/10.1136/archdischild‐2016‐310935; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carcas AJ, Abad Santos F, Sánchez Perruca L, Dal‐Ré R. Electronic medical record in clinical trials of effectiveness of drugs integrated in clinical practice. Med Clin (Barc) 2015; 145: 452–457. [DOI] [PubMed] [Google Scholar]
- 19. Mc Donald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kasenda B, von Elm E, You J, Blümle A, Tomonaga Y, Saccilotto R, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA 2014; 311: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 21. Identifying and recruiting participants for health research: a public dialogue for the Health Research Authority. London: OPM Group, July 2015 [online].Available at http://www.opm.co.uk/wp‐content/uploads/2015/07/hra‐sciencewise‐dialogue‐report‐final‐july‐2015‐recruiting‐participants.pdf (last accessed 24 May 2017).
- 22. Kraft SA, Cho MK, Constantine M, Lee SS, Kelley M, Korngiebel D, et al. A comparison of institutional review board professionals' and patients' views on consent for research on medical practices. Clin Trials 2016; 13: 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Survey Questionnaire
Data S2 Test results
Data S3 Anonymous participant level data


