Abstract
Background
Given the extent of the surgical indications for pulmonary lobectomy in breathless patients, preoperative care and evaluation of pulmonary function are increasingly necessary. The aim of this study was to assess the contribution of preoperative pulmonary rehabilitation (PR) for reducing the incidence of postoperative pulmonary complications in non‐small cell lung cancer (NSCLC) patients with chronic obstructive pulmonary disease (COPD).
Methods
The records of 116 patients with COPD, including 51 patients who received PR, were retrospectively analyzed. Pulmonary function testing, including slow vital capacity (VC) and forced expiratory volume in one second (FEV 1), was obtained preoperatively, after PR, and at one and six months postoperatively. The recovery rate of postoperative pulmonary function was standardized for functional loss associated with the different resected lung volumes. Propensity score analysis generated matched pairs of 31 patients divided into PR and non‐PR groups.
Results
The PR period was 18.7 ± 12.7 days in COPD patients. Preoperative pulmonary function was significantly improved after PR (VC 5.3%, FEV 1 5.5%; P < 0.05). The FEV 1 recovery rate one month after surgery was significantly better in the PR (101.6%; P < 0.001) than in the non‐PR group (93.9%). In logistic regression analysis, predicted postoperative FEV 1, predicted postoperative %FEV 1, and PR were independent factors related to postoperative pulmonary complications after pulmonary lobectomy (odds ratio 18.9, 16.1, and 13.9, respectively; P < 0.05).
Conclusions
PR improved the recovery rate of pulmonary function after lobectomy in the early period, and may decrease postoperative pulmonary complications.
Keywords: Chronic obstructive pulmonary disease, lung cancer, pulmonary rehabilitation
Introduction
Although there have been advances in the management of non‐small cell lung cancer (NSCLC), complete anatomical resection remains the most effective treatment in patients with early‐stage NSCLC. However, many patients with NSCLC also have chronic obstructive pulmonary disease (COPD), which increases their risk of postoperative complications and mortality.1 The preoperative physiological assessment of patients undergoing pulmonary lobectomy should begin with lung function assessment that includes spirometry to measure slow vital capacity (VC), and forced expiratory volume in one second (FEV1), and predicted postoperative pulmonary function should be calculated. In addition, the diffusing capacity for carbon monoxide (DLCO) or cardiovascular evaluation can be performed when appropriate.2 In NSCLC patients with COPD, pulmonary lobectomy may lead to a deterioration in residual pulmonary function resulting from the loss of functioning parts of the lungs, but in limited cases, pulmonary function may be improved, for example, by eliminating non‐functional emphysematous lung areas, especially after upper lobectomy.3, 4 However most COPD patients have poor respiratory function and progressive physical disability. Therefore, some regional functional differences may make NSCLC excision impossible in COPD patients even though cancer resection is anatomically possible.
Preoperative pulmonary rehabilitation (PR) may improve exercise tolerance, symptoms, and quality of life in COPD patients following lung volume reduction surgery or lung transplantation.5, 6, 7 The benefit of PR for patients undergoing lung resection for NSCLC is well established and is gaining momentum as part of the enhanced recovery pathway.8, 9, 10 Although it is well understood that PR may improve preoperative pulmonary function and functional status, its effect on reducing perioperative pulmonary complications has not been clearly demonstrated.7, 11, 12, 13 A few studies have shown a significant benefit of PR on clinical outcomes in patients with NSCLC, but these studies also included many non‐COPD patients.14, 15 No study has shown that PR reduces postoperative pulmonary complications specifically in patients with COPD and NSCLC.
To decrease morbidity and the postoperative complication rate, improving or maintaining patients’ preoperative general condition and pulmonary function is considered essential for COPD patients scheduled to undergo lung surgery. In this study, the clinical effects of PR on postoperative pulmonary complications were retrospectively evaluated in NSCLC patients with COPD who underwent pulmonary lobectomy.
Methods
Patients
The medical records of 589 consecutive patients with NSCLC who underwent pulmonary lobectomy between September 2005 and January 2016 at our institute were retrospectively reviewed. Patients lost to follow‐up or those with complications that prevented postoperative pulmonary function testing at either one or six months following surgery were excluded. Cases of sublobar resection, pneumonectomy, or concomitant resection with the thoracic chest wall were also excluded. Finally, 116 patients met the selection criteria and were enrolled in the study, including those who had experienced complications. Our institutional review board approved this retrospective analysis (permit number: 889), and informed consent was obtained from all patients after discussion of the general risks and benefits of pulmonary lobectomy for lung cancer. The 116 patients with COPD who underwent lobectomy were divided into a PR group (n = 51) and a non‐PR group (n = 65). All patients underwent a complete preoperative pulmonary evaluation. None of the patients had received preoperative chemotherapy or radiation. A prolonged air leak was defined as air leakage lasting seven days or more.16 Delayed pneumothorax was defined as pneumothorax inclusive of increasing residual dead space on the surgical side after chest tube removal and the cause was considered a pulmonary fistula.17
Pulmonary function testing
Pulmonary function was tested at our institute using a spirometer (CHESTAC 8800, CHEST M.I. Inc., Tokyo, Japan) according to American Thoracic Society standards.18 VC and FEV1 were measured in patients preoperatively and at one and six months postoperatively. The measurements were documented in the form of the actual volume and the ratio of the actual volume to the standard volume determined by the age, gender, and height of the patient. The percentage of predicted‐FEV1 (%FEV1) is defined as FEV1 of the patient divided by the average FEV1 in the population for any person of similar age, gender, and body composition. The actual degree of postoperative functional loss in proportion to the resected lung volume was calculated as the recovery rate, using the following formula:
Briefly, predicted postoperative pulmonary function was calculated according to the formula described in a previous report.19 The calculation was based on the number of segments that remained after surgery. To evaluate the predicted postoperative value in patients with PR, the pulmonary function test values after preoperative PR were used in this calculation.
The diffuse capacity of the lungs for carbon monoxide (DLCO) was measured in cases of COPD stage II or higher, according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.20
Pulmonary rehabilitation
The inclusion criteria for PR in our institute are as follows: (i) calculated predicted postoperative FEV1 under 1000 mL, (ii) COPD staging by GOLD criteria stage II or higher, (iii) marked diffuse emphysematous changes on preoperative computed tomography, and (iv) an Eastern Cooperative Oncology Group (ECOG) performance status score of two or more. Patients that met at least one of these criteria and provided their agreement underwent PR.
In the PR group, the patients were trained to master adequate breathing and coughing techniques, instructed on incentive respiratory exercise, and practiced peripheral muscle exercise training including a cycle ergometer for two to four weeks (five days a week) under physiotherapist supervision. The patients themselves continued the training involving breathing and coughing techniques until the morning of the day of surgery. After lung resection, PR started as early as postoperative day 1. First, diaphragmatic breathing exercises, peripheral circulation exercises, aerosol therapy with bronchodilators, and exercises for chest expansion and shoulder girdle mobilization were introduced. After discharge, patients were followed‐up clinically, and spirometry was performed one and six months after surgery. The differences in PR durations between the patients resulted from various reasons, such as the choice of date of surgery or the patients’ convenience. All patients with COPD were treated by bronchodilator therapy for at least four weeks preoperatively, and smoking ceased for at least four weeks before surgery.
Surgical procedures
Patients were intubated using a double‐lumen endotracheal tube. The affected lung was deflated as soon as the pleural space was opened. The choice of surgical procedure, video‐assisted thoracoscopic surgery (VATS) or open thoracotomy, was left to the discretion of the operating surgeon. The open‐method was performed via posterolateral thoracotomy (length 6–12 cm) dividing the latissimus dorsi and anterior serratus muscles. VATS involved performing the main procedures via mini‐thoracotomy (length 4–6 cm) with a monitor and direct vision. Systematic hilar and mediastinal lymph node dissection was performed in all cases. After completing the procedure, the lung was reinflated to check for air leakage. A single straight chest tube was placed in the posterior apex, which was connected to the chest drainage system, and then ‐10 cm H2O of suction was added on the day of surgery.
Postoperative management
In general, patients were extubated at the end of the operation and transferred to the ward after a brief stay in the recovery area. The chest tube was placed on water seal on the morning of postoperative day 1. A chest X‐ray was obtained daily. Chest tube withdrawal criteria were: absence of air leakage through the chest tube at the time of the evaluation, pleural fluid drainage under 200 mL/24 hours, and postoperative chest X‐ray showing no pneumothorax. The chest tube was removed at the earliest on the third postoperative day. On the morning after chest tube withdrawal, a chest X‐ray was performed to rule out the occurrence of pneumothorax. The patients were discharged when convenient if no complications occurred during this perioperative period. All patients were followed‐up postoperatively every three to six months for five years.
Statistical analysis
JMP 12.2.0 (SAS Institute, Inc., Cary, NC, USA) software was used for statistical analysis. Groups were compared using the Pearson chi‐square test, and comparisons of functional changes after surgery within each group were made using the two‐sample Student's t‐test for paired data. In all cases, two‐tailed tests were performed. To control for potential differences in the preoperative characteristics of patients in the two groups, a propensity score matching method was used. The propensity scores were generated using logistic regression based on clinically relevant preoperative variables such as age, gender, height, pack‐years smoked, ECOG performance status score, predicted‐postoperative FEV1, and approach (VATS or open thoracotomy), that were considered possible confounders for their potential association with the outcome of interest based on clinical knowledge. Patients were matched 1:1 by nearest neighbor matching (caliper width: 0.2) without replacement. Comparisons between the matched groups were performed with McNemar's test for categorical variables and paired t‐test or Wilcoxon's rank‐sum test for continuous variables. The standardized difference was used to measure covariate balance, whereby an absolute standardized difference above 0.1 represents meaningful imbalance.
Multivariate predictors were evaluated using logistic regression analysis, and the odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. On logistic regression analysis, the conventional receiver‐operating characteristic (ROC) curve was used to determine the cut‐off value of each variable that gave maximal sensitivity and specificity with respect to predicting postoperative pulmonary complications in this study population. Differences between groups were considered significant at P < 0.05. Normally distributed continuous data were expressed as mean ± standard deviation (SD). Categorical data were expressed as counts and proportions.
Results
A total of 589 patients who underwent pulmonary lobectomy for NSCLC were retrospectively reviewed. The reasons for excluding patients from this study are shown in Figure 1. Complications preventing pulmonary function test follow‐up resulted in the exclusion of 13 cases. The most common reason for loss to follow‐up was that patients could not come to the hospital (e.g. transportation problem or work responsibilities) at either one or six months after surgery. COPD was present in 51 patients (44.0%) in the PR group and 65 patients (13.7%) in the non‐PR group. Thus, 116 patients with COPD were enrolled in this study, and their clinical characteristics are summarized in Table 1. Age and Brinkman Index were significantly higher in the PR group. Furthermore, preoperative pulmonary function and the predicted postoperative value were significantly poorer in the PR group.
Figure 1.
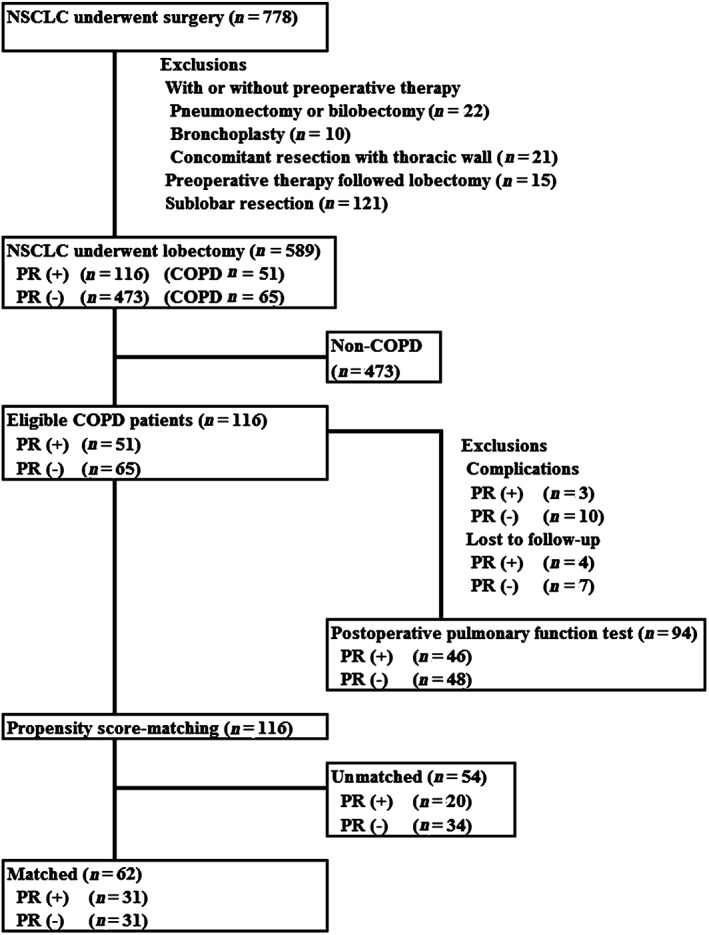
Diagram of patient selection. COPD, chronic obstructive pulmonary disease; NSCLC, non‐small cell lung cancer; PR, pulmonary rehabilitation.
Table 1.
Clinical details of NSCLC patients with COPD who underwent pulmonary resection
| COPD patients | ||||
|---|---|---|---|---|
| PR (+) | PR (−) | Standardized difference | P | |
| (n = 51) | (n = 65) | |||
| Age (years) | 74.4 ± 7.7 | 68.2 ± 8.6 | 0.760 | <0.001* |
| Gender | ||||
| Male | 46 (90.2) | 56 (86.2) | 0.124 | 0.509 |
| Female | 5 (9.8) | 9 (13.8) | — | — |
| Height (cm) | 160.1 ± 7.9 | 162.8 ± 7.0 | 0.362 | 0.038* |
| Brinkman Index | 1120.0 ± 704.4 | 950.7 ± 735.1 | 0.235 | 0.049* |
| Histology | ||||
| Adenocarcinoma | 28 (54.9) | 38 (58.5) | 0.073 | 0.702 |
| Squamous cell | 19 (37.3) | 20 (30.8) | — | — |
| Others | 4 (7.8) | 7 (10.8) | — | — |
| Tumor size (mm) | 35.0 ± 21.8 | 31.3 ± 16.7 | 0.191 | 0.404 |
| Preoperative pulmonary function | ||||
| VC (mL) | 3137.8 ± 842.0 | 3597.5 ± 754.7 | 0.575 | 0.002* |
| FEV1 (mL) | 1813.7 ± 552.8 | 2249.1 ± 499.9 | 0.826 | < 0.001* |
| FEV1% (%) | 60.5 ± 8.3 | 65.0 ± 6.0 | 0.621 | 0.001* |
| %FEV1 (%) | 90.3 ± 18.4 | 100.5 ± 20.6 | 0.522 | 0.006* |
| GOLD | ||||
| 1 | 26 (51.0) | 54 (83.1) | 0.727 | 0.003* |
| 2 | 25 (49.0) | 11 (16.9) | — | — |
| Postoperative predicted value | ||||
| Predicted VC (mL) | 2428.9 ± 816.0 | 2931.0 ± 772.1 | 0.632 | < 0.001* |
| Predicted FEV1 (mL) | 1399.2 ± 508.5 | 1849.9 ± 510.2 | 0.885 | < 0.001* |
| Predicted %FEV1 (%) | 56.4 ± 14.3 | 69.1 ± 14.7 | 0.876 | < 0.001* |
| Performance status | ||||
| 0 | 40 (78.4) | 64 (98.5) | 0.662 | < 0.001* |
| 1 | 9 (17.6) | 1 (1.5) | — | — |
| 2 | 2 (3.9) | 0 (0.0) | — | — |
| Approach | ||||
| Open thoracotomy | 33 (64.7) | 37 (56.9) | 0.160 | 0.397 |
| VATS | 18 (35.3) | 28 (43.1) | — | — |
| Operation duration (minutes) | 218.2 ± 49.5 | 224.3 ± 59.6 | 0.111 | 0.578 |
| Blood loss (mL) | 135.9 ± 128.1 | 126.5 ± 119.1 | 0.076 | 0.588 |
| Pathological staging | ||||
| IA | 12 (23.5) | 20 (30.8) | 0.164 | 0.519 |
| IB | 19 (37.3) | 20 (30.8) | — | — |
| IIA | 6 (11.8) | 7 (10.8) | — | — |
| IIB | 4 (7.8) | 5 (7.7) | — | — |
| IIIA | 10 (19.6) | 13 (20.0) | — | — |
| Postoperative stay (days) | 19.0 ± 24.8 | 13.6 ± 9.8 | 0.286 | 0.049* |
| Postoperative complications (%) | ||||
| Total | 4 (7.8) | 12 (18.5) | 0.321 | 0.101 |
| Pulmonary | 3 (5.9) | 10 (15.4) | 0.312 | 0.109 |
P < 0.05 between pulmonary rehabilitation (PR) and non‐PR groups.
COPD, chronic obstructive pulmonary disease; FEV 1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; NSCLC, non‐small cell lung cancer; VATS, video‐assisted thoracoscopic surgery; VC, vital capacity.
Propensity score analysis generated well‐matched pairs of 31 patients (Table 2); briefly, 60.8% (31/51) in the PR group and 47.7% (31/65) in the non‐PR group were matched. There were no significant differences in observed preoperative variables such as age, gender, height, Brinkman Index, and all predicted postoperative pulmonary function parameters between the groups after matching. The balance of each sample size was assessed by standardized differences, and its values on preoperative variables were mostly under 0.1. After propensity score matching, the population of COPD stage II or higher was significantly reduced (16.1% in the non‐PR group and 22.6% in the PR group). The DLCO was measured in cases of COPD stage II or higher in our institute; therefore, it was difficult to evaluate the DLCO in this study.
Table 2.
Clinical details of NSCLC patients with COPD who underwent pulmonary resection after propensity matching
| Characteristics | COPD patients after propensity score matching | |||
|---|---|---|---|---|
| PR (+) | PR (−) | Standardized difference | P | |
| (n = 31) | (n = 31) | |||
| Age (years) | 72.0 ± 8.8 | 71.3 ± 6.5 | 0.090 | 0.695 |
| Gender | ||||
| Male | 27 (87.1) | 27 (87.1) | 0.000 | 1.000 |
| Female | 4 (12.9) | 4 (12.9) | — | — |
| Height (cm) | 161.6 ± 8.1 | 161.8 ± 5.4 | 0.029 | 0.876 |
| Brinkman Index | 986.8 ± 466.3 | 1015.3 ± 820.1 | 0.043 | 0.867 |
| Histology | ||||
| Adenocarcinoma | 19 (61.3) | 19 (61.3) | 0.000 | 1.000 |
| Squamous cell | 10 (32.3) | 10 (32.3) | — | — |
| Others | 2 (6.5) | 2 (6.5) | — | — |
| Tumor size (mm) | 33.6 ± 25.5 | 31.4 ± 17.6 | 0.100 | 0.344 |
| Preoperative pulmonary function | ||||
| VC (mL) | 3332.9 ± 952.8 | 3340.7 ± 620.3 | 0.010 | 0.970 |
| FEV1 (mL) | 1967.1 ± 597.4 | 2063.6 ± 389.9 | 0.191 | 0.455 |
| FEV1% (%) | 61.7 ± 6.7 | 63.9 ± 6.5 | 0.333 | 0.191 |
| %FEV1 (%) | 92.0 ± 16.7 | 97.2 ± 19.0 | 0.290 | 0.257 |
| GOLD | ||||
| 1 | 24 (77.4) | 26 (83.9) | 0.164 | 0.063 |
| 2 | 7 (22.6) | 5 (16.1) | — | — |
| Postoperative predicted value | ||||
| Predicted VC (mL) | 2674.7 ± 908.1 | 2603.7 ± 592.3 | 0.093 | 0.642 |
| Predicted FEV1 (mL) | 1582.7 ± 540.5 | 1604.4 ± 345.3 | 0.048 | 0.850 |
| Predicted %FEV1 (%) | 61.3 ± 13.7 | 62.6 ± 13.2 | 0.097 | 0.635 |
| Performance status | ||||
| 0 | 30 (96.8) | 30 (96.8) | 0.000 | 0.317 |
| 1 | 1 (3.2) | 1 (3.2) | — | — |
| Approach | ||||
| Open thoracotomy | 20 (64.5) | 18 (58.1) | 0.131 | 0.441 |
| VATS | 11 (35.5) | 13 (41.9) | — | — |
| Operation time (minutes) | 214.1 ± 41.1 | 218.4 ± 50.3 | 0.094 | 0.717 |
| Blood loss (mL) | 124.4 ± 96.8 | 110.5 ± 90.8 | 0.148 | 0.560 |
| Pathological staging | ||||
| IA | 7 (22.6) | 8 (25.8) | 0.075 | 0.713 |
| IB | 11 (35.4) | 12 (38.7) | — | — |
| IIA | 5 (16.1) | 4 (12.9) | — | — |
| IIB | 3 (9.7) | 2 (6.5) | — | — |
| IIIA | 5 (16.1) | 5 (16.1) | — | — |
| Post‐operative stay (days) | 16.4 ± 10.3 | 12.1 ± 6.0 | 0.510 | 0.179 |
| Post‐operative complications (%) | ||||
| Total | 2 (6.5) | 5 (16.1) | 0.306 | 0.232 |
| Pulmonary | 1 (3.2) | 3 (9.7) | 0.267 | 0.165 |
COPD, chronic obstructive pulmonary disease; FEV 1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; NSCLC, non‐small cell lung cancer; PR, pulmonary rehabilitation; VATS, video‐assisted thoracoscopic surgery; VC, vital capacity.
The locations of lung cancer are summarized in Table 3, and the details of complications in COPD patients after pulmonary lobectomy are shown in Table 4.
Table 3.
Resected pulmonary location in COPD patients
| Location | Total COPD patients | After propensity score matching | ||
|---|---|---|---|---|
| PR (+) | PR (−) | PR (+) | PR (−) | |
| (n = 51) | (n = 65) | (n = 31) | (n = 31) | |
| Right upper | 11 (21.6) | 25 (38.5) | 10 (32.2) | 10 (32.2) |
| Right middle | 4 (7.8) | 6 (9.2) | 3 (9.7) | 0 (0) |
| Right lower | 14 (27.5) | 12 (18.5) | 4 (12.9) | 8 (25.8) |
| Left upper | 13 (25.5) | 14 (21.5) | 10 (32.2) | 7 (22.6) |
| Left lower | 9 (17.6) | 8 (12.3) | 4 (12.9) | 6 (19.4) |
COPD, chronic obstructive pulmonary disease; PR, pulmonary rehabilitation.
Table 4.
Details of complications in NSCLC patients with COPD after pulmonary lobectomy
| Pulmonary complications | Total COPD patients | After propensity score matching | ||
|---|---|---|---|---|
| PR (+) | PR (−) | PR (+) | PR (−) | |
| (n = 51) | (n = 65) | (n = 31) | (n = 31) | |
| Pneumonia | 1 (2.0) | 2 (3.1) | 0 (0.0) | 1 (3.2) |
| Interstitial pneumonia | 0 (0.0) | 3 (4.6) | 0 (0.0) | 1 (3.2) |
| Prolonged air leak | 2 (3.9) | 3 (4.6) | 1 (3.2) | 0 (0.0) |
| Delayed pneumothorax | 0 (0.0) | 2 (3.1) | 0 (0.0) | 1 (3.2) |
| Other complications | ||||
| Chylothorax | 0 (0.0) | 1 (1.5) | 0 (0.0) | 0 (0.0) |
| Pyothorax | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.2) |
| Wound infection | 1 (2.0) | 1 (1.5) | 1 (3.2) | 1 (3.2) |
COPD, chronic obstructive pulmonary disease; NSCLC, non‐small cell lung cancer; PR, pulmonary rehabilitation.
The duration of PR was 18.7 ± 12.7 days. Preoperative pulmonary function was significantly improved after PR (VC 5.3%, FEV1 5.5%, both P < 0.05) (Fig 2). Postoperative pulmonary function testing was performed at one month (1.2 ± 0.3 months) and six months (6.4 ± 0.5 months). The recovery rate of VC one month after surgery was not significantly different between the groups, but the recovery rate of FEV1 one month after surgery was significantly better in the PR group (101.6%; P < 0.001) compared to the non‐PR group (93.9%) (Fig 3). There was no significant difference between the groups regarding the recovery rate of postoperative VC/FEV1 at six months.
Figure 2.
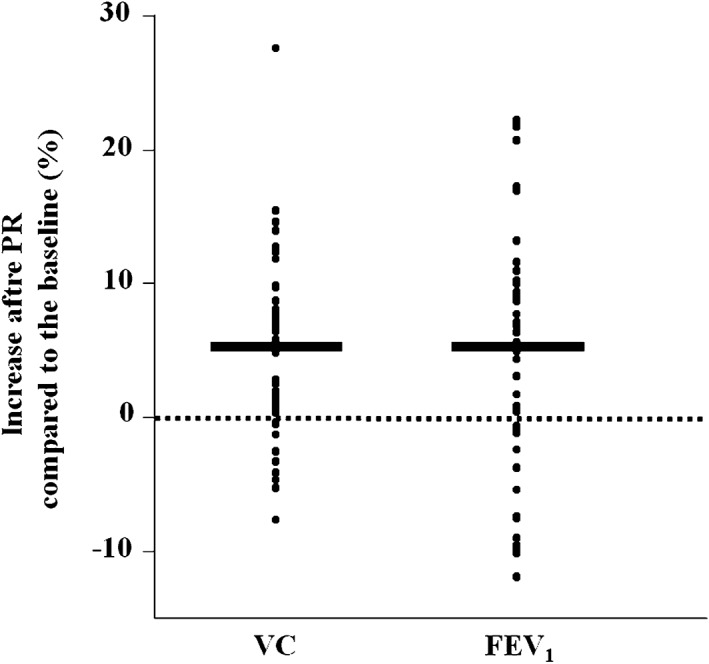
The percentage increase in pulmonary function (vital capacity [VC] and forced expiratory volume in one second [FEV 1]) after pulmonary rehabilitation (PR) compared to before PR. *P < 0.05 versus before PR (n = 51).
Figure 3.
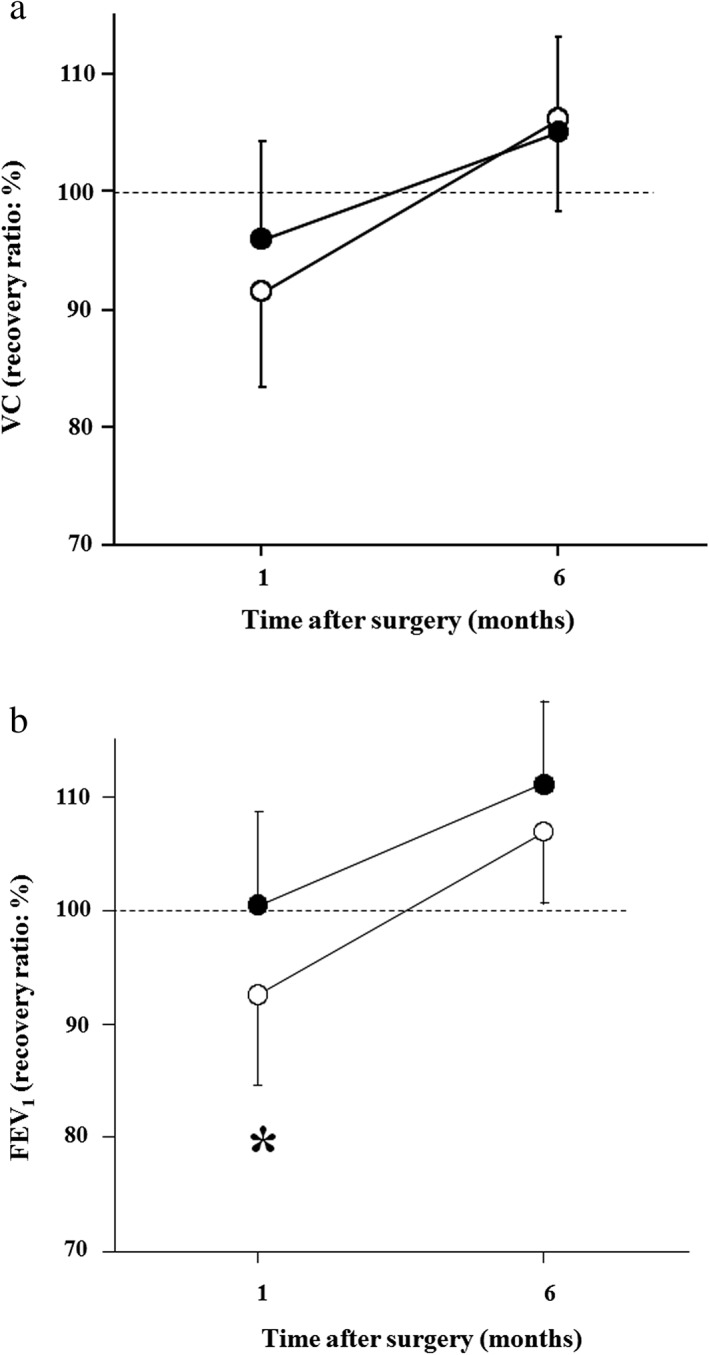
The recovery rate of (a) vital capacity (VC) and (b) forced expiratory volume in one second (FEV 1) one and six months after pulmonary lobectomy in the pulmonary rehabilitation (PR) (closed circles) and non‐PR groups (open circles). The Y‐axis shows the recovery rate as expressed by measured postoperative value/predicted postoperative value × 100 (%). Values are expressed as means ± standard deviation. *P < 0.05 versus PR.
Logistic regression analysis was performed to estimate the predictors of postoperative pulmonary complications after surgery (Table 5). The threshold was determined based on ROC analysis (as mentioned in the Methods section). Use of this cut‐off value is limited to this study, because the threshold was determined based on ROC analysis of this study population. In logistic regression analysis, age, predicted postoperative FEV1 (<1500 mL), predicted postoperative %FEV1 (≤60%), and PR were independent factors related to postoperative pulmonary complications after pulmonary lobectomy (OR 1.53, 7.10, 6.80, and 6.42, respectively; P < 0.05). These results indicate that PR could contribute to reducing postoperative pulmonary complications after pulmonary lobectomy in NSCLC patients with COPD.
Table 5.
Logistic regression analysis for predictors of the incidence of postoperative pulmonary complications in NSCLC patients with COPD after lobectomy
| Variable | Total COPD patients | After propensity score matching | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age (70 years ≦) | 2.79 | 0.53–19.8 | 0.233 | 1.53 | 1.27–78.4 | 0.036* |
| Gender (Male) | 3.35 | 0.09–67.1 | 0.462 | 1.70 | 0.45–2.23 | 0.096 |
| Brinkman Index (400 ≦) | 2.94 | 0.30–82.8 | 0.387 | 1.49 | 0.06–72.3 | 0.809 |
| Tumor size (30 mm <) | 2.74 | 0.68–13.1 | 0.160 | 2.25 | 0.18–32.9 | 0.519 |
| Approach (open thoracotomy) | 2.24 | 0.54–11.3 | 0.273 | 1.45 | 0.08–42.7 | 0.799 |
| Predicted VC (≦2000 mL) | 10.16 | 0.88–276.1 | 0.064 | 1.49 | 0.08–11.4 | 0.205 |
| Predicted FEV1 (<1500 mL) | 31.30 | 2.34–974.9 | 0.007* | 7.10 | 1.33–439.2 | 0.002* |
| Predicted %FEV1 (≦60%) | 9.67 | 1.24–92.9 | 0.030* | 6.80 | 1.425–235.0 | 0.017* |
| Rehabilitation (−) | 5.82 | 1.11–48.3 | 0.036* | 6.42 | 1.69–589.1 | 0.021* |
P < 0.05.
COPD, chronic obstructive pulmonary disease; FEV 1, forced expiratory volume in one second; NSCLC, non‐small cell lung cancer; VC, vital capacity.
Discussion
Pulmonary rehabilitation has been shown to significantly reduce dyspnea and improve exercise capacity in COPD patients prior to surgery, and may help improve these parameters and allow patients with borderline and poor lung function to undergo curative surgery.21 However, the exact effect of PR on postoperative pulmonary complications in NSCLC patients with COPD has not been elucidated. The main objective of the present study was to determine whether preoperative physiotherapy has any effect on lung function and the incidence of pulmonary complications after lung resection surgery. This study demonstrated that PR may be an option for improving pulmonary function and the recovery rate of pulmonary function after lobectomy in the early period. Furthermore, logistic regression analysis showed that PR could contribute to reducing postoperative pulmonary complications after pulmonary lobectomy in NSCLC patients with COPD.
Many investigators have established the benefits of pulmonary rehabilitation; however, the duration of a standard program for COPD was generally 6–12 weeks,22, 23 although several studies reported that short‐term PR, such as two to four weeks, still improved patients’ postoperative condition.7, 11, 12, 24 Recently, short‐term PR has been generally accepted. Because it is necessary for patients with malignant disease to undergo surgery without delay, effective short‐term preoperative PR programs should be adopted. In the present study, the PR period was generally two to four weeks. Our results suggest that this short‐term PR contributes to an improvement in pulmonary function and the recovery rate of pulmonary function after lobectomy in the early period, as well as to reducing postoperative pulmonary complications. These results indicate that short‐term PR of two to four weeks may be an acceptable duration considering the trade‐off between cancer therapy and effective PR.
Many of the common complications after thoracic surgery are pulmonary in nature. Varela et al. reported that atelectasis developed less commonly in the PR group than in a control group.25 The mechanisms by which PR improves postoperative pulmonary complication are still not clear, but coughing, deep breathing using an incentive spirometer, walking, and performing other seemingly minor activities all might contribute to decreasing the incidence of postoperative complications, such as pneumonia.26 It is possible that PR may prevent acute respiratory distress syndrome, which starts with pneumonia, but a randomized, prospective study is needed to confirm this.
DLCO, a method for evaluating pulmonary function, is a good predictor of complications. However, DLCO was not routinely measured in the present study, although it was measured in patients with GOLD stage II or higher, which indicates severely compromised respiratory function, such as idiopathic interstitial pneumonia. Because these specific predictors are more accurate, it would be worth measuring DLCO to evaluate its use as a high‐risk group marker in future studies.
In the present study, the recovery rate was used to evaluate the degree of difference between the measured postoperative pulmonary function and the expected value. The recovery rate of FEV1 at one month after surgery was significantly better in the PR than in the non‐PR group. The reason for this is unclear, but the PR effect may have continued for at least up to a month after surgery. In support of this hypothesis, Horie et al. reported that short‐term PR (two weeks of PR followed by continued one‐day PR every month up to 1.5 years) in 47 COPD patients resulted in the maintenance of pulmonary function and exercise performance over the long term.27 This result suggests the possibility that short‐term PR, such as two weeks, might have an effect for at least a month. PR was not performed in patients after discharge in the present study, thus further investigation is needed to clarify these mechanisms.
Previously published studies have shown COPD to be a well‐known risk factor for postoperative pulmonary complications.28, 29 Whereas PR improves the functional parameters responsible for inoperability,22 the selection criteria for enrolling patients in PR are still controversial. A recent study demonstrated that, even in patients with early‐stage COPD, the prevalence of postoperative pulmonary complications was higher than in NSCLC patients with normal spirometry.29 In the present retrospective review, the non‐PR group included stage I COPD. Our finding that PR reduced postoperative pulmonary complications may indicate that PR should be performed even at stage I COPD. Nevertheless, the retrospective nature of the present study is a limitation. Considering the relationship between PR and postoperative pulmonary complications, it is very difficult to conduct a randomized, controlled clinical trial (RCT) to compare patients with NSCLC and COPD undergoing pulmonary lobectomy, because randomizing patients into two groups with and without PR may present serious ethical concerns. Therefore, to overcome this limitation and to minimize bias, the best approach is to use a large registry and propensity score‐matched data.
In summary, our results suggest that PR may be an option to improve pulmonary function and the recovery rate of pulmonary function after lobectomy in the early period. Our results also suggest that PR could contribute to reducing postoperative pulmonary complications after pulmonary lobectomy in NSCLC patients with COPD.
Disclosure
No authors report any conflict of interest.
Acknowledgments
The authors thank Dr. Eri Maeda for her suggestions and support with statistical analysis.
References
- 1. Licker MJ, Widikker I, Robert J et al. Operative mortality and respiratory complications after lung resection for cancer: Impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006; 81: 1830–7. [DOI] [PubMed] [Google Scholar]
- 2. Brunelli A, Kim AW, Berger KI, Addrizzo‐Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. (Published erratum appears in Chest 2014; 145: 437.). Chest 2013; 143 (5 Suppl.): 166S–90S. [DOI] [PubMed] [Google Scholar]
- 3. Sekine Y, Iwata T, Chiyo M et al. Minimal alteration of pulmonary function after lobectomy in lung cancer patients with chronic obstructive pulmonary disease. Ann Thorac Surg 2003; 76: 356–61. [DOI] [PubMed] [Google Scholar]
- 4. Kushibe K, Takahama M, Tojo T, Kawaguchi T, Kimura M, Taniguchi S. Assessment of pulmonary function after lobectomy for lung cancer‐‐upper lobectomy might have the same effect as lung volume reduction surgery. Eur J Cardiothorac Surg 2006; 29: 886–90. [DOI] [PubMed] [Google Scholar]
- 5. Cooper JD, Trulock EP, Triantafillou AN et al. Bilateral pneumonectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995; 109: 106–16. [DOI] [PubMed] [Google Scholar]
- 6. Ries AL, Make BJ, Lee SM et al. The effects of pulmonary rehabilitation in the national emphysema treatment trial. Chest 2005; 128: 3799–809. [DOI] [PubMed] [Google Scholar]
- 7. Mujovic N, Mujovic N, Subotic D et al. Preoperative pulmonary rehabilitation in patients with non‐small cell lung cancer and chronic obstructive pulmonary disease. Arch Med Sci 2014; 10: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kenny PM, King MT, Viney RC et al. Quality of life and survival in the 2 years after surgery for non small‐cell lung cancer. J Clin Oncol 2008; 26: 233–41. [DOI] [PubMed] [Google Scholar]
- 9. Pompili C, Brunelli A, Refai M, Xiumè F, Sabbatini A. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case‐matched study. Eur J Cardiothorac Surg 2010; 37: 525–30. [DOI] [PubMed] [Google Scholar]
- 10. Sommer MS, Trier K, Vibe‐Petersen J et al. Perioperative rehabilitation in operation for lung cancer (PROLUCA) – rationale and design. BMC Cancer 2014; 14: 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bobbio A, Chetta A, Ampollini L et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non‐small cell lung cancer. Eur J Cardiothorac Surg 2008; 33: 95–8. [DOI] [PubMed] [Google Scholar]
- 12. Benzo R, Wigle D, Novotny P et al. Preoperative pulmonary rehabilitation before lung cancer resection: Results from two randomized studies. Lung Cancer 2011; 74: 441–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bradley A, Marshall A, Stonehewer L et al. Pulmonary rehabilitation programme for patients undergoing curative lung cancer surgery. Eur J Cardiothorac Surg 2013; 44: 266–71. [DOI] [PubMed] [Google Scholar]
- 14. Sebio Garcia R, Yáñez Brage MI, Giménez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: A systematic review and meta‐analysis. Interact Cardiovasc Thorac Surg 2016; 23: 486–97. [DOI] [PubMed] [Google Scholar]
- 15. Morano MT, Araújo AS, Nascimento FB et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: A pilot randomized controlled trial. Arch Phys Med Rehabil 2013; 94: 53–8. [DOI] [PubMed] [Google Scholar]
- 16. Rivera C, Bernard A, Falcoz PE et al. Characterization and prediction of prolonged air leak after pulmonary resection: A nationwide study setting up the index of prolonged air leak. Ann Thorac Surg 2011; 92: 1062–8. [DOI] [PubMed] [Google Scholar]
- 17. Takagi K, Hata Y, Sasamoto S et al. Late onset postoperative pulmonary fistula following a pulmonary segmentectomy using electrocautery or a harmonic scalpel. Ann Thorac Cardiovasc Surg 2010; 16: 21–5. [PubMed] [Google Scholar]
- 18. American Thoracic Society . Standardization of spirometry–1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis 1987; 136: 1285–98. [DOI] [PubMed] [Google Scholar]
- 19. Zeiher BG, Gross TJ, Kern JA, Lanza LA, Peterson MW. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest 1995; 108: 68–72. [DOI] [PubMed] [Google Scholar]
- 20. Rabe KF, Hurd S, Anzueto A et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176: 532–55. [DOI] [PubMed] [Google Scholar]
- 21. Ries AL, Bauldoff GS, Carlin BW et al. Pulmonary rehabilitation: Joint ACCP/AACVPR evidence‐based clinical practice guidelines. Chest 2007; 131 (5 Suppl.): 4S–42S. [DOI] [PubMed] [Google Scholar]
- 22. Nici L. Preoperative and postoperative pulmonary rehabilitation in lung cancer patients. Thorac Surg Clin 2008; 18: 39–43. [DOI] [PubMed] [Google Scholar]
- 23. Niewoehner DE. Clinical practice. Outpatient management of severe COPD. N Engl J Med 2010; 362: 1407–16. [DOI] [PubMed] [Google Scholar]
- 24. Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2013; 43: 293–6. [DOI] [PubMed] [Google Scholar]
- 25. Varela G, Ballesteros E, Jiménez MF, Novoa N, Aranda JL. Cost‐effectiveness analysis of prophylactic respiratory physiotherapy in pulmonary lobectomy. Eur J Cardiothorac Surg 2006; 29: 216–20. [DOI] [PubMed] [Google Scholar]
- 26. Pehlivan E, Turna A, Gurses A, Gurses HN. The effects of preoperative short‐term intense physical therapy in lung cancer patients: A randomized controlled trial. Ann Thorac Cardiovasc Surg 2011; 17: 461–8. [DOI] [PubMed] [Google Scholar]
- 27. Horie J, Fujii H, Ishihara H, Horikawa E. A study of the long‐term continuous effectiveness of a pulmonary rehabilitation educational program on inpatients. West Kyushu J Rehabil Sci 2008; 1: 3–9. [Google Scholar]
- 28. Gupta H, Ramanan B, Gupta PK et al. Impact of COPD on postoperative outcomes: Results from a national database. Chest 2013; 143: 1599–606. [DOI] [PubMed] [Google Scholar]
- 29. Kim ES, Kim YT, Kang CH et al. Prevalence of and risk factors for postoperative pulmonary complications after lung cancer surgery in patients with early‐stage COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 1317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


