Abstract
Schizophrenia (SCZ) is a serious neuropsychiatric disorder that manifests through several symptoms from early adulthood. Numerous studies over the last decades have led to significant advances in increasing our understanding of the factors involved in SCZ. For example, mass spectrometry-based proteomic analysis has provided important insights by uncovering protein dysfunctions inherent to SCZ. Here, we present a comprehensive analysis of the nuclear proteome of postmortem brain tissues from corpus callosum (CC) and anterior temporal lobe (ATL). We show an overview of the role of deregulated nuclear proteins in these two main regions of the brain: the first, mostly composed of glial cells and axons of neurons, and the second, represented mainly by neuronal cell bodies. These samples were collected from SCZ patients in an attempt to characterize the role of the nucleus in the disease process. With the ATL nucleus enrichment, we found 224 proteins present at different levels, and 76 of these were nuclear proteins. In the CC analysis, we identified 119 present at different levels, and 24 of these were nuclear proteins. The differentially expressed nuclear proteins of ATL are mainly associated with the spliceosome, whereas those of the CC region are associated with calcium/calmodulin signaling.
Keywords: Schizophrenia, Proteomics, Nucleus, Nuclei, Nuclear proteome
Introduction
Schizophrenia (SCZ) is a serious, debilitating, and incurable mental disorder that affects approximately 1% of the world's population [1]. The disease normally manifests between the end of adolescence and the beginning of adulthood [2] and is characterized by a range of cognitive, behavioral, and emotional dysfunctions. SCZ is the main cause of psychiatric incapacitation [3] and, although it is usually treated as a single disease, it is likely to be a spectrum of related disorders with distinct etiology, clinical presentation, response to treatment, and development [4]. Despite its high prevalence and severity, little is known about the biochemical mechanisms involved in its development or progression, and so there are few established molecular diagnoses or specific treatments. Thus, there is currently a great interest in obtaining new knowledge about the disease and, in this arena, proteomic analyses have already yielded promising results and opened up new avenues of research [5].
Proteomics is used to analyze the protein profile of a specific cell type, tissue or organism, or changes in specific proteins, using mass spectrometry as its main tool. Due to its capacity for profiling large numbers of proteins simultaneously, proteomics is currently one of the main techniques used to understand biochemical pathways and, consequently, multifactorial disorders such as SCZ [6]. In the last 2 decades, proteomics has contributed to a growing understanding of SCZ, and a number of such studies investigating this disorder have been published revealing alterations in several biochemical pathways of the central nervous system [5]. However, it is important to note that in proteomic analyses of whole tissues, it is often not possible to detect proteins that are present at low concentrations. This may occur due to the complexity of the sample and/or due to the presence of other proteins that are present at very high concentrations, which may obscure those of lower abundance. This could lead to an inability to detect proteins which have important roles in the disease process [7]. To circumvent this problem, the study of subproteomes appears as a satisfactory alternative.
Subproteomes are obtained by fractionating the proteins of a given sample into distinct groups, taking into account some specific criteria. Although there are several ways of fractionating the protein content of a sample, one alternative is the analysis of specific organelles. This type of fractionation is attractive for cellular proteome analyses, since the protein content of the organelles is less complex and represents a specific and directed set, which provides the opportunity of investigating entire protein networks to an extent that cannot be achieved using whole cell approaches [8].
In this study, we have used a protocol for separation of organelles in order to obtain samples enriched in cell nuclei. The nucleus is the largest organelle of most cells and occupies approximately 10% of its volume, although this varies according to the cell type [9]. The nucleus is the compartment where the genetic material of eukaryotic organisms is stored, and nuclear proteins constitute 10–20% of the total cellular proteins and exert important functions related to gene expression, transcriptional control, splicing, and generation of the final gene products [10]. Therefore, the importance of investigating the nuclear proteome for the understanding of any physiological or pathological process is clear, and few such studies have been carried out thus far in the field of psychiatric disorders such as SCZ.
As an added level of enrichment, we have analyzed nuclei obtained from the anterior temporal lobe (ATL) and corpus callosum (CC) regions of postmortem brains from patients and controls. Dysfunctions in the ATL have been implicated previously in SCZ, and it consists mostly of gray matter [11]. The CC is the largest white matter region of the brain, and there are morphological, electrophysiological, and neurophysiological studies showing significant involvement of this region in patients with SCZ [12, 13, 14]. Because the brain works through communication with and across the different regions, we jointly analyzed the ATL and CC nuclear proteins to provide further information on the potential role of dysfunctions of this organelle in SCZ.
Materials and Methods
Human Samples
CC and ATL samples were collected post-mortem from 12 patients who had suffered from chronic SCZ and from 8 healthy controls (Table 1). The patient samples were from the Nordbaden Psychiatric Center, Wiesloch, Germany, and the controls were from the Institute of Neuropathology, Heidelberg University, Heidelberg, Germany. Postmortem evaluations and procedures were approved by the Ethics Committee of the Heidelberg University Medical School, and both patients and controls gave written consent prior to death that their brains could be used for research purposes.
Table 1.
Clinical data of patients and controls
| Case | Age, years | Gender | PMI, h | pH values | Duration of disease, years | Duration of medication, years | atyptyp | CPE last dose | CPE last 10 years | Cause of death | DSM-IV | Age at onset | Last medication | Cigarettes | Alcohol | Hosp | ECT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCZ | 64 | F | 11 | 6.7 | 48 | 45 | 3 | 1,536 | 7.7 | pulmonary insufficiency | 295.6 | 16 | clozapine 500 mg, haloperidol 40 mg, ciatyl 40 mg | 0 | no | 21 | yes |
| SCZ | 73 | M | 20 | 6.6 | 43 | 40 | 1 | 507.4 | 1.7 | heart infarction | 295.6 | 30 | perphenazine 32 mg, promethazine 150 mg |
30/day | no | 33 | no |
| SCZ | 43 | M | 18 | 6.9 | 22 | 20 | 2 | 464 | 2.6 | heart infarction | 295.6 | 20 | zuclopethixol 40 mg, valproate 1,200 mg, tiapride 300 mg | 0 | no | 13 | no |
| SCZ | 77 | F | 32 | 6.5 | 49 | 48 | 2 | 2,555 | 8.3 | hmg embolism | 295.6 | 28 | clozapine 400 mg, benperidol 25 mg, chlorprothixen 150 mg | 0 | no | 48 | yes |
| SCZ | 76 | F | 17 | 6.8 | 49 | 47 | 1 | 300 | 4.9 | cardio-pulmonary insufficiency | 295.6 | 27 | perazine 300 mg | 0 | no | 30 | yes |
| SCZ | 63 | F | 31 | 6.8 | 40 | 30 | 3 | 75 | 1.8 | heart infarction | 295.6 | 24 | olanzapine 15 mg | 30/day | no | 30 | yes |
| SCZ | 92 | F | 37 | 6.9 | 51 | 48 | 1 | 100 | 3.4 | cardio-pulmonary insufficiency | 295.6 | 41 | prothipendyl 160 mg, perazine 100 mg | 0 | no | 51 | no |
| SCZ | 71 | M | 28 | 6.4 | 40 | 35 | 1 | 782.4 | 10 | heart infarction | 295.6 | 30 | haloperidol 32 mg, pipamperone 40 mg |
40/day | no | 12 | no |
| SCZ | 51 | M | 7 | 6.1 | 25 | 25 | 1 | 147 | 0.6 | heart infarction | 295.6 | 19 | flupenthixol 15 mg | 30/day | no | 20 | no |
| SCZ | 51 | M | 12 | 6.7 | 28 | 25 | 2 | 450 | 1.,8 | heart infarction | 295.6 | 23 | clozapine 500 mg | 30/day | no | 17 | no |
| SCZ | 81 | M | 4 | 6.7 | 62 | 50 | 1 | 92.8 | 1.4 | heart insufficiency | 295.6 | 19 | haloperidol 40 mg, prothypendyl 80 mg |
20 | no | 48 | no |
| SCZ | 64 | F | 23 | 6.6 | 41 | 40 | 2 | 54.5 | 4.6 | heart infarction | 295.6 | 24 | zotepine 150 mg, olanzapine 10 mg |
20/day | no | 5 | yes |
| Control | 41 | M | 7 | 6.5 | heart infarction | 0 | no | ||||||||||
| Control | 91 | F | 16 | 6.7 | cardio-pulmonary insufficiency | 0 | no | ||||||||||
| Control | 69 | F | 96 | 6.4 | lung embolism | 0 | no | ||||||||||
| Control | 57 | M | 24 | 6.9 | heart infarction | 0 | no | ||||||||||
| Control | 53 | M | 18 | 7 | heart infarction | 0 | no | ||||||||||
| Control | 63 | M | 13 | 6.5 | heart infarction | 0 | no | ||||||||||
| Control | 66 | M | 16 | 6.8 | heart infarction | 0 | no | ||||||||||
| Control | 79 | M | 24 | 6.4 | heart infarction | 0 | no | ||||||||||
atyptyp, duration of atypical treatment/duration of treatment with typical neuroleptics during lifetime; CPE, medication calculated in chlorpromazine equivalents (mg); CPE last 10 years, the sum of medications during the last 10 years in kg; Hosp, hospitalization time in years; ECT, electroconvulsive therapy.
Nuclear Enrichment
Nuclear proteins were obtained from the ATL and CC brain tissues according to the protocol of Cox and Emili [15]. In this protocol, each sample (20 mg tissue) was homogenized in 10 volumes of buffer containing 250 mM sucrose (Sigma-Aldrich, St. Louis, MO, USA), 50 mM Tris-HCl, 5 mM MgCl2 (Sigma-Aldrich), 1 mM dithiothreitol (Sigma-Aldrich), 250 µg spermine, and 250 µg spermidine buffer (pH = 7.4), containing 1 tablet of protease cocktail inhibitor (Roche Diagnostics, Indianapolis, IN, USA) per 25 mL buffer. The homogenate was centrifuged at 100 g for 1 min at 4°C. The supernatant was discarded. Next, 5 volumes of the same buffer was added to the sediment. The homogenate was centrifuged at 800 g for 15 min at 4°C, and the supernatant was separated for further separation of the mitochondria and stored at −80°C as CytoI. The previous step was repeated and the supernatant was named as CytoII. The pellet was homogenized in 4 mL of the same buffer mentioned above but with a concentration of 2 M sucrose. The mixture was filtered with gauze, and the filtrate was placed on 4 mL of the last buffer. The tube was centrifuged at 80,000 g for 35 min at 4°C. The pellet contained the pure nuclei. The nuclear protein pellet was dissolved in 50 mM ammonium bicarbonate (pH 8.0) prior to protein digestion.
Mass Spectrometry
Protein extracts from nuclear enrichment of ATL and CC were digested by trypsin at a ratio of 1:80 (trypsin: total protein). The resulting peptides were lyophilized and frozen at −80°C before mass spectrometry analysis. Immediately prior to analysis, lyophilized peptides were dissolved in an aqueous solution of 0.1% formic acid and injected into a 2D nano high-performance liquid chromatography system (Eksigent, Dublin, CA, USA) coupled online to a LTQ XL-Orbitrap mass spectrometer (Thermo Scientific, Bremen, Germany). The specifics of data acquisition are described in detail in Maccarrone et al. [16].
Proteome Quantification
The program used in the identification and quantification of proteins was MASCOT Distiller (Matrix Sciences, London, UK). For the identification and quantification, this program follows a series of statistical criteria. The main test used to indicate quantitative changes between the proteins was Student's t in log space. This analysis assigns to each protein a p value of significance with regard to differences in protein levels. In addition, samples values were applied to a data normalization process. This process is based on the hypothesis that it is reasonable to expect that only a minority of the proteins in the sample will be found to be differentially expressed, considering that the overall normalization is applied in order to make the mean or median ratios in the entire dataset equal to 1. Following this logic, the data distribution is log-normal, and the statistical test used to confirm this premise is the Shapiro-Wilk W test. If the results do not pass this test, it indicates that the values are meaningless and something has systematically gone wrong with the analysis. In these cases, the values are rejected in the normality test.
Analysis in silico
Shotgun proteomics analysis can produce high amounts of data, especially in studies of complex biological mixtures, such as postmortem brain samples. As a consequence, protein-protein interaction analysis and identification of the pathways involved are fundamental to understanding cellular phenotypes in the most complete manner possible. Due to this, we used bioinformatics tools available online in these analyses. These were: the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING, http://STRING-db.org/), Kyoto Encyclopedia Genes and Genomes (KEGG, http://www.genome.ad.jp/kegg/), and Reactome (http://reactome.org/).
Results
In the results of the ATL nucleus enrichment, we identified a total of 4,293 unique peptides, which corresponded to 629 proteins. Of these, 224 were present at significantly different levels between the SCZ and control samples, and 76 were nuclear proteins (nuclear enrichment of 33%; Table 2). In the CC analysis, we identified 3,820 unique peptides, corresponding to 552 proteins with 119 present at different levels, and 24 of these were nuclear proteins (nuclear enrichment of 21%; Table 3). These differentially expressed proteins were analyzed using the online human protein reference database (http://www.hprd.org/) in order to find the biological processes and function/molecular classes with which they are related (Table 2, 3).
Table 2.
Differentially expressed proteins in schizophrenia anterior temporal lobe (ATL)
| UniProt ID | Gene name | Description | Score | Mass | Peptides | SCZ/CTRL | SD | Biological process | Molecular class | Molecular function |
|---|---|---|---|---|---|---|---|---|---|---|
| PKP2_HUMAN | PKP2 | plakophilin-2 | 76 | 97,852 | 2 | 0.05 | 5.154 | cell adhesion | unclassified | molecular function unknown |
| BIN1_HUMAN | BIN1 | Myc box-dependent-interacting protein 1 | 175 | 64,887 | 5 | 0.63 | 1.859 | cell communication and signaling | adapter molecule | receptor signaling complex scaffold activity |
| CADM1_HUMAN | CADM1 | cell adhesion molecule 1 | 102 | 48,935 | 3 | 0.44 | 4.93 | cell communication and signaling | adhesion molecule | cell adhesion molecule activity |
| CALM_HUMAN | CALM1 | calmodulin | 759 | 16,827 | 9 | 2.10 | 3.515 | cell communication and signaling | calcium binding protein | calcium ion binding |
| CDC42_HUMAN | CDC42 | cell division control protein 42 homolog | 74 | 21,696 | 2 | 4.13 | 5.878 | cell communication and signaling | GTPase | GTPase activity |
| CSRP1_HUMAN | CSRP1 | cysteine and glycine-rich protein 1 | 244 | 21,409 | 5 | 0.49 | 2.012 | cell communication and signaling | adapter molecule | receptor signaling complex scaffold activity |
| CTNB1_HUMAN | CTNNB1 | catenin beta-1 | 71 | 86,069 | 2 | 0.48 | 1.285 | cell communication and signaling | adhesion molecule | cell adhesion molecule activity |
| DPYL2_HUMAN | DPYSL2 | dihydropyrimidinase-related protein 2 | 1,892 | 62,711 | 30 | 2.40 | 2.513 | cell communication and signaling | cytoskeletal associated protein | cytoskeletal protein binding |
| PP2BA_HUMAN | PPP3CA | serine/threonine-protein phosphatase 2B catalytic subunit alpha isoform | 325 | 59,335 | 5 | 1.92 | 9.805 | cell communication and signaling | serine/threonine phosphatase | protein serine/threonine phosphatase activity |
| CANB1_HUMAN | PPP3R1 | calcineurin subunit B type 1 | 76 | 19,402 | 2 | 4.30 | 9.98 | cell communication and signaling | regulatory/other subunit | Phosphatase regulator activity |
| KAP3_HUMAN | PRKAR2B | cAMP-dependent protein kinase type II-beta regulatory subunit | 157 | 46,672 | 2 | 0.47 | 1.132 | cell communication and signaling | serine/threonine kinase | protein serine/threonine kinase activity |
| SEPT7_HUMAN | SEPT7 | septin-7 | 493 | 50,933 | 11 | 2.16 | 2.939 | cell communication and signaling | cell cycle control protein | protein binding |
| SH3G2_HUMAN | SH3GL2 | endophilin-A1 | 216 | 40,108 | 4 | 1.76 | 3.614 | cell communication and signaling | unclassified | molecular function unknown |
| SIRT2_HUMAN | SIRT2 | NAD-dependent deacetylase sirtuin-2 | 220 | 43,782 | 5 | 3.94 | 8.747 | cell communication and signaling | cell cycle control protein | deacetylase activity |
| SYUB_HUMAN | SNCB | beta-synuclein | 447 | 14,279 | 6 | 2.05 | 3.512 | cell communication and signaling | unclassified | molecular function unknown |
| 1433E_HUMAN | YWHAE | 14-3-3 protein epsilon | 595 | 29,326 | 12 | 0.40 | 4.13 | cell communication and signaling | adapter molecule | receptor signaling complex scaffold activity |
| 1433G_HUMAN | YWHAG | 14-3-3 protein gamma | 1,010 | 28,456 | 18 | 0.56 | 3.229 | cell communication and signaling | adapter molecule | receptor signaling complex scaffold activity |
| 1433T_HUMAN | YWHAQ | 14-3-3 protein theta | 400 | 28,032 | 7 | 0.41 | 2.421 | cell communication and signaling | adapter molecule | receptor signaling complex scaffold activity |
| SEPT2_HUMAN | SEPT2 | septin-2 | 173 | 41,689 | 3 | 1.86 | 2.96 | cell cycle | GTPase | GTPase activity |
| DPYL1_HUMAN | CRMP1 | dihydropyrimidinase-related protein 1 | 368 | 62,487 | 6 | 3.50 | 1.543 | cell growth and/or maintenance | enzyme: hydrolase | protein binding |
| DYL1_HUMAN | DYNLL1 | dynein light chain 1, cytoplasmic | 141 | 10,530 | 4 | 1.97 | 8.851 | cell growth and/or maintenance | motor protein | motor activity |
| E41L3_HUMAN | EPB41L3 | band 4.1-like protein 3 | 473 | 121,458 | 13 | 0.56 | 7.25 | cell growth and/or maintenance | structural protein | structural molecule activity |
| FLNA_HUMAN | FLNA | filamin-A | 612 | 283,301 | 8 | 0.36 | 4.413 | cell growth and/or maintenance | anchor protein | cytoskeletal anchoring activity |
| LMNA_HUMAN | LMNA | lamin-A/C | 387 | 74,380 | 7 | 0.11 | 3.386 | cell growth and/or maintenance | structural protein | structural molecule activity |
| LMNB2_HUMAN | LMNB2 | lamin-B2 | 173 | 67,762 | 3 | 0.18 | 5.639 | cell growth and/or maintenance | structural protein | structural molecule activity |
| MAP1A_HUMAN | MAP1A | microtubule-associated protein 1A | 187 | 306,923 | 4 | 0.35 | 7.653 | cell growth and/or maintenance | cytoskeletal associated protein | cytoskeletal protein binding |
| MARE2_HUMAN | MAPRE2 | microtubule-associated protein RP/EB family member 2 | 338 | 37,236 | 6 | 2.41 | 4.902 | cell growth and/or maintenance | cytoskeletal associated protein | cytoskeletal protein binding |
| MYH9_HUMAN | MYH9 | myosin-9 | 330 | 227,646 | 4 | 0.23 | 1.904 | cell growth and/or maintenance | structural protein | structural molecule activity |
| VIME_HUMAN | VIM | vimentin | 2,238 | 53,676 | 37 | 0.35 | 5.159 | cell growth and/or maintenance | cytoskeletal protein | structural constituent of cytoskeleton |
| LEG1_HUMAN | LGALS1 | galectin-1 | 79 | 15,048 | 2 | 0.65 | 2.212 | immune response | ligand | receptor binding |
| LDHA_HUMAN | LDHA | L-lactate dehydrogenase A chain | 385 | 36,950 | 8 | 2.48 | 2.252 | metabolism; energy pathways | enzyme: dehydrogenase | catalytic activity |
| COX41_HUMAN | COX4I1 | cytochrome c oxidase subunit 4 isoform 1, mitochondrial | 126 | 19,621 | 3 | 0.43 | 1.693 | metabolism; energy pathways | enzyme: oxidoreductase | oxidoreductase activity |
| COX5B_HUMAN | COX5B | cytochrome c oxidase subunit 5B, mitochondrial | 170 | 13,915 | 4 | 7.19 | 4.156 | metabolism; energy pathways | enzyme: oxidoreductase | oxidoreductase activity |
| GSTP1_HUMAN | GSTP1 | glutathione S-transferase P | 147 | 23,569 | 2 | 1.64 | 1.268 | metabolism; energy pathways | enzyme: glutathione transferase | glutathione transferase activity |
| NDUS8_HUMAN | NDUFS8 | NADH dehydrogenase (ubiquinone) iron-sulfur protein 8, mitochondrial | 96 | 24,203 | 2 | 1.52 | 1.993 | metabolism; energy pathways | enzyme: oxidoreductase | oxidoreductase activity |
| PDE2A_HUMAN | PDE2A | cGMP-dependent 3',5'-cyclic phosphodiesterase | 89 | 107,360 | 2 | 9.65 | 2.299 | metabolism; energy pathways | enzyme: phosphodiesterase | phosphoric diester hydrolase activity |
| ODPA_HUMAN | PDHA1 | pyruvate dehydrogenase E1 component subunit alpha, somatic form, mitochondrial | 219 | 43,952 | 7 | 0.35 | 5.372 | metabolism; energy pathways | enzyme: dehydrogenase | catalytic activity |
| PGK1_HUMAN | PGK1 | phosphoglycerate kinase 1 | 510 | 44,985 | 11 | 1.86 | 6.605 | metabolism; energy pathways | enzyme: phos-photransferase | catalytic activity |
| PRDX1_HUMAN | PRDX1 | peroxiredoxin-1 | 198 | 22,324 | 4 | 3.30 | 1.57 | metabolism; energy pathways | enzyme: peroxidase | peroxidase activity |
| CALX_HUMAN | CANX | calnexin | 182 | 67,982 | 5 | 1.85 | 3.216 | protein folding | chaperone | chaperone activity |
| CH60_HUMAN | HSPD1 | 60-kDa heat shock protein, mitochondrial | 1,089 | 61,187 | 21 | 1.90 | 2.418 | protein folding; apoptosis; regulation of immune response; signal transduction | heat shock protein | heat shock protein activity |
| CALR_HUMAN | CALR | calreticulin | 197 | 48,283 | 2 | 0.31 | 1.984 | protein metabolism | chaperone | chaperone activity |
| TCPD_HUMAN | CCT4 | T-complex protein 1 subunit delta | 105 | 58,401 | 3 | 1.56 | 2.874 | protein metabolism | chaperone | chaperone activity |
| DNJC5_HUMAN | DNAJC5 | DnaJ homolog subfamily C member 5 | 93 | 22,933 | 2 | 3.89 | 5.26 | protein metabolism | chaperone | chaperone activity |
| HSP71_HUMAN | HSPA1A | heat shock 70-kDa protein 1 | 390 | 70,294 | 8 | 1.84 | 2.307 | protein metabolism | chaperone | chaperone activity |
| HSP72_HUMAN | HSPA2 | heat shock-related 70-kDa protein 2 | 485 | 70,263 | 13 | 1.91 | 2.814 | protein metabolism | heat shock protein | heat shock protein activity |
| HSP76_HUMAN | HSPA6 | heat shock 70-kDa protein 6 | 328 | 71,440 | 7 | 1.77 | 2.398 | protein metabolism | heat shock protein | heat shock protein activity |
| HSP7C_HUMAN | HSPA8 | heat shock cognate 71-kDa protein | 1,156 | 71,082 | 22 | 1.84 | 2.293 | protein metabolism | heat shock protein | heat shock protein activity |
| CH10_HUMAN | HSPE1 | 10-kDa heat shock protein | 130 | 10,925 | 3 | 2.31 | 1.134 | protein metabolism | heat shock protein | heat shock protein activity |
| PSA4_HUMAN | PSMA4 | proteasome subunit alpha type-4 | 71 | 29,750 | 3 | 0.44 | 1.528 | protein metabolism | ubiquitin proteasome system protein | ubiquitin-specific protease activity |
| SYUA_HUMAN | SNCA | alpha-synuclein | 879 | 14,451 | 12 | 1.88 | 3.76 | protein metabolism | chaperone | chaperone activity |
| EF1A1_HUMAN | EEF1A1 | elongation factor 1-alpha 1 | 312 | 50,451 | 9 | 0.58 | 2.182 | regulation of cell cycle | transcription regulatory protein | transcription regulator activity |
| H2AV_HUMAN | H2AFV | histone H2A.V | 143 | 13,501 | 2 | 0.17 | 1.264 | regulation of gene expression, epigenetic | DNA-binding protein | DNA binding |
| BASP_HUMAN | BASP1 | brain acid soluble protein 1 | 1,796 | 22,680 | 24 | 0.51 | 1.805 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | transcription regulatory protein | transcription regulator activity |
| CAND1_HUMAN | CAND1 | cullin-associated NEDD8-dissociated protein 1 | 118 | 137,999 | 2 | 1.81 | 4.005 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | transcription regulatory protein | transcription regulator activity |
| H33_HUMAN | H3F3A | histone H3.3 | 217 | 15,376 | 5 | 0.50 | 3.531 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | DNA-binding protein | DNA binding |
| H12_HUMAN | HIST1H1C | histone H1.2 | 237 | 21,352 | 3 | 0.57 | 4.622 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | DNA-binding protein | DNA binding |
| H2A1B_HUMAN | HIST1H2AB | histone H2A type 1-B/E | 249 | 14,127 | 6 | 0.38 | 2.971 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | DNA-binding protein | DNA binding |
| H2A1D_HUMAN | HIST1H2AD | histone H2A type 1-D | 226 | 14,099 | 6 | 0.33 | 1.799 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | DNA-binding protein | DNA binding |
| H2B1B_HUMAN | HIST1H2BB | histone H2B type 1-B | 190 | 13,942 | 3 | 0.41 | 5.283 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | DNA-binding protein | DNA binding |
| H2B1C_HUMAN | HIST1H2BC | histone H2B type 1-C/E/F/G/I | 165 | 13,811 | 3 | 0.43 | 1.266 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | DNA-binding protein | DNA binding |
| H4_HUMAN | HIST1H4A | histone H4 | 637 | 11,360 | 11 | 0.46 | 3.406 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | DNA-binding protein | DNA binding |
| ROA2_HUMAN | HNRNPA2B1 | heterogeneous nuclear ribonucleoproteins A2/B1 | 452 | 37,464 | 7 | 0.16 | 3.769 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | ribonucleoprotein | transcription factor binding |
| HNRPC_HUMAN | HNRNPC | heterogeneous nuclear ribonucleoproteins C1/C2 | 223 | 33,707 | 2 | 0.24 | 1.52 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | RNA-binding protein | RNA binding |
| HNRH1_HUMAN | HNRNPH1 | heterogeneous nuclear ribonucleoprotein H | 269 | 49,484 | 4 | 0.18 | 2.678 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | ribonucleoprotein | ribonucleoprotein |
| HNRH2_HUMAN | HNRNPH2 | heterogeneous nuclear ribonucleoprotein H2 | 293 | 49,517 | 3 | 0.17 | 2.343 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | ribonucleoprotein | RNA binding |
| HNRPK_HUMAN | HNRNPK | heterogeneous nuclear ribonucleoprotein K | 225 | 51,230 | 4 | 0.31 | 3.12 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | ribonucleoprotein | ribonucleoprotein |
| HNRPU_HUMAN | HNRNPU | heterogeneous nuclear ribonucleoprotein U | 114 | 91,198 | 3 | 0.41 | 1.193 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | ribonucleoprotein | RNA binding |
| MATR3_HUMAN | MATR3 | matrin-3 | 125 | 95,078 | 2 | 0.37 | 1.661 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | RNA-binding protein | RNA binding |
| NONO_HUMAN | NONO | non-POU domain-containing octamer-binding protein | 116 | 54,311 | 2 | 0.20 | 1.072 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | RNA-binding protein | RNA binding |
| PURA_HUMAN | PURA | transcriptional activator protein Pur-alpha | 157 | 35,003 | 2 | 0.41 | 2.617 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | transcription factor | transcription factor activity |
| SFRS3_HUMAN | SFRS3 | splicing factor, arginine/serine-rich 3 | 81 | 19,546 | 2 | 0.35 | 1.529 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | RNA-binding protein | RNA binding |
| SSBP_HUMAN | SSBP1 | single-stranded DNA-binding protein, mitochondrial | 92 | 17,249 | 2 | 7.35 | 3.86 | regulation of nucleobase, nucleoside, nucleotide and nucleic acid metabolism | DNA-binding protein | DNA binding |
| SEPT5_HUMAN | SEPT5 | septin-5 | 327 | 43,206 | 11 | 0.52 | 5.659 | signal transduction | GTPase | GTP binding |
| ANXA2_HUMAN | ANXA2 | annexin A2 | 319 | 38,808 | 5 | 0.46 | 6.797 | signal transduction; cell communication | calcium-binding protein | calcium ion binding |
| EAA1_HUMAN | SLC1A3 | excitatory amino acid transporter 1 | 307 | 59,705 | 6 | 0.22 | 6.411 | transport | transport/cargo protein | transporter activity |
Score, MASCOT Identification Score (cutoff for this dataset: 55); mass, the molecular mass of the protein (Da); peptides, number of identified peptides by mass spectrometry; SCZ/CTRL, fold change ratio between schizophrenia and control samples; SD, standard deviation among quantified peptides.
Table 3.
Differentially expressed proteins in schizophrenia CC
| UniProt ID | Gene name | Protein name | Score | Mass | Peptides | SCZ/CTRL | SD | Biological process | Molecular class | Molecular function |
|---|---|---|---|---|---|---|---|---|---|---|
| ARF1_HUMAN | ARF1 | ADP-ribosylation factor 1 | 298 | 20,741 | 7 | 4.26 | 5.333 | cell communication and signaling | GTPase | GTPase activity |
| BASP_HUMAN | BASP1 | brain acid soluble protein 1 | 766 | 22,680 | 14 | 5.96 | 3.633 | reg. nucleic acid metabolism | transcription regulatory protein | transcription regulator activity |
| CALM_HUMAN | CALM1 | calmodulin | 349 | 16,827 | 7 | 2.63 | 1.817 | cell communication and signaling | calcium-binding protein | calcium ion binding |
| KCC2A_HUMAN | CAMK2A | calcium/calmodulin-dependent protein kinase type II subunit alpha | 469 | 54,566 | 10 | 3.08 | 8.707 | cell communication and signaling | serine/threonine kinase | protein serine/threonine kinase activity |
| KCC2B_HUMAN | CAMK2B | calcium/calmodulin-dependent protein kinase type II subunit beta | 180 | 73,593 | 4 | 5.90 | 5.656 | cell communication and signaling | serine/threonine kinase | protein serine/threonine kinase activity |
| KCC2G_HUMAN | CAMK2G | calcium/calmodulin-dependent protein kinase type II subunit gamma | 195 | 63,311 | 4 | 7.19 | 4.726 | cell communication and signaling | serine/threonine kinase | protein serine/threonine kinase activity |
| CDC42_HUMAN | CDC42 | cell division control protein 42 homolog | 85 | 21,696 | 2 | 2.09 | 3.534 | cell communication and signaling | GTPase | GTPase activity |
| COF1_HUMAN | CFL1 | cofilin-1 | 192 | 18,719 | 6 | 5.54 | 4.899 | cell growth and maintenance | cytoskeletal associated protein | cytoskeletal protein binding |
| CSRP1_HUMAN | CSRP1 | cysteine and glycine-rich protein 1 | 458 | 21,409 | 7 | 4.07 | 5.108 | cell communication and signaling | adapter molecule | receptor signaling complex scaffold activity |
| DPYL2_HUMAN | DPYSL2 | dihydropyrimidinase-related protein 2 | 1,679 | 62,711 | 25 | 7.07 | 5.889 | cell communication and signaling | cytoskeletal associated protein | cytoskeletal protein binding |
| H2AV_HUMAN | H2AFV | histone H2A.V | 422 | 13,501 | 5 | 0.42 | 2.387 | reg. nucleic acid metabolism | DNA-binding protein | DNA binding |
| H14_HUMAN | HIST1H1E | histone H1.4 | 513 | 21,852 | 11 | 0.12 | 3.04 | reg. nucleic acid metabolism | DNA-binding protein | DNA binding |
| H31_HUMAN | HIST1H3A | histone H3.1 | 353 | 15,509 | 7 | 0.17 | 8.548 | reg. nucleic acid metabolism | DNA-binding protein | DNA binding |
| H4_HUMAN | HIST1H4A | histone H4 | 979 | 11,360 | 13 | 0.19 | 3.693 | reg. nucleic acid metabolism | DNA-binding protein | DNA binding |
| HMGB1_HUMAN | HMGB1 | high-mobility group protein B1 | 315 | 25,049 | 3 | 0.01 | 5.9 | reg. nucleic acid metabolism | DNA-binding protein | DNA binding |
| ROA2_HUMAN | HNRNPA2B1 | heterogeneous nuclear ribonucleoproteins A2/B1 | 325 | 37,464 | 6 | 0.43 | 5.195 | reg. nucleic acid metabolism | Ribonucleoprotein | Transcription factor binding |
| HNRPR_HUMAN | HNRNPR | heterogeneous nuclear ribonucleoprotein R | 106 | 71,184 | 2 | 0.03 | 1.653 | reg. nucleic acid metabolism | RNA-binding protein | RNA binding |
| HNRPU_HUMAN | HNRNPU | heterogeneous nuclear ribonucleoprotein U | 373 | 91,198 | 8 | 0.19 | 5.492 | reg. nucleic acid metabolism | ribonucleoprotein | RNA binding |
| HP1B3_HUMAN | HP1BP3 | heterochromatin protein 1-binding protein 3 | 78 | 61,454 | 2 | 3.40 | 5.984 | reg. nucleic acid metabolism | DNA-binding protein | DNA binding |
| HSP71_HUMAN | HSPA1A | heat shock 70-kDa protein 1A/1B | 310 | 70,294 | 5 | 1.58 | 1.484 | protein metabolism | chaperone | chaperone activity |
| LMNA_HUMAN | LMNA | prelamin-A/C | 1,140 | 74,380 | 24 | 0.41 | 7.189 | cell growth and maintenance | structural protein | structural molecule activity |
| LMNB2_HUMAN | LMNB2 | lamin-B2 | 207 | 67,762 | 2 | 0.05 | 2.067 | cell growth and maintenance | structural protein | structural molecule activity |
| NPM_HUMAN | NPM1 | nucleophosmin | 172 | 32,726 | 2 | 6.64 | 1.167 | protein metabolism | chaperone | chaperone activity |
| PTMA_HUMAN | PTMA | prothymosin alpha | 177 | 12,196 | 3 | 10.62 | 2.334 | cell growth and maintenance | unclassified | molecular function unknown |
| S100B_HUMAN | S100B | protein S100-B | 290 | 10,820 | 3 | 0.30 | 1.203 | cell communication and signaling | calcium-binding protein | calcium ion binding |
Score, MASCOT Identification Score (cutoff for this dataset: 55); mass, the molecular mass of the protein (Da); peptides, number of identified peptides by mass spectrometry; SCZ/CTRL, fold change ratio between schizophrenia and control samples; SD, standard deviation among quantified peptides.
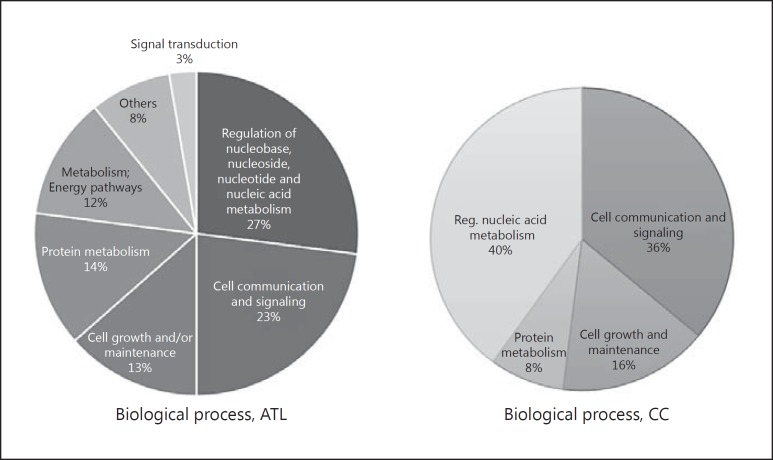
The differentially expressed proteins related to both the nuclei of the ATL region cells and the CC participated in biological processes related mainly to processes such as regulation of nucleobase, nucleoside, nucleotide, and nucleic acid metabolism (27% ATL, 40% CC) and cell communication and signaling (23% ATL, 36% CC) (Fig. 1). These processes are related to the main functions of the nucleus, such as gene expression, transcriptional control, splicing, and release of gene products [10].
Fig. 1.
Biological processes related to the anterior temporal lobe (ATL) and corpus callosum (CC) differentially expressed proteins.
Discussion
Proteomic Similarities between Regions
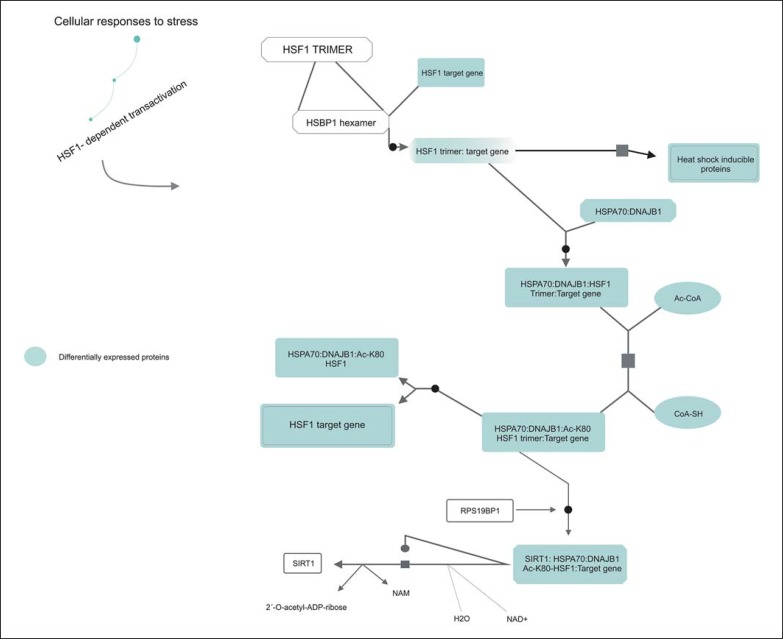
The ATL is enriched in gray matter, while the cerebral region of the CC corresponds to the largest portion of white matter in the brain. This results from the preponderance of neurons in the ATL [17], whereas glial cells and neuronal axons are more predominant in the CC [18]. It is important to compare proteomic profiles of these two regions of the brain mainly because this gives a more integrated view of brain function and not only what happens only with the neurons, as is typical of most other brain proteomic studies. In the comparison of these two regions, 64 of these were specific to the ATL, 13 to the CC, and 12 were common to both regions, i.e. 13.5% of the proteins are shared for the regions, as it can be seen in the Venn diagram (Fig. 2). According to analysis carried out using the Reactome software, these latter proteins were related to cellular stress response, with a chain of reactions related to heat-shock proteins (HSPs) (p value 3.18E-11) (Fig. 3). This type of reaction is triggered by cellular stressors such as exposure to high temperatures, hypoxia, and free radicals, and these factors can cause damage to cellular proteins and induce this type of response [19, 20, 21, 22].
Fig. 2.
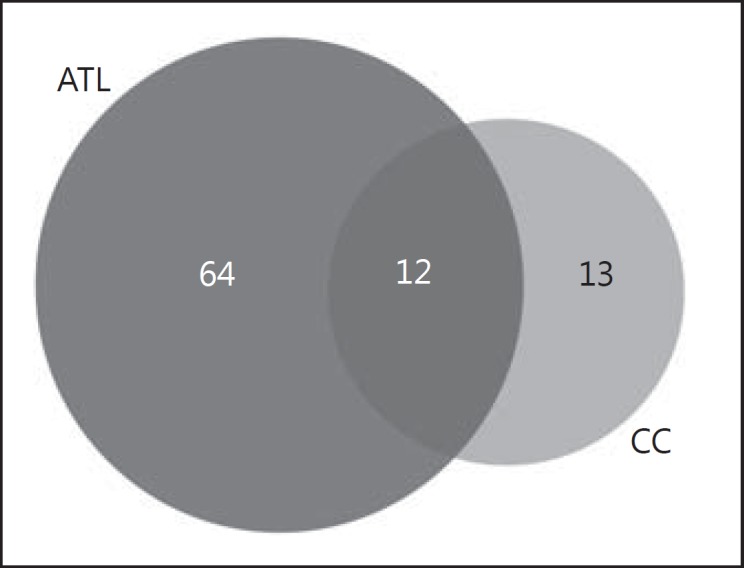
Venn diagram comparison between differentially expressed proteins in anterior temporal lobe (ATL) and corpus callosum (CC).
Fig. 3.
Analysis of Reactome of HSF1 proteins found differentially expressed in both regions by Reactome.
Since maintaining homeostasis is important for the proper functioning of cellular metabolism, this type of stress response must be effective and coordinated [23]. For this to occur, the main molecule responsible for transcription-mediated stress response, the heat shock transcription factor HSF1, must be present at optimal functional levels [24, 25]. Under normal physiological conditions, this molecule is present in its inactive form, mediated by a series of protein-protein interactions. However, in the presence of stressors, this molecule becomes activated by a series of reactions, including its phosphorylation and interaction with DNA, promoting the cellular responses to stress (Fig. 3) [26, 27, 28, 29, 30, 31].
The protein that mediates most of these reactions, the heat shock 70-kDa protein 1A/1B (HSPA1A), was found to be increased in the nuclear compartments of both the ATL and CC. This protein belongs to the family of heat shock protein 70 (HSP70), which has been implicated previously in SCZ [32]. A recent study showed that a polymorphism in the HSPA1A protein gene is associated with increased risk of developing paranoid SCZ [33], and another study found increased mRNA expression of this gene in postmortem samples from the prefrontal cortex of patients with SCZ [34].
The analysis of differentially expressed proteins in the ATL nuclei showed additional changes in 4 more heat shock proteins, with 3 of these belonging to the HSP70 family: heat shock-related 70-kDa protein 2 (HSPA2), heat shock 70-kDa protein 6 (HSPA6), heat shock cognate 71-kDa protein (HSPA8), and 10-kDa heat shock protein (HSPE1). All these proteins were found at higher levels in the patients compared to controls, indicating a large response to cellular stress in the former. There is a debate about the effects displayed in the analysis of postmortem brains in studies of psychiatric disorders. One of the main disputed points is whether or not such effects are a cause or consequence of the disease, or a consequence of prolonged treatment of these patients with different antipsychotic medications throughout their lives and through different stages of disease development. However, such data have appeared recurrently in proteomic data of postmortem brain of patients with SCZ and cannot be ignored. Nevertheless, corroborative studies of some format involving first-onset antipsychotic-naïve patients may help to resolve some of these issues.
Another family of proteins that were found differentially expressed in both the ATL (histone H3.3, histone H1.2, histone H2A type 1-B/E, histone H2A type 1-D, histone H2B type 1-B, histone H2B type 1-C/E/F/G/I, histone H4) and CC (histone H2A.V; histone H1.4; histone H3.1; histone H4), is the family of histone proteins. These proteins are linked to DNA, and they change the transcription mechanism of these molecules, thus modifying gene expression. Histones are also associated with neuronal functions such as synaptic plasticity, a function that is known to be altered in patients with SCZ [35, 36]. Mass spectrometry studies have shown that posttranslational modifications in the nucleosome, which are characterized by the junction of DNA and a complex of histone proteins, regulate histone-DNA interaction, and are part of epigenetic mechanisms of genetic regulation [37]. Many recent studies associate histone dysregulation, and consequently dysregulation of epigenetic processes, with SCZ [38, 39, 40], and there has been much discussion on possible therapeutic targets based on targeted epigenetics in the treatment of this disease (reviewed in [41]). However, as more thorough epigenetic studies in postmortem brain tissue are still arduous and limited, little is known about this association.
Nuclear Proteins Altered in the ATL
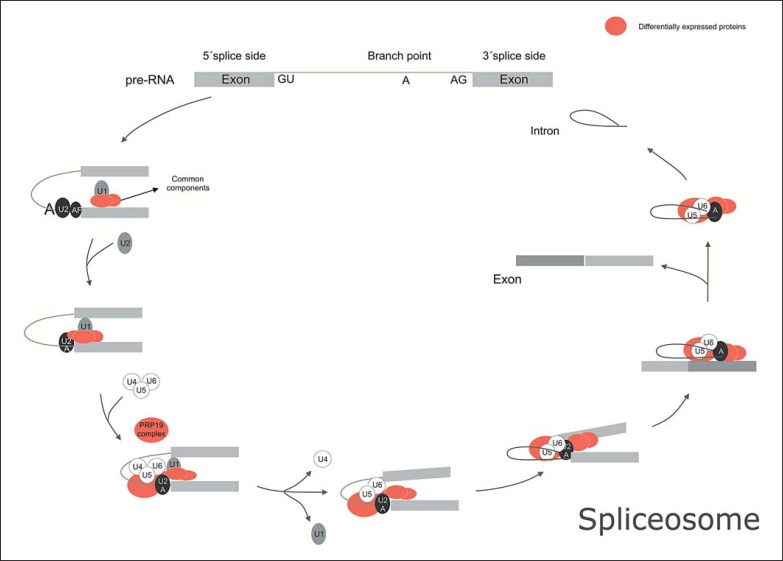
In silico analysis of the proteins found at different levels in cell nuclei of the gray matter region showed that these proteins are mainly associated with spliceosome complex (p value 5.9E-6) (online suppl. Table 1; see www.karger.com/doi/10.1159/000477299 for all online suppl. material). The spliceosome is a complex of 5 multi-megadalton ribonucleoproteins (snRNPs), which are abundant RNA-binding proteins that carry out processing of pre-mRNA transcripts. This complex removes introns of these molecules and rearranges exons, so that these conform and can give rise to the correctly configured mRNA transcripts [42]. Due to the exon rearrangement, a single pre-mRNA can give rise to different functioning mRNAs, and any perturbations of this process may lead to disease development or contribute to the severity of preexisting diseases [43].
The proteins related to this pathway that were found at altered levels in this study were heterogeneous nuclear ribonucleoproteins C1/C2 (HNRNPC), heterogeneous nuclear ribonucleoprotein K (HNRNPK), heterogeneous nuclear ribonucleoprotein U (HNRNPU), splicing factor, arginine/serine-rich 3 (SRSF3), and HSPA1A, HSPA2, HSPA6 and HSPA8, as described above. These proteins are the main constituents of the snRNP complex, which is the main component of the splicing operation (Fig. 4; KEGG).
Fig. 4.
Spliceosome of anterior temporal lobe (ATL) showing differentially expressed proteins according to program STRING.
Eight of the proteins belonging to the hnRNP family were found deregulated in a study of oligodendrocyte cells treated with clozapine, an antipsychotic used in the treatment of SCZ [44]. Moreover, studies of silencing and super-expression of proteins showed a crucial role of hnRNP proteins in the myelination of neurons by oligodendrocytes, independently of the indirect regulation of quaking proteins as previously proposed [45, 46]. This dysfunction in myelination was related to dysregulation of the synaptic connection [47, 48, 49]. One of these proteins was HNRNPC, which was found at decreased levels in SCZ patients compared to controls in this study. It is known that the regulation process of myelination undergoes a precise control dependent on the alternative splicing [45], and if the hnRNP protein complex is deregulated, this may cause aberrant alternative splicing, as reported in neurodegenerative and neuropsychiatric diseases such as frontotemporal dementia with Parkinsonism, amyotrophic lateral sclerosis and SCZ [50, 51, 52].
The deregulation of hnRNP proteins, in addition to being related to oligodendrocyte cells, is also related to the dysfunction of the neurotransmitter system. In a study performed using polymerase chain reaction analysis of SCZ prefrontal cortex tissue, alterations in splicing of mRNA molecules related to the GABAergic system were found [51]. Another study has shown that proteins related to dopamine receptors are also regulated by alternative splicing and that perturbed splicing of these proteins can cause dysfunctions in the dopaminergic system [53]. These systems are known to be altered in patients with SCZ [54]. Thus far, there are only a few recent studies that correlate perturbations in splicing and SCZ. However, the converging evidence suggests that this could play a major role in the disease process and therefore warrants further study, particularly accounting for effects on both neuronal and oligodendrocyte cells.
It should be considered that some of the alterations may be related to heterogeneity of the postmortem tissues including potential differences in cell types and cell density. Therefore, it may not be possible to identify which specific cell types the changes are associated with. However, it is likely that to the changes are associated with glial cells when analyzing white matter and with neurons when analyzing gray matter, considering the natural abundance of such cells in these tissues. It should also be considered that data generated using postmortem tissue has other disadvantages such as potential differences in postmortem intervals or agonal states. Therefore, functional analyses, such as super-expression and knockout studies, should be done to test hypotheses resulting from such investigations using postmortem tissues. For example, microdissection of specific cell types could be used to assess significant cell-specific differences between patients and controls.
Nuclear Proteins of White Matter (CC)
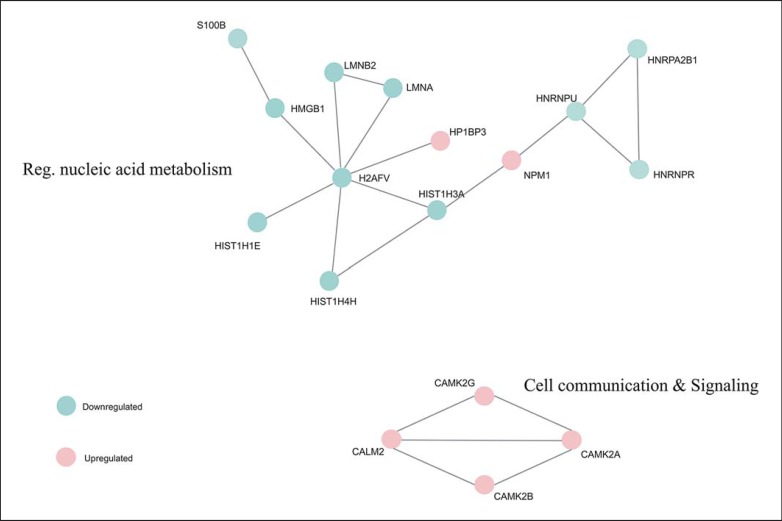
After enriching the pathways of the 24 proteins found differentially expressed in the nucleus of the CC region, only 4 proteins were present in all of the enriched pathways (online suppl. Table 2) and were present in the same interaction network (Fig. 5). These 4 proteins were calmodulin (CALM1), calcium/calmodulin-dependent protein kinase type II subunit alpha (CAMK2A), calcium/calmodulin-dependent protein kinase type II subunit beta (CAMK2B), and calcium/calmodulin-dependent protein kinase type II subunit gamma (CAMK2G), which are all calcium dependent for their activation and response, as well as being constituents of the serine/threonine protein kinase family, which has been widely associated with metabolic events such as muscle contraction, cellular metabolism/proliferation, gene expression, and neurotransmitter release (reviewed in [55]).
Fig. 5.
Deregulated network related to differentially expressed proteins in corpus callosum by STRING.
The correlation between deregulation of calcium and SCZ began in the 1970s [56], and several studies confirming and discussing various aspects of this correlation have been performed. Among the many hypotheses that correlate calcium signaling to SCZ, there is the deregulation of the dopaminergic system, which is one of the hypotheses of SCZ development of the disease [57]. Calcium participates in the uptake of dopamine by synaptic vesicles and, in this process, calcium is associated with CaMKII proteins [26]. In this study, we found increased levels of the alpha, beta, and gamma CAMK2 proteins, suggesting that there may be a high uptake of dopamine by synaptic vesicles and, consequently, a greater release of the neurotransmitter in the synaptic cleft [54]. The major focus in most studies in SCZ has been on the proteins of neuronal cells, as can be seen in the results presented above. However, one study quantified the calmodulin proteins in nuclei of neuronal and glial cells and showed that the abundance of these molecules is greater in the latter cell type [58]. Even so, little research has been done investigating the role of the deregulation of calmodulin proteins in glial cells.
A few recent studies have associated calcium/calmodulin-dependent protein with glial cells in functions such as actin cytoskeleton remodeling, glutamate and glycine transport, and regulation of neurotrophic factors [59, 60, 61, 62]. This involves the activation of the PI3K protein which, according to Pérez-Garcia et al. [62], is regulated by calmodulin proteins associated with calcium. The process of activation of the PI3K protein involves its autophosphorylation, which was found to be reduced in a recent study of postmortem CC from patients with SCZ performed by our group [63]. Given that neurotrophic factors are decreased in patients [64], we can associate the increased amount of CAMK found in this study to a form of cellular compensation which aims to normalize the activation of the neurotrophic factors. Another possible association is that the high concentration of CaMK proteins may desensitize the P13K activation process, causing the observed disruption in this pathway.
Also present in the network identified above are the nucleic acid metabolism-related proteins, which are proteins of the HSP family, and proteins linked to the genetic transcription, which belong to the family of hnRNPs. These results are related to those found in the ATL region, showing a possible relationship between the deregulation of the white and gray matter, with changes in cell stress and regulation of genetic expression.
Conclusion
The results found in this study provide an overview of the participation of nuclear proteins in the pathophysiology of the disease. When it comes to the gray matter, the results point to the dysregulation of the spliceosome, an area which has not been investigated previously. In the case of the white matter, the role of calcium/calmodulin protein deregulation in glial cells has only been partly explored in previous studies, and may be related to altered production of neurotrophic factors. These results represent the first in-depth study comparing effects on gray and white matter in SCZ and lay the groundwork for further studies in this area to help increase our understanding of this complex disease. This could lead to identification of novel biomarkers and drug targets which, in turn, may result in development of newer and better treatment options for people suffering from this disease for improved therapeutic outcomes.
Disclosure Statement
The authors declare no conflict of interest.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgements
V.M.S.C. and D.M.S. are supported by FAPESP (São Paulo Research Foundation), grants 2016/07332-7, 2013/08711-3, and 2014/10068-4. D.M.S. is also supported by The Brazilian National Council for Scientific and Technological Development (CNPq), grant 460289/2014-4.
References
- 1.Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- 2.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz SC, Murray A. Assessing cognitive impairment in patients with schizophrenia. J Clin Psychiatry. 2016;77:3–7. doi: 10.4088/JCP.14074su1c.01. [DOI] [PubMed] [Google Scholar]
- 4.Sadock BJ, Sadock VA. Compêndio de Psiquiatria: Ciência do Comportamento e Psiquiatria Clínica, Artmed. Porto Alegre: Artmed; 2007. [Google Scholar]
- 5.Nascimento JM, Martins-de-Souza D. The proteome of schizophrenia. NPJ Schizophr. 2015;1:14003. doi: 10.1038/npjschz.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins-de-Souza D, Guest PC, Rahmoune H, Bahn S. Proteomic approaches to unravel the complexity of schizophrenia. Expert Rev Proteomics. 2012;9:97–108. doi: 10.1586/epr.11.70. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw RA, Burlingame AL. From proteins to proteomics. IUBMB Life. 2005;57:267–272. doi: 10.1080/15216540500091536. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SW, Fahy E, Ghosh SS. Global organellar proteomics. Trends Biotechnol. 2003;21:82–88. doi: 10.1016/S0167-7799(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 9.Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell. Vol. 4. New York: Garland Science; 2002. pp. 191–234. [Google Scholar]
- 10.Narula K, Datta A, Chakraborty N, Chakraborty S. Comparative analyses of nuclear proteome: extending its function. Front Plant Sci. 2013;4:100. doi: 10.3389/fpls.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison PJ. Using our brains: the findings, flaws, and future of postmortem studies of psychiatric disorders. Biol Psychiatry. 2011;69:102–103. doi: 10.1016/j.biopsych.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Guo H, Christoff J, Campos V, Li Y. Normal corpus callosum in Emx1 mutant mice with C57BL/6 background. Biochemical and biophysical research communications. Biochem Biophys Res Commun. 2000;276:649–653. doi: 10.1006/bbrc.2000.3533. [DOI] [PubMed] [Google Scholar]
- 13.Rotarska-Jagiela A, Schönmeyer R, Oertel V, et al. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage. 2008;39:1522–1532. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 14.Innocenti GM, Ansermet F, Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry. 2003;8:261–274. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- 15.Cox B, Emili A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc. 2006;1:1872–1878. doi: 10.1038/nprot.2006.273. [DOI] [PubMed] [Google Scholar]
- 16.Maccarrone G, Rewerts C, Lebar M, et al. Proteome profiling of peripheral mononuclear cells from human blood. Proteomics. 2013;13:893–897. doi: 10.1002/pmic.201200377. [DOI] [PubMed] [Google Scholar]
- 17.Purves D, Augustine GJ, David F, et al. Neuroscience. Vol. 4. Sunderland: Sinauer Associates; 2008. pp. 15–16. [Google Scholar]
- 18.Fitsiori A, Nguyen D, Karentzos A, et al. The corpus callosum: white matter or terra incognita. Br J Radiol. 2011;84:5–18. doi: 10.1259/bjr/21946513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Liu PCC, Santoro N, Thiele DJ. Conservation of a stress response: human heat shock transcription factors functionally substitute for yeast HSF. EMBO J. 1997;16:6466–6477. doi: 10.1093/emboj/16.21.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voellmy R, et al. Chaperone regulation of the heat shock protein response. Adv Exp Med Biol. 2007;594:89–99. doi: 10.1007/978-0-387-39975-1_9. [DOI] [PubMed] [Google Scholar]
- 21.Shamovsky I, Ivannikov M, Kandel ES, et al. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:4–8. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 22.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- 23.Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 24.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baler R, Dahl G, Voellmyl R. Activation of human heat shock genes is accompanied by oligomerization, modification, and rapid translocation of heat shock transcription factor HSF1. Mol Cell Biol. 1993;13:2486–2496. doi: 10.1128/mcb.13.4.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo J, Rungger D. Multiple layers of regulation of human heat shock transcription factor 1. Mol Cell Biol. 1995;15:4319–4330. doi: 10.1128/mcb.15.8.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotto J, Kline M, Morimoto RI. Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation. J Biol Chem. 1996;271:3355–3359. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- 28.Zou J, Guo Y, Guettouche T, et al. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 29.Knauf U, Newton EM, Kyriakis J, Kingston RE. Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev. 1996;10:2782–2793. doi: 10.1101/gad.10.21.2782. [DOI] [PubMed] [Google Scholar]
- 30.Kline MP, Morimoto RI. Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol. 1997;17:2107–2115. doi: 10.1128/mcb.17.4.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;14:1–14. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JJ, Mandelli L, Lim S, et al. Association analysis of heat shock protein 70 gene polymorphisms in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2008;258:239–244. doi: 10.1007/s00406-007-0791-6. [DOI] [PubMed] [Google Scholar]
- 33.Kowalczyk M, Owczarek A, Suchanek R. Heat shock protein 70 gene polymorphisms are associated with paranoid schizophrenia in the Polish population. Cell Stress Chaperones. 2014;19:205–215. doi: 10.1007/s12192-013-0446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arion D, Unger T, Lewis DA, et al. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2007;2:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bano D, Piazzesi A, Salomoni P, Nicotera P. The histone variant H3.3 claims its place in the crowded scene of epigenetics. Aging. 2017;9:602–614. doi: 10.18632/aging.101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martins-De-Souza D, Dias-Neto E, Schmitt A, et al. Proteome analysis of schizophrenia brain tissue. World J Biol Psychiatry. 2010;11:110–120. doi: 10.3109/15622970903490626. [DOI] [PubMed] [Google Scholar]
- 37.Beck HC. Mass spectrometry in epigenetic research. Methods Mol Biol. 2010;593:263–282. doi: 10.1007/978-1-60327-194-3_13. [DOI] [PubMed] [Google Scholar]
- 38.Ibi D. Epigenetic signaling in schizophrenia. Cell Signal. 2016;27:2131–2136. doi: 10.1016/j.cellsig.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chase KA, Gavin D, Guidotti A, Sharma R. Histone methylation at H3K9; evidence for a restrictive epigenome in schizophrenia. Schizophr Res. 2014;149:15–20. doi: 10.1016/j.schres.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavin D, Sharma RP. Histone modifications, DNA methylation, and schizophrenia. Neurosci Biobehav Rev. 2011;34:882–888. doi: 10.1016/j.neubiorev.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasan A, Mitchell A. Epigenetic dysregulation in schizophrenia: molecular and clinical aspects of histone deacetylase inhibitors. Eur Arch Psychiatry Clin Neurosci. 2013;263:273–284. doi: 10.1007/s00406-013-0395-2. [DOI] [PubMed] [Google Scholar]
- 42.Will CL, Luhrmann R. Spliceosome structure and function. Cold Spring Harb Perspect Biol. 2011;3:1–24. doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward AJ, Cooper TA. The pathobiology of splicing. J Pathol. 2011;220:152–163. doi: 10.1002/path.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassoli JS, Iwata K, Steiner J, et al. Effect of MK-801 and clozapine on the proteome of cultured human oligodendrocytes. Front Cell Neurosci. 2016;10:1–14. doi: 10.3389/fncel.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao L, Mandler MD, Yi H, Feng Y. Quaking I controls a unique cytoplasmic pathway that regulates alternative splicing of myelin-associated glycoprotein. Proc Natl Acad Sci USA. 2010;107:19061–19066. doi: 10.1073/pnas.1007487107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwata K, Matsuzaki H, Manabe T, Mori N. Altering the expression balance of hnRNP C1 and C2 changes the expression of myelination-related genes. Psychiatry Res. 2011;190:364–366. doi: 10.1016/j.psychres.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 47.Saia-Cereda VM, Cassoli JS, Schmitt A, et al. Proteomics of the corpus callosum unravel pivotal players in the dysfunction of cell signaling, structure, and myelination in schizophrenia brains. Eur Arch Psychiatry Clin Neurosci. 2015;265:601–612. doi: 10.1007/s00406-015-0621-1. [DOI] [PubMed] [Google Scholar]
- 48.Cassoli JS, Guest PC, Malchow B, et al. Disturbed macro-connectivity in schizophrenia linked to oligodendrocyte dysfunction: from structural findings to molecules. NPJ Schizophr. 2015;1:15034. doi: 10.1038/npjschz.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davalieva K, Kostovska IM, Dwork AJ. Proteomics research in schizophrenia. Front Cell Neurosci. 2016;10:1–22. doi: 10.3389/fncel.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin CL, Bristol LA, Jin L, et al. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- 51.Huntsman M, Tran B-V, Potkin SG, Jr, et al. Altered ratios of alternatively spliced long and short γ2 subunit mRNAs of the γ-amino butyrate type A receptor in prefrontal cortex of schizophrenics. Proc Natl Acad Sci USA. 1998;95:15066–15071. doi: 10.1073/pnas.95.25.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Souza I, Poorkaj PP, Hong M, et al. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc Natl Acad Sci USA. 1999;96:5598–5603. doi: 10.1073/pnas.96.10.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park E, Iaccarino C, Lee J, et al. Regulatory roles of heterogeneous nuclear ribonucleoprotein M and Nova-1 protein in alternative splicing of dopamine D2 receptor Pre-mRNA. J Biol Chem. 2011;286:25301–25308. doi: 10.1074/jbc.M110.206540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- 55.Naz H, Islam A, Ahmad F, Hassan I. Calcium/calmodulin-dependent protein kinase IV: a multifunctional enzyme and potential therapeutic target. Prog Biophys Mol Biol. 2016;121:54–65. doi: 10.1016/j.pbiomolbio.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 56.Jimerson D, Post R, Carman J, et al. CSF calcium: clinical correlates in affective illness and schizophrenia. Biol Psychiatry. 1979;14:37–51. [PubMed] [Google Scholar]
- 57.Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- 58.Vendrell M, Aliguk R, Bachs O, Serratosa J. Presence of calmodulin and calmodulin-binding proteins in the nuclei of brain cells. J Neurochem. 1991;57:622–628. doi: 10.1111/j.1471-4159.1991.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 59.Szabo M, Dulka K, Gulya K. Calmodulin inhibition regulates morphological and functional changes related to the actin cytoskeleton in pure microglial cells. Brain Res Bull. 2015;120:41–50. doi: 10.1016/j.brainresbull.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Underhill SM, Wheeler XDS, Amara SG. Differential regulation of two isoforms of the glial glutamate transporter EAAT2 by DLG1 and CaMKII. J Neurosci. 2015;35:5260–5270. doi: 10.1523/JNEUROSCI.4365-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gadea A, Lo E, Herna A, Marı A. Role of Ca2+ and calmodulin-dependent enzymes in the regulation of glycine transport in Muller glia. J Neurochem. 2002;80:634–645. doi: 10.1046/j.0022-3042.2001.00735.x. [DOI] [PubMed] [Google Scholar]
- 62.Perez-Garcia JM, Valentin C, et al. Glial cell line-derived neurotrophic factor increases intracellular calcium concentration. Role of calcium/calmodulin in the activation of the phosphatidylinositol 3-kinase pathway. J Biol Chem. 2004;279:6132–6142. doi: 10.1074/jbc.M308367200. [DOI] [PubMed] [Google Scholar]
- 63.Saia-Cereda VM, Cassoli JS, Schmitt A, et al. Differential proteome and phosphoproteome may impact cell signaling in the corpus callosum of schizophrenia patients. Schizophr Res. 2016;177:70–77. doi: 10.1016/j.schres.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 64.Toyooka K, Asama K, Watanabe Y, Muratake T. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249–257. doi: 10.1016/s0165-1781(02)00127-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data