Abstract
The objective of the present study is to describe a cohort of complex esophageal atresia and the yield of genetic tests performed for such patients. We selected 45 patients with complex esophageal atresia (EA), namely those having at least one associated anomaly. We reviewed their medical records to assess clinical features, other diagnoses, and genetic investigations. Most of the patients had a diagnosis of VACTERL association (56%) with no genetic variant identified. Interestingly, 5 patients in the cohort (11%) had a right pulmonary hypoplasia or agenesis. A majority of our cohort (73%) had genetic testing; 60% were karyotyped (abnormal in 4 of the 27 patients tested), 31% had aCGH (abnormal in 1 of the 14 patients tested), and 31% had diepoxybutane (DEB) testing for Fanconi anemia (abnormal in 2 of the 14 patients tested). One patient had exome sequencing studies, but no candidate gene was identified. Various anomalies were associated with EA, and overall a genetic variant could be identified in 7 of the 33 patients tested. Chromosomal studies such as aCGH and chromosomal breakage studies should be considered, and their yield varied between 7 and 14%. Other genetic investigations such as exome sequencing could possibly have even higher yields but will need to be assessed in a large cohort. Improved genetic diagnoses in EA may improve the management of these patients by directing specific surveillance and management schemes.
Keywords: aCGH, Diepoxybutane test, Esophageal atresia, Tracheo-esophageal fistula, VACTERL
Esophageal atresia (EA) with or without tracheo-esophageal fistula (TEF) is a relatively common congenital defect occurring in about 2.4/10,000 births [Nassar et al., 2012]. Different anatomical classifications have been proposed. The Gross classification includes 5 subtypes mainly depending on the presence and the location of TEF. Type A is an isolated form of EA, type B has a proximal TEF, type C, which is the most frequent type, is associated with distal TEF, while type D comprises both proximal and distal fistulae. Finally, type E is defined as the presence of a TEF without an EA [Gross and Piotti, 1953].
About half of the patients with EA are known to have associated anomalies [Shaw-Smith, 2006]. Despite its relatively high prevalence, its etiology remains poorly understood. Some environmental factors, such as fetal alcohol exposure or maternal diabetes, have been associated with an increased risk for EA/TEF [Martínez-Frías and Rodríguez-Pinilla, 1991; Martínez-Frías, 1994]. EA is also a clinical feature seen in several genetic syndromes caused by chromosomal anomalies (e.g., trisomy 13, 18, and 21) or by specific gene mutations (e.g., CHARGE syndrome, Fanconi anemia). Despite this complex etiology, genetic causes can be identified in less than 10% of the patients with EA [Genevieve et al., 2007].
The aim of the present retrospective study is to describe a series of patients with complex EA/TEF, to identify useful genetic testing, and to propose an algorithm to facilitate investigation of these patients.
Patients and Methods
A total of 181 patients have been seen at the EA-TEF Clinic at CHU Sainte-Justine, Montreal, between 2005 and 2015. For this retrospective study, we selected patients with complex EA/TEF, namely those having at least one associated anomaly, with the exclusion of patients with only minor cardiac anomalies, such as the frequently associated right aortic arch and aberrant right subclavian artery [Berthet et al., 2015], since genetic testing was usually not performed in these cases or in cases of completely isolated EA. A total of 45 patients, mainly Caucasian French-Canadian individuals, were included. The mean age was 11.6 years (2–25 years), and there were 21 (47%) females and 24 (53%) males. We reviewed medical records of the selected patients and compiled data about clinical features, associated diagnoses, and genetic investigations performed.
To determine if diagnoses found in this cohort had already been reported in association to EA/TEF, we searched in PubMed and OMIM for matching terms, “esophageal atresia” or “tracheo-esophageal fistula,” and the diagnosis.
Results
Clinical Features of the Cohort Analyzed
A majority of patients (n = 35; 78%) had a type C EA. Other types were divided as following: 6 patients (13%) had a type A, 1 patient (2%) had a type D, and 3 patients (7%) had a type E. There was no type B in this cohort.
A wide range of anomalies, affecting various organ systems, were observed (Table 1), with the most frequent being cardiovascular defects (76%). We noted 5 individuals (11%) with right pulmonary hypoplasia or agenesis. Developmental delay or intellectual disability was found in 11 patients (24%). Among other anomalies, we observed an intrauterine growth restriction in 8 patients (18%) and a failure to thrive in 7 patients (16%).
Table 1.
Associated anomalies in 45 patients
| Anomalies | Patients, n (%) |
|---|---|
| Cardiovascular | 34 (75.6) |
| Skeletal (excluding vertebrae) | 22 (48.9) |
| Gastrointestinal | 21 (46.7) |
| Kidney | 21 (46.7) |
| Vertebral | 21 (46.7) |
| Respiratory | 17 (37.8) |
| Behavior, cognition, and development | 15 (33.3) |
| Genitourinary (excluding kidney) | 12 (26.7) |
| Deafness/hearing loss | 12 (26.7) |
| Neurological | 10 (22.2) |
| Auricular (excluding deafness/hearing loss) | 7 (15.6) |
| Other | 21 (46.7) |
Genetic Findings
Most of the individuals (n = 33; 73%) had at least 1 genetic test. Table 2 shows the distribution of genetic tests performed in these patients (patients who had more than 1 test were counted once for each test). A genetic variant was identified in 7 patients (21% of those tested). Karyotyping was the most frequently performed test and often the first offered, but aCGH is progressively replacing karyotypes in the clinic for most indications and, for 5 of the 14 patients in whom aCGH was performed, no karyotyping had been done previously. Among the 9 patients who had FISH analysis for the chromosomal region 22q11.2, only 2 did not have prior karyotyping. Karyotyping and the diepoxybutane (DEB) test for chromosomal breakage studies to search for Fanconi anemia showed the highest detection rate with, respectively, 15 and 14% of abnormal results. For all patients in which the karyotype showed abnormalities, no additional genetic investigations were performed. Targeted analyses were performed in 3 patients and included polymerase chain reaction (PCR) for X fragile syndrome and molecular analysis of NF1, SPRED1, and PTEN in one patient, GLI3 in another, and EFTUD2 in a last patient. These analyses identified a mutation of GLI3 in an individual suspected to have Greig cephalopolysyndactyly syndrome on the basis of the preaxial polydactyly and dysmorphisms such as hypertelorism. Exome sequencing was performed on a single patient, and no variant suspected of causing his phenotype was found. He presented with hypoplastic ears, hypertelorism, macrostomia, abnormalities of the bones of the skull base, and a ventricular septal defect.
Table 2.
Genetic testing in our cohort of 45 patients
| Genetic testing | Patients tested, n (%) | Positive results, n (%) |
|---|---|---|
| Karyotype | 27 (60.0) | 4 (14.8) |
| CGH | 14 (31.1) | 1 (7.1) |
| DEB test | 14 (31.1) | 2 (14.3) |
| FISH 22q11.2 | 9 (20.0) | 0 (0.0) |
| Targeted testing | 3 (6.7) | 0 (0.0) |
| Subtelomeric FISH | 2 (4.4) | 0 (0.0) |
| FISH trisomy 13, 18, 21 | 1 (2.2) | 0 (0.0) |
| FISH monosomy 5q, 7q | 1 (2.2) | 0 (0.0) |
| FISH trisomy 8 | 1 (2.2) | 0 (0.0) |
| Exome sequencing | 1 (2.2) | 0 (0.0) |
CHG, comparative genomic hybridization; DEB, diepoxybutane.
Twenty-seven patients received a diagnosis based on their clinical features, so that 34 (76%) of the individuals had a diagnosis (Table 3). A majority (56%) of patients received a diagnosis of VACTERL (vertebral, anorectal, cardiac, tracheo-esophageal, renal and limb malformations) association from their physicians. Two patients had another diagnosis in addition to VACTERL association, namely Klippel-Feil syndrome and 47,XYY mosaicism. aCGH identified 2 deletions (2q35 and 16p23.3) in a single patient. No information was available about the inheritance of these variants, as the parents declined further testing. A search in CNV databases, DECIPHER [Firth et al., 2009] and ClinGen [Rehm et al., 2015], did not reveal patients with similar deletions. Therefore, we were not able to conclude if these variants were pathogenic.
Table 3.
Diagnosis in our cohort of 45 patients
| Diagnosis | Patients, n (%) |
|---|---|
| VACTERL | |
| Diagnosed by physician (includes those with 2/6 criteria) | 25 (55.6) |
| With ≥3/6 criteria | 23 (51.1) |
| Klippel-Feil syndrome | 1 (2.2) |
| Moebius syndrome | 1 (2.2) |
| Poland syndrome | 1 (2.2) |
| Meier-Gorlin syndrome | 1 (2.2) |
| Trisomy 21 | 3 (6.7) |
| Fanconi anemia | 2 (4.4) |
| 47,XYY mosaicism | 1 (2.2) |
Individuals with VACTERL Association
Patients who received a diagnosis of VACTERL association by their physician had 2–6 of the 6 associated components with an average of 4. The usual definition of VACTERL association requires at least 3 out of 6 features, but diagnostic criteria may vary from one physician to another [Solomon, 2011; Solomon et al., 2012]. For the purpose of our study, we considered as having VACTERL for only those having a minimum of 3 criteria. Some individuals affected with a confirmed genetic syndrome other than VACTERL also met criteria for VACTERL association, but they were not counted in this category as the other syndrome explained their features. Cardiac anomalies (83%) were the most frequent VACTERL acronym malformations (Table 4). Among other malformations, genitourinary (35%), skeletal - except vertebrae and limbs - (35%), and respiratory (35%) systems were the most affected systems. We noted 1 patient (4%) in that group with unilateral pulmonary hypoplasia. Sixteen of the 23 patients with VACTERL association (70%) had at least 1 genetic test, mainly karyotyping (65%), DEB test (35%), and aCGH (30%). Only one of this subset of patients had an abnormal result, with a karyotype showing 47,XYY mosaicism.
Table 4.
Associated anomalies in VACTERL patients
| Anomalies | Patients (n = 23) | Solomon et al. [2010a] (n = 60) | Brosens et al. [2014] (n = 139) |
|---|---|---|---|
| VACTERL acronym anomalies | |||
| Vertebral | 17 (73.9) | 47 (78) | 83 (60) |
| Anorectal | 10 (43.5) | 33 (55) | 72 (52) |
| Cardiac | 19 (82.6) | 48 (80) | 83 (60) |
| Tracheo-esophageal | 23 (100) | 31 (52) | 139 (100) |
| Renal | 18 (78.3) | 43 (72) | 74 (53) |
| Limb | 7 (30.4) | 28 (47) | 53 (38) |
| Other anomalies | |||
| Skeletal (excluding vertebrae and limbs) | 8 (34.8) | – | – |
| Genitourinary (excluding renal) | 8 (34.8) | – | – |
| Respiratory | 8 (34.8) | – | – |
| Gastrointestinal (excluding tracheo-esophageal and anorectal) | 7 (30.4) | – | – |
| Behavior, cognition, and development | 3 (13.0) | – | – |
| Neurological | 3 (13.0) | – | – |
Percentages are given in parentheses.
Fanconi Anemia Patients
The 2 patients diagnosed with Fanconi anemia had multiple anomalies affecting various systems. They both presented with deafness/hearing loss, congenital heart defect (atrial septal defect and patent ductus arteriosus), failure to thrive, and epilepsy. The first patient also showed microcephaly, ossicular defects, ocular anomalies (microphthalmia, strabismus, and cataracts), radial hypoplasia, thumb hypoplasia, umbilical hernia, testicle atrophy, developmental delay, and attention deficit hyperactivity disorder. The second individual had an aberrant subclavian artery, thumb duplication, duodenal atresia, hiatal hernia, single kidney, and cryptorchism.
Discussion
Anomalies Associated with EA/TEF
As previously reported, many malformations can occur in association to EA/TEF. VACTERL features have been reported in several individuals, but other anomalies not included in the VACTERL association are also frequently seen [Genevieve et al., 2007; de Jong et al., 2008; Solomon et al., 2010a; Solomon, 2011; Brosens et al., 2014]. In the present cohort, a large spectrum of associated anomalies was found, principally in cardiovascular, musculoskeletal, gastrointestinal, genitourinary, and respiratory systems.
Diagnoses in this Cohort
Most of the diagnoses found in this cohort were previously associated with EA/TEF. It includes the VACTERL association, trisomy 21, Fanconi anemia, and Klippel-Feil syndrome [Brosens et al., 2014]. On the other hand, according to our literature review, Moebius syndrome, Poland syndrome, and 47,XYY mosaicism have never been reported in association to EA/TEF. The patient with Meier-Gorlin syndrome in our cohort was already reported [Guernsey et al., 2011] and appears to be the only patient with this condition reported to also have EA/TEF. More data would be necessary to determine if EA/TEF could be a minor feature of these syndromes. A GLI3 mutation has already been reported in a patient with EA [Yang L et al., 2014]. However, the variant found in the patient from this cohort appeared to be a non-deleterious polymorphism present in 0.6% of the European population (ExAC; http://exac.broadinstitute.org), even though it was previously thought to be associated with Greig cephalopolydactyly [Kalff-Suske et al., 1999; Krauss et al., 2009]. Finally, neither deletions 2q35 nor 16p23.3 were reported as being associated with EA/TEF, and it has been impossible to establish if these variants have been inherited from a parent, so the significance of these findings remains to be determined if more patients with this association are identified.
VACTERL Association
The distribution of VACTERL features amongst patients with this diagnosis was similar to what has previously been reported (Table 4). Brosens et al. [2014] selected patients with 3 or more VACTERL features in a cohort of patients with tracheo-esophageal anomalies, as in our study, but they did not exclude patients with other proven genetic syndromes. Solomon et al. [2010a], however, studied patients with VACTERL association that did not correspond to a known genetic syndrome but did not select their patients in a cohort of EA/TEF, so they found a rate of tracheo-esophageal anomalies lower than our study and the study of Brosens et al. [2014]. We found similar results to the study of Solomon et al. [2010a] for cardiac (80%) and renal (72%) defects, while Brosens et al. [2014] reported lower incidences (60 and 53%, respectively). For vertebral anomalies, our incidence of 74% was between those found by Solomon et al. [2010a] (78%) and Brosens et al. [2014] (60%). With incidences of, respectively, 44 and 30% for anorectal and limb malformations, our results were lower than Solomon et al. [2010a] (55 and 47%) and Brosens et al. [2014] (52 and 38%).
As we observed in our cohort, many other malformations than the main ones in VACTERL association have been described in patients with EA. As features of some syndromes (e.g., CHARGE) overlap with those of VACTERL, it might suggest an underlying genetic syndrome in some of those patients [Solomon, 2011].
Until now, VACTERL association has been recognized as a multifactorial disease, while its specific etiology (genetic or other) remains undetermined in most cases. The environment is a major factor which has been identified in some individuals as the causal factor for this association of malformations. Notably, a well-known associated exposition is maternal diabetes [Solomon, 2011]. Otherwise, it has been shown that some signaling pathways, such as sonic hedgehog, Hox and retinoic acid pathways could also be implicated in the etiology [Solomon, 2011; Brosens et al., 2014]. Individuals with VACTERL features were identified with mutations of ZIC3 and FOXF1 [Chung et al., 2011; Hilger et al., 2015], FGF8 [Zeidler et al., 2014], PTEN [Reutter and Ludwig, 2013], and HOXD13 [Garcia-Barcelo et al., 2008]. A study of 69 twins affected by VACTERL association did not show a higher concordance rate in monozygotic twins than in dizygotic twins, and therefore suggested that inherited genetic factors play a limited role in this condition [Bartels et al., 2012b]. Moreover, while Solomon et al. [2010b] found evidence of inheritance in patients with VACTERL association, with 9% of them having a primary relative affected by one or more VACTERL features, Bartels et al. [2012a] instead described no higher prevalence of VACTERL features in first-degree relatives.
Fanconi Anemia
The phenotype of Fanconi anemia is highly variable, which makes it a difficult condition to diagnose on a clinical basis. As it was observed in the 2 patients with Fanconi anemia in this cohort, this disease includes multiple congenital malformations, and 5–10% of the patients with Fanconi anemia meet VACTERL criteria [Giampietro et al., 1993; Faivre et al., 2005]. Recent cases with features overlapping VACTERL were identified with mutations in FANCB [Mikat et al., 2016], FANCL [Vetro et al., 2015], and FANCI [Savage et al., 2016]. Apart from the features frequently reported in this disorder, the 2 patients in our cohort also presented with epilepsy, while the usual Fanconi anemia spectrum does not include epilepsy as a main feature. To our knowledge, only 1 case of Fanconi anemia with epilepsy was identified in the literature, the genetic etiology being a compound heterozygosity for a point mutation of FANCA and a large intragenic deletion encompassing exon 9 of the other FANCA allele [Alonso et al., 2011]. In our cohort, both children were diagnosed by DEB chromosomal breakage analysis and neither had molecular genetic testing or aCGH. It could be interesting to see if those patients could carry a similar deletion that could explain their seizure phenotype.
Even if EA/TEF is not a frequent feature of Fanconi anemia, with an incidence of 1–14% [de Jong et al., 2010; Brosens et al., 2014], it might be relevant to perform chromosomal breakage analysis in every patient presenting with this defect. Notably, 2 patients were reported with EA as the only associated malformation [Perel et al., 1998]. This suggests we should consider ordering chromosome breakage studies more often in EA. In this cohort, the DEB test was positive in 2 of the 14 patients who had this test, making it the genetic test with the highest detection rate (14%) in this cohort. Previous studies did not show such high diagnostic yield, neither in patients with isolated EA [Esmer et al., 2004] nor in those with VACTERL association [Esmer et al., 2004; Solomon et al., 2012]. Diagnosing Fanconi anemia early carries significant clinical importance since it can influence therapy for hematological anomalies [de Jong et al., 2010].
Pulmonary Agenesis
An interesting finding in this cohort is the presence of 5 patients with right pulmonary agenesis or hypoplasia. Except for 1 patient who met VACTERL criteria, the others received no diagnosis. This congenital defect has already been reported in association with EA/TEF in the literature in less than 40 individuals, which thus seems relatively infrequent [Stark et al., 2007; Yadav et al., 2012; Verma et al., 2013]. As esophageal and respiratory structures both derive from the same embryological structure, the foregut, it might suggest the implication of genes regulating the development of this structure. For example, haploinsufficiency of the forkhead gene Foxf1 has previously been linked to congenital lung and foregut malformations [Mahlapuu et al., 2001]. It would be interesting to further study this association with the aim of identifying a genetic cause in patients with this phenotype.
Genetic Testing
Genetic variants have been identified in 21% of the patients following genetic testing. It must be pointed out that in most of the patients only karyotyping was performed. Only 31% of the patients had a chromosomal microarray, although it has a much better diagnostic yield than karyotyping for patients with multiple congenital anomalies [Miller et al., 2010]. Sequencing of only 3 genes previously associated with EA/TEF (GLI3, EFTUD2, and PTEN) has been performed in some patients in this cohort. The other targeted tests aimed to explain other features present in the patient in which they were performed. Many other genes have been found to be involved in syndromes with EA/TEF as a variable feature, such as CHD7 (CHARGE syndrome), MYCN (Feingold syndrome), and SOX2 (microphthalmia and EA) [Brosens et al., 2014]. Analysis of those genes could yield answers concerning EA/TEF's etiology in our cohort, but a suggestive phenotype generally has to be recognized by physicians to prompt such investigations. Recent next-generation sequencing technologies such as whole-exome sequencing could be an interesting alternative since it is not limited to specific genes and does not inevitably require a prior diagnostic hypothesis. Moreover, it could not only identify mutations in genes already associated with EA/TEF, but it could also identify unsuspected syndromes or new causal genes. In this cohort, a single patient underwent whole-exome sequencing, and no variant could be linked to his phenotype, but further studies will be necessary to determine the detection rate of such technology.
Suggested Investigations in EA/TEF
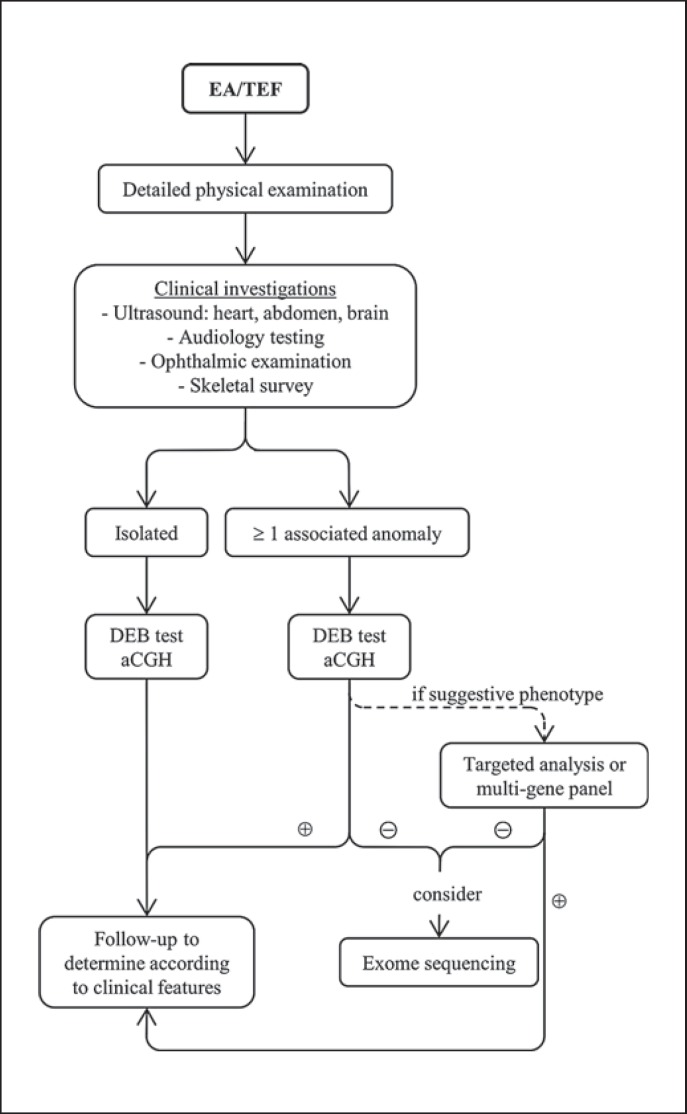
According to the literature, up to 60% of the patients with EA/TEF had associated anomalies [Shaw-Smith, 2006]. For this reason, a thorough physical examination and clinical investigations should be performed in every patient presenting with EA/TEF (Fig. 1). Also, every patient should be seen by a clinical geneticist, who would then decide on subsequent appropriate genetic testing. In this cohort of complex EA/TEF, we found 5 patients (11%) with chromosomal anomalies (e.g., trisomy 21), while it has been reported in 6–10% [Felix et al., 2007] of the patients with EA/TEF. Thus, we suggest aCGH in all patients, as well as to consider chromosomal breakage studies (e.g., the DEB assay) in complex EA, which showed a high detection rate in this cohort. For patients with associated anomalies, targeted analysis or multi-gene panel should be performed if a suggestive phenotype is identified. Finally, if these genetic tests remain negative, or as an alternative to targeted genetic testing, whole-exome sequencing could be considered in patients with multiple anomalies since it has a diagnostic rate of 25–54% in patients with multiple congenital anomalies [Iglesias et al., 2014; Yang Y et al., 2014; Valencia et al., 2015].
Fig. 1.
Algorithm for the management of patients with EA/TEF. DEB, diepoxybutane; EA, esophageal atresia; TEF, tracheo-esophageal fistula.
Limitations of the Present Study
This study has some limitations that must be mentioned. First of all, our cohort did not include all EA patients diagnosed at our institutions, only those with associated malformations; therefore, we cannot use it to calculate the absolute frequency of malformations in EA, and we cannot compare our findings to other cohorts which included all EA patients. Also, as we did a retrospective analysis of medical records and did not perform any prospective genetic investigations for this study, our results thus depend on the observations, imaging, and tests requested by different physicians over the years. VACTERL association is usually diagnosed by exclusion, but not all patients with VACTERL association in this cohort had genetic testing, and this might have led to a misdiagnosis. For example, we cannot exclude the possibility that some patients diagnosed with VACTERL would in fact have CHARGE syndrome because there are some overlapping features. Finally, it is possible that some medical records were incomplete because some patients might have undergone investigations in other pediatric hospitals.
In conclusion, this study described various malformations that can occur in association with EA/TEF. It is important for clinicians to be aware of the possibility of a genetic syndrome causing these multiple congenital anomalies. Even if most patients remain without a genetic explanation for their phenotype, genetic testing identified a variant in 16% of our cohort (21% of those tested). Chromosomal microarray might be a good test to perform as a first intention test in patients showing malformations associated with EA/TEF to exclude a chromosomal anomaly, and a chromosomal breakage analysis could permit an early diagnosis of Fanconi anemia. Identifying genes causing EA/TEF in humans by panel or exome sequencing could eventually lead to better diagnosis and management of patients with this condition, but such a study needs to be performed in a large cohort to determine the cohort-specific yield.
Statement of Ethics
Institutional Review Board approval was obtained.
Disclosure Statement
The authors declare no conflicts of interest.
Acknowledgments
We thank the families and the funding sources (FRSQ clinician-chercheur to P.M.C., CIHR clinician-scientist to P.M.C., Réseau de Médecine Génétique appliquée (RMGA) to Z.K.).
References
- Alonso L, Sevilla J, Gonzalez-Vicent M, Abad L, Gonzalez-Mediero I, Diaz MA. Pulmonary glial heterotopia in a child diagnosed with Fanconi anemia and epilepsy. J Pediatr Hematol Oncol. 2011;33:462–464. doi: 10.1097/MPH.0b013e318215cef0. [DOI] [PubMed] [Google Scholar]
- Bartels E, Jenetzky E, Solomon BD, Ludwig M, Schmiedeke E, et al. Inheritance of the VATER/VACTERL association. Pediatr Surg Int. 2012a;28:681–685. doi: 10.1007/s00383-012-3100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E, Schulz AC, Mora NW, Pineda-Alvarez DE, Wijers CH, et al. VATER/VACTERL association: identification of seven new twin pairs, a systematic review of the literature, and a classical twin analysis. Clin Dysmorphol. 2012b;21:191–195. doi: 10.1097/MCD.0b013e328358243c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet S, Tenisch E, Miron MC, Alami N, Timmons J, et al. Vascular anomalies associated with esophageal atresia and tracheoesophageal fistula. J Pediatr. 2015;166:1140–1144. doi: 10.1016/j.jpeds.2015.01.038. [DOI] [PubMed] [Google Scholar]
- Brosens E, Ploeg M, van Bever Y, Koopmans AE, IJsselstijn H, et al. Clinical and etiological heterogeneity in patients with tracheo-esophageal malformations and associated anomalies. Eur J Med Genet. 2014;57:440–452. doi: 10.1016/j.ejmg.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Chung B, Shaffer LG, Keating S, Johnson J, Casey B, Chitayat D. From VACTERL-H to heterotaxy: variable expressivity of ZIC3-related disorders. Am J Med Genet A. 2011;155A:1123–1128. doi: 10.1002/ajmg.a.33859. [DOI] [PubMed] [Google Scholar]
- de Jong EM, Felix JF, Deurloo JA, van Dooren MF, Aronson DC, et al. Non-VACTERL-type anomalies are frequent in patients with esophageal atresia/tracheo-esophageal fistula and full or partial VACTERL association. Birth Defects Res A Clin Mol Teratol. 2008;82:92–97. doi: 10.1002/bdra.20437. [DOI] [PubMed] [Google Scholar]
- de Jong EM, Felix JF, de Klein A, Tibboel D. Etiology of esophageal atresia and tracheoesophageal fistula: “mind the gap”. Curr Gastroenterol Rep. 2010;12:215–222. doi: 10.1007/s11894-010-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmer C, Sánchez S, Ramos S, Molina B, Frias S, Carnevale A. DEB test for Fanconi anemia detection in patients with atypical phenotypes. Am J Med Genet A. 2004;124A:35–39. doi: 10.1002/ajmg.a.20327. [DOI] [PubMed] [Google Scholar]
- Faivre L, Portnoï MF, Pals G, Stoppa-Lyonnet D, Le Merrer M, et al. Should chromosome breakage studies be performed in patients with VACTERL association? Am J Med Genet A. 2005;137:55–58. doi: 10.1002/ajmg.a.30853. [DOI] [PubMed] [Google Scholar]
- Felix JF, Tibboel D, de Klein A. Chromosomal anomalies in the aetiology of oesophageal atresia and tracheo-oesophageal fistula. Eur J Med Genet. 2007;50:163–175. doi: 10.1016/j.ejmg.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Firth HV, Richards SM, Bevan AP, Clayton S, Corpas M, et al. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am J Hum Genet. 2009;84:524–533. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Barceló MM, Wong KK, Lui VC, Yuan ZW, So MT, et al. Identification of a HOXD13 mutation in a VACTERL patient. Am J Med Genet A. 2008;146A:3181–3185. doi: 10.1002/ajmg.a.32426. [DOI] [PubMed] [Google Scholar]
- Geneviève D, de Pontual L, Amiel J, Sarnacki S, Lyonnet S. An overview of isolated and syndromic oesophageal atresia. Clin Genet. 2007;71:392–399. doi: 10.1111/j.1399-0004.2007.00798.x. [DOI] [PubMed] [Google Scholar]
- Giampietro PF, Adler-Brecher B, Verlander PC, Pavlakis SG, Davis JG, Auerbach AD. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116–1120. [PubMed] [Google Scholar]
- Gross RE, Piotti E. The Surgery of Infancy and Childhood: Its Principles and Techniques. Philadelphia: Saunders; 1953. [Google Scholar]
- Guernsey DL, Matsuoka M, Jiang H, Evans S, Macgillivray C, et al. Mutations in origin recognition complex gene ORC4 cause Meier-Gorlin syndrome. Nat Genet. 2011;43:360–364. doi: 10.1038/ng.777. [DOI] [PubMed] [Google Scholar]
- Hilger AC, Halbritter J, Pennimpede T, van der Ven A, Sarma G, et al. Targeted resequencing of 29 candidate genes and mouse expression studies implicate ZIC3 and FOXF1 in human VATER/VACTERL association. Hum Mutat. 2015;36:1150–1154. doi: 10.1002/humu.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias A, Anyane-Yeboa K, Wynn J, Wilson A, Truitt Cho M, et al. The usefulness of whole-exome sequencing in routine clinical practice. Genet Med. 2014;16:922–931. doi: 10.1038/gim.2014.58. [DOI] [PubMed] [Google Scholar]
- Kalff-Suske M, Wild A, Topp J, Wessling M, Jacobsen EM, et al. Point mutations throughout the GLI3 gene cause Greig cephalopolysyndactyly syndrome. Hum Mol Genet. 1999;8:1769–1777. doi: 10.1093/hmg/8.9.1769. [DOI] [PubMed] [Google Scholar]
- Krauss S, So J, Hambrock M, Köhler A, Kunath M, et al. Point mutations in GLI3 lead to misregulation of its subcellular localization. PLoS One. 2009;4:e7471. doi: 10.1371/journal.pone.0007471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlapuu M, Enerbäck S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- Martínez-Frías ML. Epidemiological analysis of outcomes of pregnancy in diabetic mothers: identification of the most characteristic and most frequent congenital anomalies. Am J Med Genet. 1994;51:108–113. doi: 10.1002/ajmg.1320510206. [DOI] [PubMed] [Google Scholar]
- Martínez-Frías ML, Rodríguez-Pinilla E. Tracheoesophageal and anal atresia in prenatal children exposed to a high dose of alcohol. Am J Med Genet. 1991;40:128. doi: 10.1002/ajmg.1320400129. [DOI] [PubMed] [Google Scholar]
- Mikat B, Roll C, Schindler D, Gembruch U, Klempert I, et al. X-linked recessive VACTERL-H due to a mutation in FANCB in a preterm boy. Clin Dysmorphol. 2016;25:73–76. doi: 10.1097/MCD.0000000000000111. [DOI] [PubMed] [Google Scholar]
- Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar N, Leoncini E, Amar E, Arteaga-Vázquez J, Bakker MK, et al. Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol. 2012;94:893–899. doi: 10.1002/bdra.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perel Y, Butenandt O, Carrere A, Saura R, Fayon M, et al. Oesophageal atresia, VACTERL association: Fanconi's anaemia related spectrum of anomalies. Arch Dis Child. 1998;78:375–376. doi: 10.1136/adc.78.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm HL, Berg JS, Brooks LD, Bustamante CD, Evans JP, et al. ClinGen-the Clinical Genome Resource. N Engl J Med. 2015;372:2235–2242. doi: 10.1056/NEJMsr1406261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutter H, Ludwig M. VATER/VACTERL association: evidence for the role of genetic factors. Mol Syndromol. 2013;4:16–19. doi: 10.1159/000345300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage SA, Ballew BJ, Giri N; NCI DCEG Cancer Genomics Research Laboratory, Chandrasekharappa SC, et al. Novel FANCI mutations in Fanconi anemia with VACTERL association. Am J Med Genet A. 2016;170A:386–391. doi: 10.1002/ajmg.a.37461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw-Smith C. Oesophageal atresia, tracheo-oesophageal fistula, and the VACTERL association: review of genetics and epidemiology. J Med Genet. 2006;43:545–554. doi: 10.1136/jmg.2005.038158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD. VACTERL/VATER association. Orphanet J Rare Dis. 2011;6:56. doi: 10.1186/1750-1172-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD, Pineda-Alvarez DE, Raam MS, Bous SM, Keaton AA, et al. Analysis of component findings in 79 patients diagnosed with VACTERL association. Am J Med Genet A. 2010a;152A:2236–2244. doi: 10.1002/ajmg.a.33572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD, Pineda-Alvarez DE, Raam MS, Cummings DA. Evidence for inheritance in patients with VACTERL association. Hum Genet. 2010b;127:731–733. doi: 10.1007/s00439-010-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon BD, Bear KA, Kimonis V, de Klein A, Scott DA, et al. Clinical geneticists' views of VACTERL/VATER association. Am J Med Genet A. 2012;158A:3087–3100. doi: 10.1002/ajmg.a.35638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark Z, Patel N, Clarnette T, Moody A. Triad of tracheoesophageal fistula-esophageal atresia, pulmonary hypoplasia, and duodenal atresia. J Pediatr Surg. 2007;42:1146–1148. doi: 10.1016/j.jpedsurg.2007.01.044. [DOI] [PubMed] [Google Scholar]
- Valencia CA, Husami A, Holle J, Johnson JA, Qian Y, et al. Clinical impact and cost-effectiveness of whole exome sequencing as a diagnostic tool: a pediatric center's experience. Front Pediatr. 2015;3:67. doi: 10.3389/fped.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Mahajan JK, Laxmi Narsimha Rao K. Esophageal atresia and tracheoesophageal fistula with unilateral pulmonary agenesis - hypoplasia. J Neonatal Surg. 2013;2:21. [PMC free article] [PubMed] [Google Scholar]
- Vetro A, Iascone M, Limongelli I, Ameziane N, Gana S, et al. Loss-of-function FANCL mutations associate with severe Fanconi anemia overlapping the VACTERL association. Hum Mutat. 2015;36:562–568. doi: 10.1002/humu.22784. [DOI] [PubMed] [Google Scholar]
- Yadav PS, Pant N, Chadha R, Choudhury SR. Oesophageal atresia and tracheoesophageal fistula with right pulmonary agenesis and duplication of the azygos vein. Arch Dis Child. 2012;97:513. doi: 10.1136/archdischild-2011-301248. [DOI] [PubMed] [Google Scholar]
- Yang L, Shen C, Mei M, Zhan G, Zhao Y, et al. De novo GLI3 mutation in esophageal atresia: reproducing the phenotypic spectrum of Gli3 defects in murine models. Biochim Biophys Acta. 2014;1842:1755–1761. doi: 10.1016/j.bbadis.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Yang Y, Muzny DM, Xia F, Niu Z, Person R, et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler C, Woelfle J, Draaken M, Mughal SS, Große G, et al. Heterozygous FGF8 mutations in patients presenting cryptorchidism and multiple VATER/VACTERL features without limb anomalies. Birth Defects Res A Clin Mol Teratol. 2014;100:750–759. doi: 10.1002/bdra.23278. [DOI] [PubMed] [Google Scholar]