Abstract
Safe maximal surgical resection is the initial treatment of choice for pediatric brainstem low-grade gliomas. Optimal therapy for incompletely resected tumors or that progress after surgery is uncertain. We reviewed the clinical characteristics, therapy, and outcomes of all children with nontectal brainstem low-grade gliomas treated at the University of Michigan between 1993 and 2013. Median age at diagnosis was 6 years; histology was confirmed in 23 of 25 tumors, 64% were pilocytic astrocytoma. Nineteen patients underwent initial tumor resection; 14/19 received no upfront adjuvant therapy. Eight patients in the study had progressive disease; 5 initially resected tumors received chemotherapy at tumor relapse, all with partial or complete radiographic responses. Ten-year progression-free survival is 71% and overall survival, 100%. This single-institution retrospective study demonstrates excellent survival rates for children with brainstem low-grade gliomas. The efficacy of the well-tolerated chemotherapy in this series supports its role in the treatment of unresectable or progressive brainstem low-grade gliomas.
Keywords: brainstem tumors, low-grade glioma, progressive tumors, chemotherapy, overall survival
Neoplasms that arise in the brainstem constitute approximately 15% of pediatric brain tumors.1 The majority (80%) of these brainstem tumors are malignant diffuse intrinsic pontine gliomas, but most of the balance are brainstem low-grade gliomas.2 Children with diffuse intrinsic pontine gliomas present with rapidly progressive cranial nerve deficits, weakness or ataxia, have characteristic magnetic resonance imaging (MRI) findings and abysmal outcomes, with very short survival.3 Conversely, brainstem low-grade gliomas are generally slow growing with an indolent course and more favorable prognosis.
Brainstem low-grade gliomas can be classified by location, as tectal midbrain, tegmental midbrain, focal pontine, pontomedullary, medullary, and cervicomedullary. Children with tectal gliomas typically present with hydrocephalus from obstruction of the cerebral aqueduct and can usually be managed with cerebrospinal fluid diversion (shunt or endoscopic third ventriculostomy) and radiographic surveillance, without other treatment.4 Brainstem low-grade gliomas in locations other than tectal midbrain often require more definitive therapy. With the advent of the MRI and advances in operative techniques, neurosurgical resection is attempted for many nontectal brainstem low-grade gliomas, and outcomes have greatly improved. However, complete resection is not always possible because of the critical location of these tumors. As well, in a subset of patients with minimal signs or symptoms, the potential for operative deficits may be great enough to favor a conservative approach of watchful waiting. Unresected or incompletely resected brainstem low-grade glioma tumors have the potential for gradual growth, estimated in one series at an average of 4 mm increase in tumor diameter per year.5 There is lack of consensus regarding optimal adjuvant therapy for tumors that are incompletely resected or those that progress after initial resection. Both radiotherapy and chemotherapy can be effective treatment, but optimal management in various specific settings remains uncertain.
The goal of this study was to evaluate the tumor characteristics, clinical features, management and outcomes of all children with nontectal brainstem low-grade gliomas treated at our institution over a 20-year period (1993–2013). This included the safety and efficacy of surgical intervention, adjuvant and salvage chemotherapy and/or radiation therapy, survival outcomes, treatment sequelae, and molecular characterization of the tumors.
Methods
Study Subjects
A retrospective review of the medical records was conducted on all children <21 years of age with a diagnosis of nontectal brainstem lowgrade gliomas, treated and followed at the University of Michigan C. S. Mott Children’s Hospital from 1993 to 2013. The study was approved by the institutional review board. Subjects were eligible for the study if they had imaging findings consistent with a brainstem lowgrade glioma—a well circumscribed or exophytic lesion that is hypointense on T1-weighted images, hyperintense on T2-weighted images and enhancing with contrast, or if there was a histologic confirmation of a low-grade glioma in brainstem. Tumor location was identified as nontectal midbrain, pontine, pontomedullary, medullary or cervicomedullary. Patients with tectal gliomas were excluded from the study because these tumors are usually treated with cerebrospinal fluid diversion and radiographic surveillance, without need for definitive tumor therapy. Tumor treatment may have included surgical resection or biopsy, chemotherapy and radiotherapy.
Data Collected
Data pertaining to the clinical presentation, tumor imaging characteristics and histopathologic diagnosis, treatment and treatment-related complications or toxicities including long-term neurologic sequelae, and survival outcomes, were collected for all patients. The extent of surgical resection was based on the neurosurgeon’s intraoperative estimate and findings on postoperative imaging and defined as gross total resection, no residual disease; near total resection, >90% resection; subtotal resection, 50% to 90% resection; partial resection, <50% resection; and biopsy only. Chemotherapy was used as upfront adjuvant or neoadjuvant therapy or when there was tumor progression; chemotherapy regimens included (1) carboplatin/vincristine; (2) thioguanine/procarbazine/lomustine/vincristine; (3) cyclic oral temozolomide (TMZ) at a dosing schedule of 150 to 200 mg/m2/day × 5 days in 28-day cycles. Administration of carboplatin/vincristine and thioguanine/procarbazine/lomustine/vincristine were patterned on Children’s Cancer Group protocol A9952.6 Conventionally fractionated radiation to the site of the primary tumor using 6-to 15-MeV photons was administered to those treated with radiotherapy as upfront therapy or at tumor progression. No patients were treated with proton therapy.
BRAF Mutation Analysis
Unstained slides and a corresponding hematoxylin-eosin (H&E) slides were prepared from formalin-fixed paraffin-embedded tissue blocks. Areas containing high tumor content were selected by a pathologist (N.A.B). Genomic DNA was extracted from 2–6 unstained slides within the selected area using the Pinpoint Slide DNA Isolation System (Zymo Research). RNA was extracted from 10-mm scrolls using TRIzol LS (Thermo Fisher Scientific). BRAFV600E mutation testing was performed on extracted DNA as previously described.7 KIAA1549-BRAF fusion testing was performed on RNA using a reverse transcription–polymerase chain reaction assay designed with forward primers within exons 15 and 16 of KIAA1549 and reverse primers within exons 9 and 11 of BRAF. The assay was validated to detect the 3 most common fusion transcripts—exon 16/exon 9, exon 15/exon 9, and exon 16/exon 11—as well as the more unusual exon 15/exon 11 transcript. Together, these cover approximately 98% of KIAA1549-BRAF fusions occurring in pilocytic astrocytoma according to the COSMIC database (http://cancer.sanger.ac.uk/cosmic).
Analysis
The primary outcome measures were 10-year progression-free survival and overall survival. Progression-free survival was defined as the time from diagnosis to progression or death; overall survival was defined as the time from diagnosis to death or end of study period. Kaplan Meier curves were used to estimate both progression-free survival and overall survival.
Results
Between 1993 and 2013, a total of 25 children with a diagnosis of nontectal brainstem low-grade glioma consecutively treated at the University of Michigan C. S. Mott Children’s Hospital were eligible for the study. Three additional children with brainstem low-grade gliomas were excluded from the analysis because they had received the majority of their care at other institutions and datasets were insufficient. There were 13 boys/12 girls, with a median age at diagnosis of 6 years (range, 4 months to 16 years); none of the children had a diagnosis of neurofibromatosis type 1 (or type 2). Patient demographics, presenting symptoms, tumor location, and histopathologic diagnosis are shown in Table 1. Sixty-four percent of the tumors were pilocytic astrocytoma and 68% were dorsally exophytic tumors. Histopathologic diagnosis was made in 23/25 patients. No tissue was available in 2/25 patients in whom the diagnosis was based on MRI findings that were strongly suggestive of a brainstem low-grade glioma (Figure 1).
Table 1.
Characteristics of Study Subjects (N = 25).
| Characteristic | |
|---|---|
| Age | |
| Median age at diagnosis | 6 years (range: 4 mo–16 y) |
| Gender | |
| Male | 13 |
| Female | 12 |
| Tumor location | |
| Nontectal midbrain | 6 |
| Pontine | 3 |
| Pontomedullary | 7 |
| Medullary | 3 |
| Cervicomedullary | 6 |
| Histology | 23/25 |
| Pilocytic astrocytoma | 16 (64%) |
| WHO Grade II astrocytoma | 3 (12%) |
| Ganglioglioma | 2 (8%) |
| Mixed oligoastrocytoma | 2 (8%) |
| Diagnosis by MRI only | 2/25 (8%) |
| Presenting features | |
| Headache | 12 (48%) |
| Vomiting | 11 (44%) |
| Hydrocephalus | 14 (56%) |
| Cranial nerve or extremity paresis | 16 (64%) |
| (Including dysphagia) | |
| Failure-to-thrive | 5 (20%) |
| Central sleep apnea | 1 (4%) |
Figure 1.
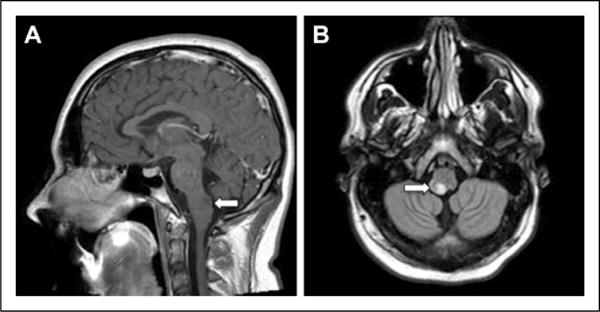
Magnetic resonance images (MRIs) in an 11-year-old boy who has been followed closely, with no biopsy for confirmation and has not had any therapeutic intervention (Table 2). (A) T1-weighted sagittal non contrast image and (B) T1 axial flair image showing a dorsally exophytic medullary brainstem lesion.
Twenty-two patients underwent an initial surgery, either biopsy (3) or surgical resection (19); extent of resection was gross total resection/near total resection (10), subtotal resection (7), partial resection (2) (Table 2). One additional patient underwent a gross total resection after tumor progression following upfront radiotherapy and chemotherapy. Among the 19 patients who underwent tumor resection at diagnosis, 14 patients received no further upfront treatment, whereas 5 patients received adjuvant therapy (chemotherapy [3], radiotherapy [2]). Among the 6 patients who underwent no tumor resection at diagnosis (biopsy [3], no surgery [3]), 1 patient received no initial therapy and 5 received (neo)adjuvant therapy (radiotherapy [3], radiotherapy + chemotherapy [2]).
Table 2.
Clinical Presentation and Outcome of the Study Subjects.
| Surgery at diagnosis |
Extent of resection |
Age at diagnosis in years |
Tumor location | Histology | BRAF alteration | Neurologic deficits at presentation |
Hydrocephalus/ VP shunt |
Adjuvant therapy |
Progression/ PFS |
Salvage therapy |
Disease outcome |
Follow-up, (in years) |
Sequelae | Clinical outcome (compared with status at diagnosis) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes, n = 22 | GTR = 4 | 6 | Cervicomedullary, DE | Ganglioglioma | Inadequate | - R hemiparesis - Ataxia |
No/no | None | No | – | NED | 11 | Right hemiparesis | No change |
| 16 | Pontomedullary, DE | PA | NA | - Ataxia | Yes/yes | None | No | – | NED | 3 | -Tracheostomy - Multiple cranial nerve palsy - Ataxia - G tube dependent |
Worse | ||
| 5 | Pontomedullary, DE | PA | Negative | - Dysphagia - FTT |
Yes/no | None | No | – | NED | 9 | None | Better | ||
| 1.8 | Medullary, DE | PA | Negative | - Abnormal tongue movements -FTT | Yes/no | None | No | – | NED | 12 | Hemiatrophy and fasciculation of tongue | Better | ||
| NTR = 6 | 5 | Pontine, focal | PA | NA | - Right sensorineural hearing loss | No/no | None | Yes/3 mo | CT VCR/carboplatin |
NED | 2.5 | - U/L deafness - Right facial nerve paresis |
Worse | |
| 1.6 | Cervicomedullary, DE | PA | Inadequate | - Dysphagia -FTT - Quadriparesis |
Yes/no | None | Yes/4 mo | Resection + CT VCR/carboplatin |
NED | 18 | Central sleep apnea | Better | ||
| 0.8 | Cervicomedullary, DE | PA | Inadequate | - Torticollis - R sixth nerve palsy -RUE monoparesis |
No/no | None | No | – | SD | 10 | None | Better | ||
| 6 | Pontomedullary, DE | PA | Inadequate | - L sixth nerve palsy - L hearing loss - L seventh nerve palsy - L hemiparesis |
Yes/no | None | No | – | SD | 12 | - L seventh nerve palsy - L sensorineural hearing loss - L hemiparesis |
No change | ||
| 5.5 | Pontomedullary, DE | Ganglioglioma | KIAA1549- BRAF | - Dysphagia | No/no | None | No | – | SD | 6 | None | Better | ||
| 2 | Midbrain tegmentum | PA | KIAA 1549- BRAF | - L hemiparesis | No/no | None | Yes/4 mo | CT VCR/carboplatin |
NED | 1.6 | None | Better | ||
| STR = 7 | 2 | Midbrain tegmentum, LE | PA | NA | - Dysphagia - Diplopia - Ataxia - FTT |
Yes/yes | None | Yes/15 mo | CT TPCV |
SD | 10.5 | - Dysphagia - Left hemiparesis |
Worse | |
| STR = 7 | 16 | Cervicomedullary, DE | PA | Inadequate | - None | No/no | None | No | – | SD | 2 | None | No change | |
| 11 | Pontine, focal DE | PA | Negative | - None | Yes/no | None | Yes/16 y | Resection +RT | SD | 17 | None | No change | ||
| 4.25 | Cervicomedullary, DE | PA | NA | - Apnea - Ataxia - Dysphagia - Right hemiparesis |
No/no | None | Yes/6 mo | CT VCR/carboplatin |
SD | 14.5 | - Sleep apnea - Bulbar dysfunction - Right hemiparesis - Tracheostomy - G tube dependent |
Worse | ||
| 16 | Pontomedullary, DE | PA | Inadequate | None | Yes/no | RT | No | – | SD | 15 | Mild ataxia | Worse | ||
| 5 | Pontomedullary, LE | PA | KIAA 1549- BRAF | - None | Yes/no | CT TPCV |
No | – | NED | 2 | None | No change | ||
| 8 | Cervicomedullary, DE | Mixed OA | NA | - Obstructive sleep apnea - Stridor |
No/no | RT | No | – | SD | 8 | Obstructive sleep apnea | No change | ||
| PR = 2 | 14 | Pontine, focal | Grade II Astro | Inadequate | - R seventh nerve palsy - B/L abductor palsy |
No/no | CT TMZ |
No | – | SD | 7 | - R seventh nerve palsy - B/L abductor palsy |
No change | |
| 0.3 | Pontomedullary, DE | Mixed OA | NA | - Dysphagia - FTT |
Yes/yes | CT VCR/carboplatin |
Yes/3 y | RT | SD | 13 | - Central sleep apnea - T racheostomy |
Worse | ||
| Biopsy = 3 | 11 | Midbrain tegmentum, DE | Grade II Astro | NA | - Dysphagia - B/L ptosis - Ataxia - Dysarthria |
Yes/no | RT+CT TPCV |
No | – | SD | 6 | None | Better | |
| 13 | Midbrain tegmentum | PA | KIAA 1549- BRAF | - Ataxia - Right hemiparesis |
Yes/yes | RT | No | – | SD | 13 | Right hemiparesis | Better | ||
| 8 | Midbrain | Grade II Astro | NA | -Left hemiparesis | No/no | RT | No | – | SD | 4 | None | Better | ||
| None, n = 3 | 11 | Medullary, DE | NA | - None | No/no | None | No | – | SD | 2 | None | No change | ||
| 14 | Midbrain tegmentum | PA | Inadequate | -Left hemiparesis - Right third nerve paresis | Yes/yes | RT+ 1 cycle CT VCR/carboplatin | Yes/3 mo after RT, during CT | Resection after failure of RT and CT | NED | 5.5 | - Left hemiparesis - Right third nerve paresis |
No change | ||
| 7 | Medullary, DE | NA | - Hyponasal speech | Yes/yes | RT | No | – | SD | 15 | Hyponasal speech | No change |
Abbreviations: CCNU, lomustine; CT, chemotherapy; DE, dorsally exophytic; FTT, failure to thrive; GTR, gross total resection; LE, laterally exophytic; NA, not available; NED, no evidence of disease; NTR, near total resection; OA, oligoastrocytoma; PA, pilocytic astrocytoma; PFS, progression-free survival; PR, partial resection; RT, radiotherapy; SD, stable disease; STR, subtotal resection; TMZ, temozolomide; TPCV, thioguanine, procarbazine, lomustine, vincristine
A total of 8/25 patients experienced progressive disease at a median of 5 months from diagnosis (range, 3 months to 16 years). Seven of these had undergone tumor resection at diagnosis (3 near total resection, 3 subtotal resection, and 1 partial resection + adjuvant chemotherapy). One of the 8 patients with progressive disease underwent no initial surgery but had a gross total resection after progression following failed radiotherapy plus 1 cycle of chemotherapy. Only 2 of the 8 patients with progressive disease received adjuvant therapy at diagnosis (chemotherapy [1] and radiotherapy + chemotherapy [1]).
Salvage therapy for the 8 patients with progressive disease included a first surgery/resection (gross total resection) in 1 patient; repeat resection in 2 patients (followed by radiotherapy in 1 and by chemotherapy in 1); chemotherapy only in 4 patients; radiotherapy only in 1 patient. None of these 8 patients have since progressed and are alive with stable disease in 4 or no evidence of disease in 4. Likewise, the 17 of 25 patients who did not progress are all alive with stable disease or no evidence of disease. Thus, the Kaplan-Meier estimates of 10-year survival for the entire cohort are progression-free survival of 71% and overall survival of 100% (Figure 2). Although none of the 4 patients who underwent a gross total resection at diagnosis experienced tumor progression, neither gross total resection nor any other extent of resection correlated with progression-free survival (P = .44) (Figure 3). A schema of the therapy used and outcomes for all those with progressive disease is outlined in Figure 4.
Figure 2.
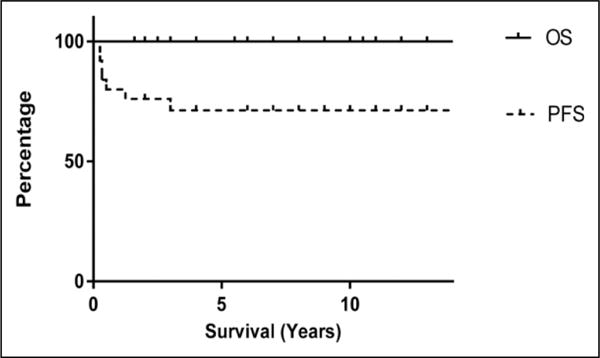
Kaplan-Meier Survival curves for all patients.
Figure 3.
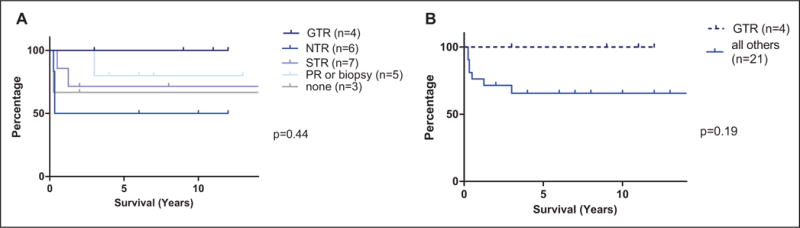
Progression-free survival based on extent of resection: (A) gross total resection vs the individual groups and (B) gross total resection vs the rest.
Figure 4.
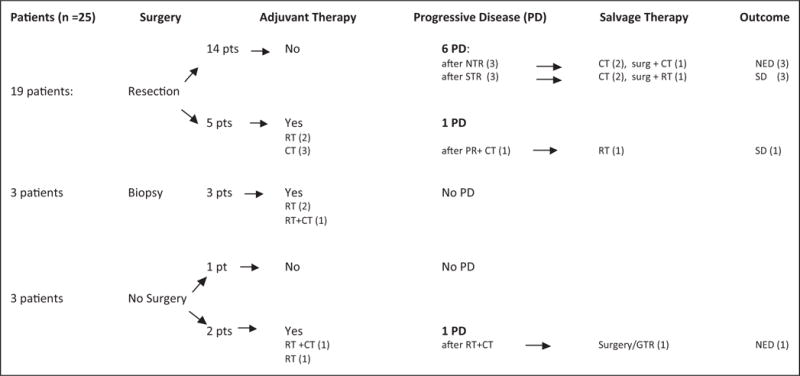
Brainstem low-grade glioma therapy schema and salvage therapy/outcomes after progressive disease.
Chemotherapy was generally well tolerated. Only 1 of the 10 patients who received chemotherapy (5, adjuvant at diagnosis; 5, salvage at progression), experienced grade 3 myelosuppression that delayed chemotherapy and required dose reduction for prolonged myelosuppression. Two patients developed manageable hypersensitivity reactions to carboplatin toward their end of therapy. One patient developed grade 3 constipation on vincristine that required dose reduction.
At last follow up, at a median of 9 years, 5 patients had persistent, moderately severe adverse neurologic sequelae that were first noted shortly after surgical resection. All 5 had undergone surgical resection at outside institutions without pediatric neurosurgical expertise, prior to transfer to our institution; one also received subsequent adjuvant radiotherapy, and 3 salvage chemotherapy at tumor progression. Among the 9 children who received radiotherapy (at a median age of 11 years, range, 3.3–16 years), 5 had no adverse sequelae during the study period, one developed hypothyroidism and one, growth hormone deficiency, both requiring hormonal supplementation and 4 sustained neurocognitive dysfunction of varying severity. One patient had language impairment in the form of mild fluent dysphasia, and another had learning difficulties that required special education support in school. Two others had more severe deficits, one with severe psychiatric disturbance that precluded independent living, and the other with moderate cognitive impairment (IQ 65 on formal neuropsychometric testing).
Determination of tumor BRAF mutation status was attempted in this cohort and was successful in 7 tumors; but tissue was not available/inadequate or of poor quality in the remaining tumors. We detected a KIAA1549-BRAF fusion in 4/7 tumors whereas all 7 were negative for the BRAFV600E mutation (Table 2).
Discussion
Brainstem low-grade gliomas are relatively rare brain tumors of childhood and, despite their benign histopathology, have the potential to cause significant neurologic morbidity because of their location.2, 8–10 Whenever feasible, surgical gross total resection has been proposed as the treatment of choice for pediatric brainstem low-grade gliomas. Several studies that report sustained disease control after complete tumor resection support this view, as does our finding of no recurrences among the 5 patients (4 at diagnosis, 1 at progression) whose tumors were totally resected.11–14
Although our data support that a complete resection is desirable, because these tumors arise near critical brainstem structures, cranial nerve nuclei, sensory and motor long tracts, an aggressive attempt at total resection can leave the child with significant neurologic deficits.15 Five patients in our cohort developed new major neurologic deficits following surgical resection of their tumors. Three of these involved the pons, perhaps suggesting increased potential for morbidity from tumor resection in this location. Moreover, these patients underwent surgery at nonpediatric centers before transfer to our institution for further management, underscoring the critical importance of optimal pediatric neurosurgical expertise in the management of brainstem low-grade gliomas in children. The decision about the extent of an attempted resection rests on the neurosurgeon’s expertise/experience and knowledge of risk for operative injury weighed against the safety and efficacy of other available therapies for tumor control.
When a brainstem low-grade glioma cannot be totally resected but the extent of residual tumor is modest and the child has minimal deficits, expectant management with close radiographic surveillance seems a reasonable approach. But for patients with significant and symptomatic residual tumor, the prudence of watchful waiting is less certain. The decision to employ adjuvant therapy upfront after an incomplete resection or biopsy hinges on the risk of irreversible injury from further tumor growth balanced against the potential toxicity of adjuvant therapies, as well as their effectiveness for rescue at tumor relapse.
A retrospective review of 96 patients treated at Hospital for Sick Children in Toronto, reported a favorable outcome after upfront observation as a first-line management in some patients with brainstem low-grade gliomas and significant residual tumor after resection. Although 54% of 42 tumors progressed after observation following incomplete resection, there was no difference in their progression-free survival compared to a subgroup of 24 patients who received upfront chemotherapy or radiotherapy in the presence of significant residual disease. Moreover, there was no survival disadvantage for the observation group in whom the progression-free survival and overall survival were 57% and 93%, comparable to 57% and 89% for the entire cohort.16 A very good progression-free survival of 70% was similarly noted for cervicomedullary low-grade gliomas following surgical resection alone, by Robertson et al.17 Lundar et al demonstrated a favorable 47% progression-free survival in 15 patients with low-grade midbrain tumors that were surgically resected upfront. No further adjuvant therapy or repeat resection was required in these patients, the majority of whom had not undergone a total resection.12 Thus, expectant surveillance is a safe approach for many brainstem low-grade gliomas with residual tumor after diagnosis. However, a significant fraction of these tumors do progress and require additional treatment, for which both chemotherapy and radiotherapy have been effective therapies.
The radiotherapy required for pediatric brainstem low-grade gliomas can be delivered to a focal tumor volume, and taken together with the infratentorial location of these tumors, this should decrease the potential for severe neurologic/neurocognitive sequelae compared with irradiation of supratentorial tumors or the whole brain. No neurocognitive deficits were reported among 4 children with focal pontine tumors conformally irradiated to a dose of 54 Gy by Edward et al.18 However, Freeman et al found school performance difficulties, seizures, hearing, and neuroendocrine deficits in 7 of 8 long-term survivors of brainstem gliomas treated with radiotherapy.19 As well, young children treated with radiotherapy for posterior fossa ependymomas, tumors very close to brainstem, were reported to have emotional/behavioral function below the mean, as well as attention deficits and academic achievement problems, even with the use of conformal radiotherapy.20 Development of anaplastic astrocytomas and late-occurring stroke are additional concerns in children who receive cranial irradiation for low-grade tumors.21–23 Moreover, in the largest study of long-term outcomes of pediatric low-grade gliomas, from the SEER database (4040 children with low-grade gliomas, 12% brainstem low-grade gliomas), for the entire cohort, not only was the 20-year overall survival inferior in children who received radiotherapy, but the increased 2.4 hazard ratio of death due to nontumor causes in the irradiated group suggests that radiotherapy-induced mortality accounted for some of the deaths.24
In our study, at a median follow-up of 9 years, among the 9 patients who received radiotherapy, 5 patients had no adverse sequelae attributable to radiotherapy, but 2 patients acquired neuroendocrine deficits and 4 patients developed varying degrees of neurocognitive or psychiatric dysfunction. These long-term sequelae further emphasize prudence when considering radiotherapy for these tumors, even with focal administration.
Despite these limitations constraining its use with impunity, radiotherapy has substantial efficacy in the treatment of brainstem low-grade gliomas, either as initial therapy (with or without surgical resection) and for salvage of relapsed tumors.25–28 In the Toronto series of 96 children with brainstem low-grade gliomas, radiotherapy was used in 22 patients (either as adjuvant or salvage treatment) with 5-year progression-free survival and overall survival of 66% and 83%, respectively, comparable to that of the entire cohort.16 From another single institution review of 52 pediatric brainstem low-grade gliomas at St Jude Children’s Research Hospital, in which 5-year event-free survival and overall survival were 59% and 98%, upfront radiotherapy provided prolonged progression-free survival in 17/21 patients with incompletely resected or biopsied tumors, and more than 70% of relapsed patients were successfully salvaged with radiotherapy.9 The use of radiotherapy was more limited, but also effective in our study. Six of seven patients who received upfront adjuvant radiotherapy have not experienced tumor progression (after incomplete resection [2], biopsy [3], or no surgery [1]); all but one was older than 7 years at the time of irradiation. Additionally, 2 patients were successfully salvaged with radiotherapy (+/− re-resection) at progression, at ages 27 years and 4 years. Nevertheless, given the potential long-term side effects of cranial irradiation, postponement, at least until the child is older and can tolerate it with fewer sequelae, seems a desirable goal.
Chemotherapy may also be effective as adjuvant therapy against relapse/progression of brainstem low-grade gliomas, and as salvage therapy for recurrent or progressive tumors. In these settings, chemotherapy may obviate high-risk surgical tumor resection or repeat resection, and may permit deferral of radiotherapy in a young child until an older age when it is better tolerated. Chemotherapy seems particularly advantageous for the treatment of tumors in young children deemed high risk for surgical resection, but with neurologic deficits that may worsen with any further tumor growth.
However, data for the safety and efficacy of chemotherapy for brainstem low-grade gliomas is limited. This data in children is derived mainly from studies of incompletely resected or progressive low-grade gliomas in any brain location, the majority of which are hypothalamic/optic pathway tumors, with few brainstem gliomas. Various combinations of chemotherapeutic agents have been successfully used in these studies, but outcomes for the small numbers of brainstem tumors are not separately reported.6, 8, 29–34 A randomized phase III CCG study of 102 incompletely resected or progressive low-grade gliomas demonstrated a 50% event-free survival with no difference between the 2 regimens (carboplatin and vincristine vs thioguanine, procarbazine, CCNU [lomustine], and vincristine).6 A large multicenter German HIT 96 study also successfully used carboplatin/vincristine to avoid radiotherapy in a large majority of children with low-grade gliomas, and reported 75% 5-year progression-free survival.8 In both studies, the carboplatin and vincristine regimen was generally well tolerated, with hypersensitivity reaction to carboplatin being the most common reason for patients to come off therapy.
Rhonge et al reported the outcome of 16 unresectable or recurrent brainstem low-grade gliomas treated with carboplatin/vincristine at 2 Canadian centers. The chemotherapy was well tolerated and afforded a 5-year 70% progression-free survival and 100% overall survival.2 Our data also support efficacy of chemotherapy for brainstem low-grade gliomas. Ten of our 25 patients received chemotherapy (6 carboplatin/vincristine; 3 thioguanine, procarbazine, lomustine, vincristine; 1 temozolomide), either as adjuvant therapy in 5, or at the time of tumor progression in 5. There was tumor progression in 2/5 patients who received adjuvant chemotherapy, but all 5 patients who received chemotherapy for salvage of progressive tumor are alive and progression-free at a median of 8 years’ follow-up after initial progression.
There have been recent major advances in understanding the molecular mechanisms that underlie the oncogenesis of pediatric low-grade gliomas. These include very frequent BRAF gene fusions in pilocytic astrocytomas, as well as other BRAF mutations and various downstream MAP kinase pathway alterations in other low-grade gliomas, depending on location and including brainstem low-grade gliomas.35 Molecular evaluation was possible in 7 tumors in this cohort, 4 of whom had the KIAA1549-BRAF fusion; all 7 patients were negative for the BRAFV600E mutation. These findings are consistent with those found among pediatric pilocytic astrocytomas and other low-grade gliomas in previous studies, but meaningful clinical correlations in our brainstem low-grade glioma cohort is not possible because of the small number of molecular analyses. However, with the emerging development of personalized medicine, integration of this molecular information into diagnostic evaluation of brainstem low-grade gliomas as well as of other pediatric low-grade gliomas, will be increasingly important for treatment decision making, as more targeted therapies become available.
Conclusions
Although there remain challenges in the management of brainstem low-grade gliomas, it is heartening to confirm the findings of others, that the combination of skilled surgery, radiotherapy, and chemotherapy results in excellent survival of children with brainstem low-grade gliomas, with a 10-year 71% progression-free survival and 100% overall survival of our study patients, all alive with stable disease or no evidence of disease.
Findings of this study support the view that totally resected brainstem low-grade glioma tumors have an excellent chance of cure without other therapy, but also that many tumors with residual disease after resection or even biopsy may be safely followed and further treated at the time of progression without jeopardizing ultimate tumor control and long-term survival. An exception to this latter conclusion may be for patients with significant neurologic deficits that are likely to worsen with any tumor progression.
There is already good evidence supporting the efficacy of radiotherapy for brainstem low-grade gliomas, also corroborated by the results of our study. The findings in our cohort also strengthen previously limited data, that chemotherapy useful in the treatment of low-grade gliomas in other locations is valuable for brainstem low-grade gliomas, as well. These results reinforce a role for chemotherapy in the treatment of these tumors, especially when it is important to avoid the potential long-term sequelae of radiotherapy in younger children, and to obviate aggressive surgery for tumors at very high risk for surgical morbidity. Nevertheless, in view of the overall favorable prognosis of these tumors, sometimes even after minimal surgical intervention and with no adjuvant therapy, the potential for treatment-related long-term morbidity should be kept prominently in mind when making therapeutic decisions. Going forward, molecular characterization of these tumors will be important to individualize targeted therapies with the hope of reducing adverse effects of chemotherapy and radiation therapy.
Acknowledgments
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Author Contributions
SAU and PLR contributed in caring for the patients, study design, obtaining the data, data analysis, writing the manuscript, and reviewing the final manuscript. CK contributed in caring for the patients, data analysis, and reviewing the manuscript. SV, BLB, NAB, and DW contributed in performing data collection, data analysis, and writing the manuscript. HCW, KED, and ASL performed data collection. KM, HJG, DAH, and COM contributed in caring for the patients, data analysis and reviewing the manuscript.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
The study was reviewed and approved by the Institutional Review Board of the University of Michigan (IRB approval number: HUM00088512).
References
- 1.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronghe M, Hargrave D, Bartels U, et al. Vincristine and carboplatin chemotherapy for unresectable and/or recurrent low-grade astrocytoma of the brainstem. Pediatr Blood Cancer. 2010;55:471–477. doi: 10.1002/pbc.22557. [DOI] [PubMed] [Google Scholar]
- 3.Frazier JL, Lee J, Thomale UW, Noggle JC, Cohen KJ, Jallo GI. Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr. 2009;3:259–269. doi: 10.3171/2008.11.PEDS08281. [DOI] [PubMed] [Google Scholar]
- 4.Griessenauer CJ, Rizk E, Miller JH, et al. Pediatric tectal plate gliomas: clinical and radiological progression, MR imaging characteristics, and management of hydrocephalus. J Neurosurg Pediatr. 2014;13:13–20. doi: 10.3171/2013.9.PEDS13347. [DOI] [PubMed] [Google Scholar]
- 5.van den Bent MJ, Snijders TJ, Bromberg JE. Current treatment of low grade gliomas. Memo. 2012;5:223–7. doi: 10.1007/s12254-012-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown NA, Weigelin HC, Bailey N, et al. Requisite analytic and diagnostic performance characteristics for the clinical detection of BRAF V600E in hairy cell leukemia: a comparison of 2 allele-specific PCR assays. Appl Immunohistochem Mol Morphol. 2015;23:590–600. doi: 10.1097/PAI.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 8.Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14:1265–1284. doi: 10.1093/neuonc/nos202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klimo P, Jr, Pai Panandiker AS, Thompson CJ, et al. Management and outcome of focal low-grade brainstem tumors in pediatric patients: the St. Jude experience. J Neurosurg Pediatr. 2013;11:274–281. doi: 10.3171/2012.11.PEDS12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed KA, Laack NN, Eckel LJ, Orme NM, Wetjen NM. Histologically proven, low-grade brainstem gliomas in children: 30-year experience with long-term follow-up at Mayo Clinic. Am J Clin Oncol. 2014;37:51–56. doi: 10.1097/COC.0b013e31826b9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman HJ, Soloniuk DS, Humphreys RP, et al. Management and outcome of low-grade astrocytomas of the midline in children: a retrospective review. Neurosurgery. 1993;33:964–971. doi: 10.1227/00006123-199312000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Lundar T, Due-Tonnessen BJ, Egge A, et al. Neurosurgical treatment of pediatric low-grade midbrain tumors: a single consecutive institutional series of 15 patients. J Neurosurg Pediatr. 2014;14:598–603. doi: 10.3171/2014.9.PEDS1462. [DOI] [PubMed] [Google Scholar]
- 13.Pierre-Kahn A, Hirsch JF, Vinchon M, et al. Surgical management of brain-stem tumors in children: results and statistical analysis of 75 cases. J Neurosurg. 1993;79:845–852. doi: 10.3171/jns.1993.79.6.0845. [DOI] [PubMed] [Google Scholar]
- 14.Pollack IF, Hoffman HJ, Humphreys RP, Becker L. The longterm outcome after surgical treatment of dorsally exophytic brain-stem gliomas. J Neurosurg. 1993;78:859–863. doi: 10.3171/jns.1993.78.6.0859. [DOI] [PubMed] [Google Scholar]
- 15.Jallo GI, Shiminski-Maher T, Velazquez L, Abbott R, Wisoff J, Epstein F. Recovery of lower cranial nerve function after surgery for medullary brainstem tumors. Neurosurgery. 2005;56:74–77. doi: 10.1227/01.neu.0000144782.39430.12. [DOI] [PubMed] [Google Scholar]
- 16.Fried I, Hawkins C, Scheinemann K, et al. Favorable outcome with conservative treatment for children with low grade brainstem tumors. Pediatr Blood Cancer. 2012;58:556–560. doi: 10.1002/pbc.23200. [DOI] [PubMed] [Google Scholar]
- 17.Robertson PL, Allen JC, Abbott IR, Miller DC, Fidel J, Epstein FJ. Cervicomedullary tumors in children: a distinct subset of brainstem gliomas. Neurology. 1994;44:1798–1803. doi: 10.1212/wnl.44.10.1798. [DOI] [PubMed] [Google Scholar]
- 18.Edwards MS, Wara WM, Ciricillo SF, Barkovich AJ. Focal brainstem astrocytomas causing symptoms of involvement of the facial nerve nucleus: long-term survival in six pediatric cases. J Neurosurg. 1994;80:20–25. doi: 10.3171/jns.1994.80.1.0020. [DOI] [PubMed] [Google Scholar]
- 19.Freeman CR, Bourgouin PM, Sanford RA, Cohen ME, Friedman HS, Kun LE. Long term survivors of childhood brain stem gliomas treated with hyperfractionated radiotherapy. Clinical characteristics and treatment related toxicities. The Pediatric Oncology Group. Cancer. 1996;77:555–562. doi: 10.1002/(SICI)1097-0142(19960201)77:3<555::AID-CNCR19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Willard VW, Conklin HM, Boop FA, Wu S, Merchant TE. Emotional and behavioral functioning after conformal radiation therapy for pediatric ependymoma. Int J Radiat Oncol Biol Phys. 2014;88:814–821. doi: 10.1016/j.ijrobp.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277–5282. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 22.Dirks PB, Jay V, Becker LE, et al. Development of anaplastic changes in low-grade astrocytomas of childhood. Neurosurgery. 1994;34:68–78. [PubMed] [Google Scholar]
- 23.Krieger MD, Gonzalez-Gomez I, Levy ML, McComb JG. Recurrence patterns and anaplastic change in a long-term study of pilocytic astrocytomas. Pediatr Neurosurg. 1997;27:1–11. doi: 10.1159/000121218. [DOI] [PubMed] [Google Scholar]
- 24.Bandopadhayay P, Bergthold G, London WB, et al. Long-term outcome of 4, 040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer. 2014;61:1173–1179. doi: 10.1002/pbc.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farmer JP, Montes JL, Freeman CR, Meagher-Villemure K, Bond MC, O’Gorman AM. Brainstem gliomas. A 10-year institutional review. Pediatr Neurosurg. 2001;34:206–214. doi: 10.1159/000056021. [DOI] [PubMed] [Google Scholar]
- 26.Kortmann RD, Timmermann B, Taylor RE, et al. Current and future strategies in radiotherapy of childhood low-grade glioma of the brain. Part I: treatment modalities of radiation therapy. Strahlenther Onkol. 2003;179:509–520. doi: 10.1007/s00066-003-9104-9. [DOI] [PubMed] [Google Scholar]
- 27.Sandri A, Sardi N, Genitori L, et al. Diffuse and focal brain stem tumors in childhood: prognostic factors and surgical outcome. Experience in a single institution. Childs Nerv Syst. 2006;22:1127–1135. doi: 10.1007/s00381-006-0083-x. [DOI] [PubMed] [Google Scholar]
- 28.Schild SE, Stafford SL, Brown PD, et al. The results of radiotherapy for brainstem tumors. J Neurooncol. 1998;40:171–177. doi: 10.1023/a:1006193306286. [DOI] [PubMed] [Google Scholar]
- 29.Castello MA, Schiavetti A, Padula A, et al. Does chemotherapy have a role in low-grade astrocytoma management? A report of 13 cases. Med Pediatr Oncol. 1995;25:102–108. doi: 10.1002/mpo.2950250210. [DOI] [PubMed] [Google Scholar]
- 30.Gajjar A, Heideman RL, Kovnar EH, et al. Response of pediatric low grade gliomas to chemotherapy. Pediatr Neurosurg. 1993;19:113–118. doi: 10.1159/000120714. discussion 9–20. [DOI] [PubMed] [Google Scholar]
- 31.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 32.Pons MA, Finlay JL, Walker RW, Puccetti D, Packer RJ, McEl-wain M. Chemotherapy with vincristine (VCR) and etoposide (VP-16) in children with low-grade astrocytoma. J Neurooncol. 1992;14:151–158. doi: 10.1007/BF00177619. [DOI] [PubMed] [Google Scholar]
- 33.Lefkowitz IB, Packer RJ, Sutton LN, et al. Results of the treatment of children with recurrent gliomas with lomustine and vincristine. Cancer. 1988;61:896–902. doi: 10.1002/1097-0142(19880301)61:5<896::aid-cncr2820610507>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 34.Kuo DJ, Weiner HL, Wisoff J, Miller DC, Knopp EA, Finlay JL. Temozolomide is active in childhood, progressive, unresectable, low-grade gliomas. J Pediatr Hematol Oncol. 2003;25:372–378. doi: 10.1097/00043426-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Collins VP, Jones DT, Giannini C. Pilocytic astrocytoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129:775–788. doi: 10.1007/s00401-015-1410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


