Abstract
Background
Lung cancer prognosis is related to various factors; however, the comprehensive relationship between these factors, including microRNAs (miRNAs), and lung cancer prognosis has not been determined. Thus, the aim of this study was to identify the key factors associated with lung cancer prognosis.
Methods
We retrospectively analyzed prognosis and relevant factors in 216 non‐small cell lung cancer (NSCLC) patients diagnosed from January 2008 to December 2014. Paraffin‐embedded lung tissue samples were used to detect miRNA‐21 and miRNA‐155. Clinical information was collected, including tumor malignancy, tumor node metastasis (TNM) classification, pathological type, site of the original tumor, and lung involvement. The association between factors and overall survival was analyzed. A Cox proportional hazard model was used to perform univariate and multivariate analyses.
Results
Age at surgery (hazard ratio [HR] 1.021, 95% confidence interval [CI] 1.003–1.040), TNM stages II (HR 3.858, 95% CI 2.449–6.078) and III (HR 3.099, 95% CI 1.911–5.023), and miRNA‐21 (HR 1.002, 95% CI 1.001–1.003) and miRNA‐155 expression (HR 1.131, 95% CI 1.064–1.202) were independent prognostic factors in NSCLC patients. MiRNA‐21 expression with TNM stages II (HR 1.282, 95% CI 1.197–1.372) and III (HR 1.247, 95% CI 1.149–1.354) interacted to affect prognosis in NSCLC patients.
Conclusions
Older age, higher clinical TNM stage, and increased miRNA‐21 and miRNA‐155 expression in NSCLC tissue were independently associated with poor survival in NSCLC patients. MiRNA‐21 expression and clinical TNM stage interacted to affect prognosis.
Keywords: MicroRNA, non‐small‐cell lung cancer, prognostic factor
Introduction
Primary lung cancer (LC) is a common malignant tumor, originating in lung tissue and characterized by uncontrolled malignant tumor cell growth. About 80% of lung cancers are non‐small‐cell lung cancers (NSCLC).1 NSCLC may spread beyond the lung by tumor cell metastasis into tissue in other parts in the body. Severe respiratory failure, and the associated symptoms of metastasis are the common cause of death in patients with advanced LC. LC is one of leading causes of cancer‐related death worldwide, affecting nearly 1.8 million people and resulting in the death of 1.6 million in 2012. LC is the primary cause of cancer‐related death in men and the second in women after breast cancer.2 After diagnosis, only about 17.4% of LC patients survive five years in developed countries, and the rate is worse in developing countries.3, 4
Lung cancer prognosis is related to various factors, including tumor malignancy, tissue origin and classification, and invasiveness.5, 6 microRNAs (miRNAs), which are small non‐coding RNAs, have been reported to be associated with LC prognosis.7, 8, 9 There are a wide variety of biological processes of tumorigenesis and development, in which miRNAs act as key regulators, including tumor cell proliferation, differentiation, invasion, angiogenesis, epithelial mesenchymal transition (EMT), and apoptosis.10 The comprehensive relationship between these factors, including miRNAs, and LC prognosis has not been determined. Thus, the aim of this study was to identify the association between these factors and LC prognosis using multivariate analysis.
Methods
Subjects and samples
This retrospective study was conducted at Xuanwu Hospital, Beijing, China. Formalin‐fixed, paraffin‐embedded (FFPE) NSCLC tissues were obtained from patients who underwent surgical resection from January 2008 to December 2014 at the Department of Thoracic Surgery at this hospital. The inclusion criteria were: confirmed diagnosis and typical characteristics of NSCLC; complete basic information including age, gender, histology, tumor size, tumor type, tumor node metastasis (TNM) stage, and treatment after surgery; no previous anticancer treatment administered before surgical resection; complete contact information and follow‐up medical records; no diagnosis of any other severe diseases, including cancers; and willingness to participate in the study. Patients or samples unable to meet these requirements were excluded from the study. The seventh edition of the TNM Staging System of the American Joint Committee on Cancer and Union for International Cancer Control was used for tumor staging.
The institutional review board of Xuanwu Hospital (clinical research 2007018) approved the study. Written informed consent was obtained from all study participants and their accompanying relatives. Patients were informed that their resected lung tissue would be used in this study.
Laboratory process
Formalin‐fixed, paraffin‐embedded LC tissues were sectioned at 5–20 μm thickness. The sections were fully deparaffinized using 1 mL xylene (Guangzhou Chemical Reagent Factory, Guangzhou, China) and vigorously centrifuged at 14 000 g at room temperature for 2 minutes in a BY‐320C centrifuge (Baiyang Medical Instrument Company, Beijing, China). After the xylene was removed by washing in absolute ethanol, the total RNA was purified and extracted using the miRNeasy FFPE kit (Qiagen China Co., Ltd., Shanghai, China), and the quantity and quality were measured using a dual‐beam ultraviolet spectrophotometer (Beckman Coulter, Fullerton, CA, USA).
miRNA expression concentration was measured by quantitative real‐time reverse transcription–PCR (RT–PCR) using the QuantiTect SYBR Green PCR Kit (Qiagen) and the 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The reactions were performed in triplicate in a 96‐well optical plate at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds, and 60°C for 60 seconds. Real‐time PCR results were expressed as cycle threshold (CT) values, which were defined by the number of PCR cycles required for the fluorescent signal to be higher than the threshold, indicating baseline variability. MiRNA expression was represented by 2−ΔCT, where ΔCT = (CT MiRNA of case or control minus CT reference sample). SnRNA U6 was used as an endogenous control to normalize all samples.11
Data analysis
Statistical analysis was performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). All continuous and categorical data were described using mean ± standard deviation ( ± s) and frequencies, respectively. Receiver operating characteristic (ROC) curves were performed to analyze whether miRNA expression could discriminate patient status. The areas under the ROC curves (AUROCs) and optimum cut‐off values were also calculated. A Cox proportional hazard model was used to perform univariate and multivariate analyses to examine associations between factors and survival. In the Cox proportional hazard model, death was considered the outcome variable, with all other variables considered independent. Survival was calculated from the date of surgery to the date of death or last follow‐up (to December 2015). Only deaths from LC, metastatic disease, or complication were included as endpoints of the study.
Statistically significant variables in the univariate analyses and those felt to be clinically relevant were entered into a multivariate regression model. Variables were entered and eliminated from the model in a stepwise manner. All testing was two‐sided, and significance was determined at P ≤ 0.05.
Results
Basic characteristics
A total of 216 NSCLC FFPE tissues were selected for the study: 110 (50.9%) squamous cell carcinoma (SCC), 73 (33.8%) adenocarcinoma (AC) and 33 (15.3%) large cell carcinoma (LCC). The average age at surgery was 55.9 ± 15.1 years. Eighty‐eight (40.7%) patients survived and 128 (59.3%) had died by the final follow‐up.
MiRNA‐21 and miRNA‐155 expression in different age groups
We analyzed miRNA‐21 and miRNA‐155 expression at different ages, TNM stages, and tumor sizes. There was no significant difference between these groups (Table 1).
Table 1.
MiRNA‐21 and MiRNA‐155 expression in NSCLC by TNM stage, age, and tumor size
| MiRNA | Item | N | ± s | F or t | P |
|---|---|---|---|---|---|
| miR‐21 | Stage I | 88 | 4.567 ± 2.597 | F = 1.037 | 0.356 |
| Stage II | 57 | 4.148 ± 2.135 | |||
| Stage III | 71 | 4.776 ± 2.579 | |||
| miR‐21 | Stage I | 88 | 4.567 ± 2.597 | F = 0.043 | 0.836 |
| Stage II and III | 128 | 4.496 ± 2.403 | |||
| miR‐155 | Stage I | 88 | 4.487 ± 2.218 | F = 0.780 | 0.460 |
| Stage II | 57 | 4.819 ± 2.398 | |||
| Stage III | 71 | 4.930 ± 2.216 | |||
| miR‐155 | Stage I | 88 | 4.4867 ± 2.218 | F = 1.491 | 0.223 |
| Stage II and III | 128 | 4.869 ± 2.290 | |||
| miR‐21 | Age ≤ 60 | 106 | 4.409 ± 2.267 | t = 0.678 | 0.498† |
| Age > 60 | 110 | 4.637 ± 2.672 | |||
| miR‐155 | Age ≤ 60 | 106 | 4.582 ± 2.334 | t = 0.837 | 0.403 |
| Age > 60 | 110 | 4.840 ± 2.196 | |||
| miR‐21 | Tumor size ≤ 3 cm | 117 | 4.239 ± 2.232 | t = 1.823 | 0.070† |
| Tumor size > 3 cm | 99 | 4.863 ± 2.714 | |||
| miR‐155 | Tumor size ≤ 3 cm | 117 | 4.447 ± 2.203 | t = 1.885 | 0.061 |
| Tumor size > 3 cm | 99 | 5.028 ± 2.304 |
Equal variances not assumed.
MiRNA, microRNA; NSCLC, non‐small cell lung cancer; TNM, tumor node metastasis.
Receiver operating characteristic analysis
Receiver operating characteristic (ROC) curves were used to analyze whether miRNA expression could discriminate the different survival status in patients (Fig 1). The AUROCs of miRNA‐21 and miRNA‐155 were 0.758 (95% confidence interval [CI] 0.693–0.823; P = 0.000) and 0.725 (95% CI 0.656–0.793; P = 0.000), respectively. The cut‐off values were 4.490 and 4.250, respectively.
Figure 1.
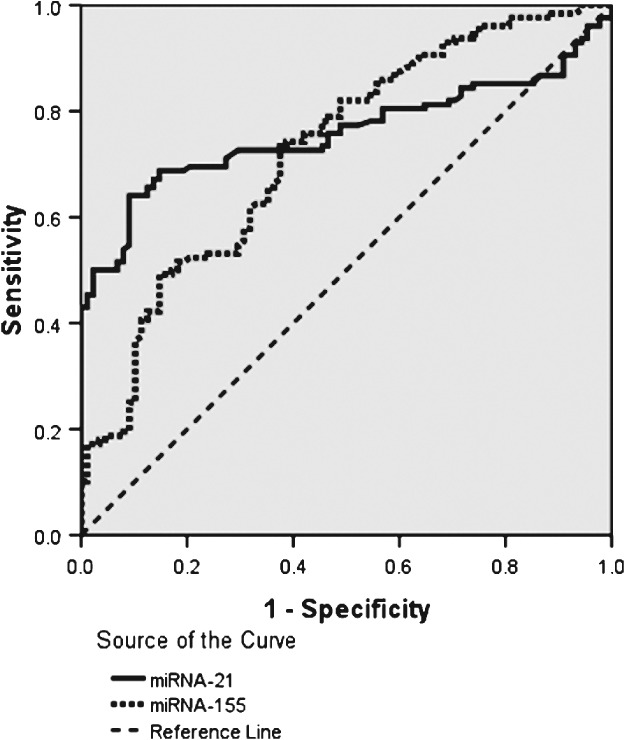
Receiver operating characteristic curve analysis for microRNA‐21 and microRNA‐155 expression in non‐small cell lung cancer tissues.
Results of univariate analysis
We used a univariate Cox proportional hazard model to examine the association between each factor and survival. Univariate analysis indicated that age at surgery, miRNA‐21, miRNA‐155, TNM stage, and severe complications before surgery were statistically related to NSCLC prognosis (P < 0.05) (Table 2).
Table 2.
Relationship between survival and single factors analyzed by univariate cox proportional hazard regression
| Item | HR (95% CI) | P |
|---|---|---|
| Age at surgery† | 1.022 (1.005–1.040) | 0.013 |
| Tumor size† | 1.063 (0.965–1.171) | 0.219 |
| miRNA‐21† | 1.196 (1.119–1.277) | 0.000 |
| miRNA‐155† | 1.163(1.073–1.259) | 0.000 |
| Gender | ||
| Female | 1 | |
| Male | 1.157 (0.766–1.747) | 0.489 |
| Location | ||
| Central | 1 | |
| Peripheral | 1.163 (0.755–1.790) | 0.494 |
| Diffuse | 0.710 (0.464–1.084) | 0.113 |
| Histology | ||
| SCC | 1 | |
| AC | 0.973 (0.581–1.629) | 0.916 |
| LCC | 0.933 (0.544–1.603) | 0.803 |
| TNM Stage | ||
| I | 1 | |
| II | 3.384 (2.179–5.258) | 0.000 |
| III | 2.211 (1.391–3.515) | 0.001 |
| Severe complication before surgery | ||
| No | 1 | |
| Yes | 1.918 (1.324–2.778) | 0.001 |
| Radiation | ||
| No | 1 | |
| Yes | 0.639 (0.342–1.191) | 0.159 |
| Chemotherapy | ||
| No | 1 | |
| Yes | 3.893 (0.962–15.766) | 0.057 |
| Regularly see doctor | ||
| No | 1 | |
| Sometimes | 1.118 (0.527–2.374) | 0.384 |
| Yes | 1.025 (0.559–1.880) | 0.309 |
Analyzed as a continuous variable.
AC, adenocarcinoma; CI, confidence interval; HR, hazard ratio; LCC, large cell carcinoma; MiRNA, microRNA; SCC, squamous cell carcinoma; TNM, tumor node metastasis.
Multivariate analysis by Cox regression
The five factors of univariate analysis were input into a multivariate Cox proportional hazard regression model. Age at surgery (hazard ratio [HR] 1.021, 95% CI 1.003–1.040), TNM stages II (HR 3.858, 95% CI 2.449–6.078) and III (HR 3.099, 95% CI 1.911–5.023), and miRNA‐21 (HR 1.002, 95% CI 1.001–1.003) and miRNA‐155 expression (HR 1.131, 95% CI 1.064–1.202) were independent prognostic factors in NSCLC patients. MiRNA‐21 expression with TNM stages II (HR 1.282, 95% CI 1.197–1.372) and III (HR1.247, 95%CI 1.149–1.354) had interaction effects on NSCLC prognosis (Table 3).
Table 3.
Relationship between survival and various factors analyzed by multivariate Cox proportional hazard regression
| Items | β | HR (95% CI) | Wald X2 | P |
|---|---|---|---|---|
| Age at surgery | 0.021 | 1.021 (1.003–1.040) | 5.012 | 0.025 |
| TNM stage II | 1.350 | 3.858 (2.449–6.078) | 33.898 | 0.000 |
| TNM stage III | 1.131 | 3.099 (1.911–5.023) | 21.051 | 0.000 |
| MiRNA‐155 | 0.123 | 1.131 (1.064–1.202) | 15.491 | 0.000 |
| MiRNA‐21 | 0.002 | 1.002 (1.001–1.003) | 7.305 | 0.007 |
| MiRNA‐21+ TNM stage II | 0.248 | 1.282 (1.197–1.372) | 50.988 | 0.000 |
| MiRNA‐21 +TNM stage III | 0.221 | 1.247 (1.149–1.354) | 27.676 | 0.000 |
CI, confidence interval; HR, hazard ratio; MiRNA, microRNA; TNM, tumor node metastasis.
The HRs of factors of survival length in patients who died from cancer‐related causes were entered into the multivariate cox proportional hazard regression model. TNM stages II (HR 3.558, 95% CI 2.207, 5.734) and III (HR 2.523, 95% CI 1.553, 4.101), and severe complications before surgery (HR 1.988, 95% CI 1.345, 2.939) were independent prognostic factors for survival length in dead NSCLC patients.
Discussion
The primary finding of this study is that four independent factors were associated with NSCLC prognosis: age at surgery, clinical TNM stage, and miRNA‐21 and miRNA‐155 expression in lung tissue. Older patients had shorter survival. Compared to the first stage, patients at higher clinical stages had shorter survival. Increased miRNA‐21 and miRNA‐155 expression in lung tissue was related to poor survival. miRNA‐21 was not only an independent factor related to poor patient survival, but also positively interacted with TNM stage to affect prognosis. Patients at higher clinical stage with higher miRNA‐21 expression in lung tissue had even shorter survival.
Ten years ago, it was reported that precursor miRNA‐21 is overexpressed in lung cancer tissues.12 Follow‐up studies indicated that miRNA‐21 expression was a significant independent factor associated with NSCLC prognosis. Markou et al. reported that overexpression of mature miRNA‐21 extracted from fresh frozen LC tissue specimens was associated with high incidence of death caused by LC, which was revealed to be an independent effective factor for disease‐specific death.13 Wang et al. reported that the level of miRNA‐21 expression was higher in NSCLC serum samples than in controls, therefore serum miRNA‐21 expression may be a useful prognostic marker for NSCLC patients.14 A meta‐analysis of 10 independent publications found that miRNA‐21 and miRNA‐155 overexpression in frozen lung tissues, paraffin‐embedded lung tissues, or patient serum were significantly associated with poor NSCLC prognosis.8 Our conclusion that higher miRNA‐21 expression in paraffin‐embedded lung tissues was an independent factor associated with poor NSCLC prognosis was consistent with the results of previous studies.
Researchers have reported that increased miRNA‐155 expression is closely related to poor prognosis in LC. However, the results of previous studies are controversial as the conclusions between studies are inconsistent. In a meta‐analysis of 1350 NSCLC cases from 10 independent publications, Wang et al. found that miRNA‐155 overexpression was significantly associated with poor NSCLC prognosis.8 They revealed that compared to European patients, higher miRNA‐155 expression levels were only associated with poor survival in LC patients from Asia and America.15 Donnem et al. reported that the relationship between miRNA‐155 and prognosis may be impacted by the histological subtype of NSCLC, indicating that miR‐155 expression had the opposite relationship in AC and SCC patients.16 Our study results support the conclusion that increased miRNA‐155 is independently associated with poor patient survival of NSCLC.
The hypothesis that miRNA expression is related to NSCLC survival has been investigated in previous studies (but was not investigated in the present study). MiRNA‐21 plays important roles in tumor cell proliferation, differentiation, and apoptosis by targeting key proteins in cancer cells.17 A meta‐analysis found that higher miR‐21 expression in NSCLC patients led to a higher risk of node metastasis.8 Zhu et al. reported that miRNA‐21 affects cancer growth by targeting the tumor suppressor gene TPM1.18 miRNA‐21 expression is reported to correlate with node metastasis in NSCLC patients; that is, NSCLC patients with high miRNA‐21 expression experience greater node metastasis than patients with low miRNA‐21 expression.19 However, it must be noted that the type of sample used to extract miRNA, such as frozen tissues, FFPE tissues, and serum, may influence the outcome.20 Decreased miRNA‐21 expression promoted apoptosis in A549 cells induced by irradiation.21 Lin et al. reported that miRNA‐21 regulates NSCLC cell invasion and chemo‐sensitivity through SMAD7, which is a key inhibitor of transforming growth factors.22 Wang et al. reported that miRNA‐21 overexpression is related to decreased sensitivity to radiotherapy in LC patients, which was also positively related to clinical stage, lymph node metastasis, and poor prognosis.23
Our results indicate that the interaction between miRNA‐21 and clinical TNM stage was associated with prognosis in NSCLC patients, which meant the combined role of miRNA‐21 and clinical stage had a more powerful effect for promoting NSCLC development than individually. Few previous studies have come to this conclusion. In addition, no previous studies have investigated the effect of interaction between miRNA‐21 and TNM stage on NSCLC prognosis. One possible explanation for this effect is that miRNA‐21 and cancer cells interact and progress to clinical stage through complex molecular mechanisms, and higher clinical stage stimulates the formation of miRNA‐21, in turn developing NSCLC. Future studies are warranted to elucidate the mechanisms.
Non‐small cell lung cancer prognosis is related to various factors. Older age, higher clinical TNM stage, and increased miRNA‐21 and miRNA‐155 expression were independently associated with poor patient survival. MiRNA‐21 expression and clinical TNM stage interacted to affect NSCLC prognosis; however, further investigation is needed to determine the mechanism. Our results can be extrapolated to different source patients and different kinds of tissue samples.
Disclosure
No authors report any conflict of interest.
Acknowledgment
This work was supported by the Beijing Science and Technology Commission “Research on Key Techniques of Early Detection and Standardized Therapy of Lung Cancer” project (D141100000214002).
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . World Cancer Report 2014, Chapter 5.1. World Health Organization, Geneva: 2014. [Google Scholar]
- 3. National Cancer Institute, Surveillance, Epidemiology, and End Results Program . Cancer Stat Facts: Lung and Bronchus Cancer 2017. [Cited 5 Jan 2017.] Available from URL: https://seer.cancer.gov/statfacts/html/lungb.html.
- 4. Yang J, Zhu J, Zhang YH et al. Lung cancer in a rural area of China: Rapid rise in incidence and poor improvement in survival. Asian Pac J Cancer Prev 2015; 16: 7295–302. [DOI] [PubMed] [Google Scholar]
- 5. Ou SH, Zell JA, Ziogas A, Anton‐Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients: A population‐based analysis of 19,702 stage I patients in the California Cancer Registry from 1989 to 2003. Cancer 2007; 110: 1532–41. [DOI] [PubMed] [Google Scholar]
- 6. Guo J, Meng R, Yin Z et al. A serum microRNA signature as a prognostic factor for patients with advanced NSCLC and its association with tissue microRNA expression profiles. Mol Med Rep 2016; 13: 4643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su K, Zhang T, Wang Y, Hao G. Diagnostic and prognostic value of plasma microRNA‐195 in patients with non‐small cell lung cancer. World J Surg Oncol 2016; 14(1): 224. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Wang Y, Li J, Tong L et al. The prognostic value of miR‐21 and miR‐155 in non‐small‐cell lung cancer: A meta‐analysis. Jpn J Clin Oncol 2013; 43: 813–20. [DOI] [PubMed] [Google Scholar]
- 9. Peng J, Liu HZ, Zhong J et al. MicroRNA‐187 is an independent prognostic factor in lung cancer and promotes lung cancer cell invasion via targeting of PTRF. Oncol Rep 2016; 36: 2609–18. [DOI] [PubMed] [Google Scholar]
- 10. Del Vescovo V, Grasso M, Barbareschi M, Denti MA. MicroRNAs as lung cancer biomarkers. World J Clin Oncol 2014; 5: 604–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Chen J, Lin Z et al. Role of deregulated microRNAs in non‐small cell lung cancer progression using fresh‐frozen and formalin‐fixed, paraffin‐embedded samples. Oncol Lett 2016; 11: 801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yanaihara N, Caplen N, Bowman E et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006; 9: 189–98. [DOI] [PubMed] [Google Scholar]
- 13. Markou A, Tsaroucha EG, Kaklamanis L, Fotinou M, Georgoulias V, Lianidou ES. Prognostic value of mature microRNA‐21 and microRNA‐205 overexpression in non‐small cell lung cancer by quantitative real‐time RT‐PCR. Clin Chem 2008; 54: 1696–704. [DOI] [PubMed] [Google Scholar]
- 14. Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang KM, De W. Prognostic significance of serum miRNA‐21 expression in human non‐small cell lung cancer. Surg Oncol 2011; 104: 847–51. [DOI] [PubMed] [Google Scholar]
- 15. Wang F, Zhou J, Zhang Y et al. The value of microRNA‐155 as a prognostic factor for survival in non‐small cell lung cancer: A meta‐analysis. PLoS One 2015; 10: e0136889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donnem T, Eklo K, Berg T et al. Prognostic impact of MiR‐155 in non‐small cell lung cancer evaluated by in situ hybridization. J Transl Med 2011; 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu YR, Qi HJ, Deng DF, Luo YY, Yang SL. MicroRNA‐21 promotes cell proliferation, migration, and resistance to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal cancer. Tumour Biol 2016; 37: 12061–70. [DOI] [PubMed] [Google Scholar]
- 18. Zhu S, Si ML, Wu H, Mo YY. MicroRNA‐21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol Chem 2007; 282: 14328–36. [DOI] [PubMed] [Google Scholar]
- 19. Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA‐21 targets tumor suppressor genes in invasion and metastasis. Cell Res 2008; 18: 350–9. [DOI] [PubMed] [Google Scholar]
- 20. Saito M, Schetter AJ, Mollerup S et al. The association of microRNA expression with prognosis and progression in early‐stage, non‐small cell lung adenocarcinoma: A retrospective analysis of three cohorts. Clin Cancer Res 2011; 17: 1875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo Q, Zhang H, Zhang L et al. MicroRNA‐21 regulates non‐small cell lung cancer cell proliferation by affecting cell apoptosis via COX‐19. Int J Clin Exp Med 2015; 8: 8835–41. [PMC free article] [PubMed] [Google Scholar]
- 22. Lin L, Tu HB, Wu L, Liu M, Jiang GN. MicroRNA‐21 regulates non‐small cell lung cancer cell invasion and chemo‐sensitivity through SMAD7. Cell Physiol Biochem 2016; 38: 2152–62. [DOI] [PubMed] [Google Scholar]
- 23. Wang XC, Wang W, Zhang ZB, Zhao J, Tan XG, Luo JC. Overexpression of miRNA‐21 promotes radiation‐resistance of non‐small cell lung cancer. Radiat Oncol 2013; 8: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]


