Abstract
Background:
The purpose of this study was to investigate the effects of an 8-week aerobic exercise and supplementation of 25(OH)D3 on GLP1 and DDP4 levels in men with type II diabetes.
Methods:
In this semiexperimental research, among 40–60-year-old men with type II diabetes who were referred to the diabetic center of Isabn-E Maryam hospital in Isfahan; of whom, 48 patients were voluntarily accepted and then were randomly divided into 4 groups: aerobic exercise group, aerobic exercise with 25(OH) D supplement group, 25(OH) D supplement group, and the control group. An aerobic exercise program was conducted for 8 weeks (3 sessions/week, each session 60 to75 min with 60–80% HRmax). The supplement user group received 50,000 units of oral Vitamin D once weekly for 8 weeks. The GLP1, DPP4, and 25(OH) D levels were measured before and after the intervention. At last, the data were statistically analyzed using the ANCOVA and post hoc test of least significant difference.
Results:
The results of ANCOVA showed a significant difference between the GLP1 and DPP4 levels in aerobic exercise with control group while these changes were not statistically significant between the 25(OH) D supplement group with control group (P < 0.05).
Conclusions:
Aerobic exercises have resulted an increase in GLP1 level and a decrease in DPP4 level. However, consumption of Vitamin D supplement alone did not cause any changes in GLP1and DPP4 levels but led to an increase in 25-hydroxy Vitamin D level.
Keywords: Aerobic exercises, insulin resistance, mellitus diabetes, Vitamin D
Introduction
Type II diabetes is identified through resistance to insulin and increase in glucose production in pancreas and in some cases by reduction in blood insulin level. In type II diabetes, the main problem is traced in the target tissues, especially in muscles, so that in those tissues, the resistance to insulin is increased and in turn, it causes hyperglycemia.[1] Diabetes is one of the most common chronic and noncontagious diseases, spreading quickly as a global epidemic. During the last three decades, the spread of diabetes has shown a rising trend in developed and developing countries.[2] The increase in blood sugar level, blood pressure, oxidative stress and dyslipidemia, makes diabetic patients prone to cardiovascular diseases, so much that cardiovascular diseases are now considered as number one cause of death among these patients.[3] Taking the common complications caused by diabetes and its epidemic effect into consideration, much research has been conducted for finding a treatment and managing diabetes and homeostasis glucose. Among these studies is a study conducted on incretins. At present, the gastric inhibitory peptides (GIP) and glucagon like peptide-1 (GLP1) are among the incretin hormones of which GLP1is considerably more active than GIP.[4] GLP1 has multiple physiologic roles in the body. It causes pancreas to release insulin and maintain glucose hemostat level without any hyperglycemia. In addition, by influencing the alpha cells of the pancreas, it stimulates a reduction in glucagon production and helps maintain the proportion of glucagon with respect to insulin. On the other hand, it has a trophic effect on the pancreas cells, and by increasing the neogenesis and reducing apoptosis, it increases the beta-cell mass.[5] By further understanding the physiologic role of GLP1, the importance of these factors in the treatment of type II diabetes has further gone under investigation. In addition to lowering the GLP1 level, its effects on diabetic patients are negligible. On the appearance of GLP1 in the blood flow, another enzyme called DPP4 is destroyed and disappeared.[6] Currently, the DPP4 enzyme inhibitor drugs keep the GLP1 active and by this will keep the blood sugar level under control.[7] In diabetic patients, the GLP1 level is reduced and the DPP4 level is increased and this in turn will cause the glucose hemostatic imbalance. At present, inhibiting DPP4 and increasing GLP1 levels are among the most important methods of controlling and managing type II diabetes.[8] It has been long since the effects of exercise activities on diabetes have been known. The results show that in type II diabetic patients, frequent muscle contractions in the absence of insulin will ease the absorption and consumption of sugar into the muscle cells. Moreover, exercise and sport activities increase glucose transporter proteins (GLUT4) and will result in insulin resistance reduction.[9] A regular exercise program can have a substantial role in reducing diabetic complications such as obesity, hypertension, hyperlipidemia, hyperinsulinemia, and an increase in the insulin target tissue. Aerobic exercises can reduce insulin and glycogen hemoglobin resistance.[10] For example, in a study by Farzanegi (2014), a significant increase in GLP1 was reported following an aerobic exercise.[11] In the past few decades, a large number of nonosteopathic Vitamin D-related diseases such as type II diabetes have been identified.[12] In addition to influencing the calcium homeostasis, Vitamin D plays a role in natural excretion of insulin and in increasing insulin and glucose homeostasis sensitivity.[13] Vitamin D increases the insulin receptor genes and reduces the insulin resistance.[14] Besides, the researches of Saremi et al. (2014) show the positive effect of simultaneous consuming of Vitamin D supplement and doing aerobic exercises on decreasing the cardiovascular risk factors, increasing insulin sensitivity, and decreasing insulin resistance.[15] In various studies, a Vitamin D deficiency was observed in higher proportion among diabetic patients. Vitamin D deficiency causes a disorder in insulin excretion and a decrease in GLUT4 activity and an increase in insulin resistance.[16] Taking the widespread of type II diabetes and its dangerous consequences into account, the importance of controlling type II diabetes by doing more exercise through Incretins, and vitamin D influences on type II diabetes treatment, the lack of similar research, the present study was conducted on the simultaneous aerobic exercises and 25(OH) D3 supplements on the proportion of GLP1 with respect to DPP4 in type II diabetic patients.
Methods
This is a semiexperimental study with four different study groups. The statistical population consists of all the men with type II diabetes among which 48 of the patients who volunteered to cooperate with the study and had the qualifying conditions for entry (these conditions included being male type II diabetes verified by doctor's diagnosis and medical records, age between 40 and 60, without any cardiovascular disease background and regular physical activities, not having used insulin, not having experienced any of the diabetes complications such as foot ulcer, blood sugar level lower than 250 (mg/dl) not having used any interfering drugs with 25(OH) D3 such as corticosteroids) were referred to one of the popular hospitals (Isabn-E Maryam hospital) in Isfahan. They were selected based on accessibility and through targeted sampling.
Following the submission of the consent letter, the examinees were referred to a laboratory after an 8–12 h of fasting to determine their GLP1, DPP4, and 25-hydroxy Vitamin D. A 10cc blood sample was then taken from the participants’ arms vessels. Then, the examinees were randomly divided into four groups (aerobic exercise group, aerobic exercises along with 25(OH) D3 group, 25(OH) D3 group, and a control group). The aerobic exercise group and the aerobic exercise with vitamin 25(OH) D3 supplements participated in a 8-weeks exercise program (3 sessions/week and each session 60–75 min and with intensity of 60–80% of maximum heart rate). The intensity and volume of the aerobic exercises were planned according to the American Diabetes Association's exercise recommendations for diabetic patients and under the supervision of a trainer coach.[17] The aerobic exercise plan for each session was a progressive plan in time and activity intensity. By this scheme, we started with a 60% of maximum heart rate in the 1st week and reached an 80% of maximum heart rate in the 8th week. The exercise intensity was calculated for each individual using the Karvonen equation (i.e., 220– age). Each exercise session was divided into three segments of “warm-up,” “main exercise,” and “cool-down.” Participants spend around 5–10 min prior and after the main exercise to warm up and cool down. Besides, none of the patients leave the research until the end of the study. The supplement user group received 50,000 units of oral Vitamin D once weekly for 8 weeks.[13] Following the completion of the 8-week exercise program along taking the supplements, to assess the possible changes in the GLP1, DPP4 and 25 (OH) D3 levels as a result of the aerobic exercises and consumption of 25 (OH) D3, another blood sampling was conducted after an 8 to 12 hours of fasting for all the four study groups. The 25(OH) D3 level was measured using the IDS company 25 (OH) D3 diagnostic kit (with 5 nmol/lit sensitivity) using the immunoassay enzyme method. The GLP1 and DPP4 measurement was performed using the diagnostic kit by Estibiopharm Company and by using a sandwich enzyme-linked immunosorbent assay. The kit sensitivity of GLP-1 and DPP4 were measured to be 0.062 pml/lit and 1 ng/ml, respectively.
Some descriptive statistical analyses (such as the mean and standard deviation) and the covariance analysis test (ANCOVA) and the post hoc test of least significant difference (LSD) were conducted to study the possible changes due to the effects of the exercise programs (before and after) and supplement intake in each of the four study groups. All of the analyses were performed by Statistical SPSS, version 19 (Inc., Chicago, IL, USA) software and the significant level was set to be P < 0.05.
Results
The changes in the variables under study in all four groups and their comparisons are shown in Table 1.
Table 1.
A comparison of the variables measured in the 4 groups of type 2 diabetic patients, before and after an 8-week intervention program
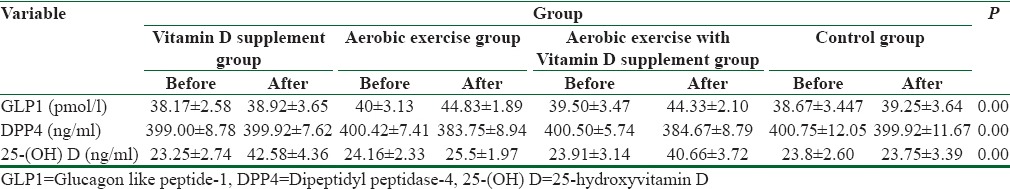
As it can be noticed in Table 1, there is a significant difference among the four different group results in GLP1 variable (F = 35.27, P = 0.00), DDP4 (F = 17.80, P = 0.00), and in 25(OH) D3 (F = 151.33, P = 0.00). Considering the significant difference among the four groups, to analyze the difference among them, we used the LSD post hoc test. The results of the test are displayed in Table 2.
Table 2.
The least significant differences Post hoc test results
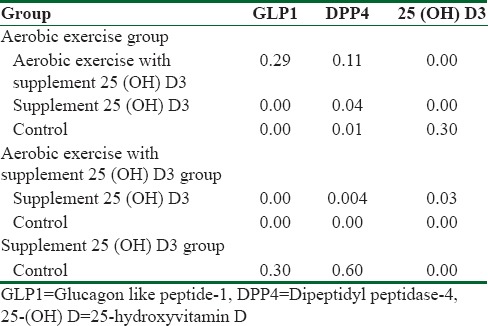
As it is shown in Table 2, the GLP1 and DPP4 levels in aerobic exercise and aerobic exercise with Vitamin D supplement groups showed significant changes with respect to the control group (P = 0.00). In other words, both aerobic exercises and aerobic exercises with Vitamin D supplement have caused a lower DPP4 level and a higher GLP1 level. On the other hand, there was no significant difference between the aerobic exercise group and aerobic exercise with Vitamin D supplement in GLP1 level (P = 0.29) and in DPP4 level (P = 0.11). That is, the 25(OH) D3 supplement has not had significant effect in GLP1 and DPP4 levels. In addition, there was a significant difference between the aerobic exercises and the supplement consumer groups in the GLP1 and DPP4 levels (P = 0.00) and (P = 0.04), respectively. On the other hand, there is a significant difference between the aerobic exercise group and the supplement user group in GLP1 level (P = 0.00) and in DPP4 level (P = 0.04). Furthermore, there was no significant difference between the Vitamin D supplement consumer group and the control group in GLP1 and DPP4 levels. This implies that the aerobic exercises have caused a decline in DPP4 and an increase in GLP1 level, and the Vitamin D supplement did not play any roles in the consequent changes. Further, a significant difference is observed in the 25(OH) D3 level between the 25(OH) D3 users and the control groups (P = 0.00) and also between the supplement user group along with the aerobic exercise groups and the control group (P = 0.000). This result shows that consumption of supplement, both with and without aerobic exercise, has led in increasing the 25(OH) D3 serum compared to the control group. Else, there is a significant difference between supplement user group with aerobic exercise and the exclusive supplement consumer groups (P = 0.03) and between the aerobic exercise group and the exclusive supplement consumer group (P = 0.000) while there was no significant difference between the aerobic exercise group and the control group in 25(OH) D3 level (P = 0.30). This means that doing aerobic exercises along with 25(OH) D3 consumption in comparison to the 25(OH) D3 consumption exclusively causes a higher increase in 25(OH) D3 plasma level.
Discussion
Among the most important findings of this study were the effects of aerobic exercises on increasing the GLP1 level and decreasing the DPP4 level and the ineffectiveness of 25(OH) D3 supplement on GLP1 and DPP4 levels. Regarding the effect of aerobic exercise on GLP1 and DPP4 levels, the results of this study are in compliance with those by Farzanegi (2014), Malin et al. (2013). However, they are not in compliance with the results by Ueda et al. (2013).[11,18,19] The differences in the results can be associated with the differences in the intensity, exercise program length, and the age and sex of the participants. According to researchers, exercise activities have the following three influences on the body: an increase in transporting of glucose to the muscles, an increase in insulin performance on the involved muscles, and positive adjustment in message paths stimulated by the insulin. These three together will cause an improvement in the glucose conditions in the body. In addition, a sport activity acts as an pseudoinsulin activity by reducing the intercellular fat reservoir, increasing fat oxidation, and protein expression (AKT) will lead to an increase in muscle capacity and regulate the amount of glucose in circulation.[20] In addition, an increase in oxide nitric (NO) production, a decrease in oxidative stress, inflammatory cytokines, and an increase in the capacity and antioxidation enzymes due to the exercise adaptation can lead to an improvement in insulin resistance in type II diabetic people.[21]
Concerning the consumption of 25 (OH) D3 supplement and the simultaneous effects of vitamin D supplement and aerobic exercises, this study showed that whether you take the vitamin D supplement with aerobic exercises or just take the vitamin D supplement alone, the 25(OH)D3 will increase in patients with type II diabetic compared to the control group. 25(OH) D3 in type II diabetic patients compared to the control group. The result of this study was in compliance with those by Saremi et al. (2013), Moosavi et al. (2015), Bazyar et al. (2014), Rapti et al. (2016).[22,23,24,25] However, it is not in compliance with the results obtained by Jorde et al. (2009), Breslavsky et al. (2013).[26,27] The difference can be the result of difference in period length and the amount of supplement. In the studies of Rapti et al. (2016), simultaneous consuming of Sitagliptin tablet (inhibitor DPP4) and Vitamin D caused the improvement in the glycemic index. Considering the powers of the inhibitoring Sitagliptin, it seems that the drug had the most effect on inhibitoring DPP4 and increasing GLP1 level.[25] Multiple mechanisms have been suggested for relating Vitamin D and type II diabetes such as the 25(OH) D3 that can make a connection to the nuclear receptor on the synthesizing gene of the membrane insulin receptors which increased the synthesis in receptors, hence, the higher the presence of the insulin dependent transporters, the more insulin is absorbed through the cellular membrane.[28] Furthermore, it has been reported that Vitamin D regulates the Renin–angiotensin system by reducing renin gene expression and by inhibiting the angiotensin receptors. The increase in the activity of this system plays a role in creating insulin resistance, inflammation, and blood pressure.[29] Another suggested mechanism is that Vitamin D deficiency will lead to an increase in parathyroid hormone (PTH) which in turn will result in lipogenesis, obesity, and insulin resistance.[30] The decline in Vitamin D will cause an increase in PTH which is followed by an increase in intercellular Ca++. The increase in intercellular Ca++ will in turn inhibits the insulin receptors in the target tissues and will block the GLIT4 channel.[31] On the other hand, some studies have reported an increase in 25(OH) D3 level following some aerobic exercise. For example, Aly et al. (2016) reported an increase in 25(OH) D3 level following 4 weeks of swimming exercises in aerobic exercise group.[32] Therefore, the complementary role of Vitamin D supplement and aerobic exercises simultaneously seem to have an effect in managing diabetes, lowering blood sugar level, and insulin resistance.
Conclusions
Overall, an 8-week aerobic exercise activities program caused an increase in GLP1 level and a decrease in DPP4 level; however, the Vitamin D supplement did not have any influence on the two hormones. It seems that exercise activities, independent from Vitamin D effects, cause an increase in GLP1 level and a decrease in DPP4 level. On the other hand, aerobic exercises and the interactive effects of aerobic exercise and 25(OH) D3 will lead into an increase in 25(OH) D3 level which can be effective in managing and reducing the type II diabetic danger factors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was extracted from a sport physiology thesis in a Bu-Ali Sina university in Hamedan and was registered in Iranian Registry of Clinical Trials IRCT2017010315812N3 and in the ethics committee under the following code: ID IR.UMSHA.REC.1395.467. By this, I would like to extend my appreciation and gratitude to a Bu-Ali Sina University administration of research and technology department and also all the participating patients in this study.
References
- 1.Rahimi N, Marandi SM, Kargarfard M. The effect of eight weeks aquatic training on lipid profile of patients who suffer from type II diabetes. J Isfahan Med Sch. 2011;29:1–9. [Google Scholar]
- 2.Harati H, Hadaegh F, Saadat N, Azizi F. Population-based incidence of Type 2 diabetes and its associated risk factors: results from a six-year cohort study in Iran. BMC Public Health. 2009;9:186. doi: 10.1186/1471-2458-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarrett RJ. Cardiovascular disease and hypertension in diabetes mellitus. Diabetes Metab Rev. 1989;5:547–58. doi: 10.1002/dmr.5610050702. [DOI] [PubMed] [Google Scholar]
- 4.Gautier JF, Choukem SP, Girard J. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab. 2008;34(Suppl 2):S65–72. doi: 10.1016/S1262-3636(08)73397-4. [DOI] [PubMed] [Google Scholar]
- 5.Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010;1:8–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A, Paliwal G, Upadhyay N, Tiwari A. Therapeutic stimulation of GLP-1 and GIP protein with DPP-4 inhibitors for type-2 diabetes treatment. J Diabetes Metab Disord. 2015;14:15. doi: 10.1186/s40200-015-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Ndefo UA, Okoli O, Erowele G. Alogliptin: A new dipeptidyl peptidase-4 inhibitor for the management of type 2 diabetes mellitus. Am J Health Syst Pharm. 2014;71:103–9. doi: 10.2146/ajhp130131. [DOI] [PubMed] [Google Scholar]
- 8.Duvnjak L, Blaslov K. Dipeptidyl peptidase-4 inhibitors improve arterial stiffness, blood pressure, lipid profile and inflammation parameters in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2016;8:26. doi: 10.1186/s13098-016-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanuso S, Jimenez A, Pugliese G, Corigliano G, Balducci S. Exercise for the management of type 2 diabetes: a review of the evidence. Acta Diabetol. 2010;47:15–22. doi: 10.1007/s00592-009-0126-3. [DOI] [PubMed] [Google Scholar]
- 10.Madden KM. Evidence for the benefit of exercise therapy in patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:233–9. doi: 10.2147/DMSO.S32951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farzanegi P. The effects of aerobic training and arbutin on GLP1 and GLP1R in diabetes Rats. Indian J Fundam Appl Life Sci. 2014;4:2231–6345. [Google Scholar]
- 12.Cimbek A, Gürsoy G, Kirnap NG, Acar Y, Kiliç Z, Güngör F, et al. Relation of obesity with serum 25 hydroxy Vitamin D3 levels in type 2 diabetic patients. J Res Med Sci. 2012;17:1119–23. [PMC free article] [PubMed] [Google Scholar]
- 13.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and Vitamin D deficient – A randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–55. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 14.Talaei A, Mohamadi M, Adgi Z. The effect of Vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol Metab Syndr. 2013;5:8. doi: 10.1186/1758-5996-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saremi A, Shavandi N, Shahrjerdi Sh, Mahmoudi Z. The effect of aerobic training with Vitamin D supplementation on cardiovascular risk factors in obese women. J Cell Tissue. 2014;4:389–8. [Google Scholar]
- 16.Bonakdaran S, Varasteh AR. Correlation between serum 25 hydroxy Vitamin D3 and laboratory risk markers of cardiovascular diseases in type 2 diabetic patients. Saudi Med J. 2009;30:509–14. [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36:10–4. [Google Scholar]
- 18.Malin SK, Huang H, Mulya A, Kashyap SR, Kirwan JP. Lower dipeptidyl peptidase-4 following exercise training plus weight loss is related to increased insulin sensitivity in adults with metabolic syndrome. Peptides. 2013;47:142–7. doi: 10.1016/j.peptides.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueda SY, Miyamoto T, Nakahara H, Shishido T, Usui T, Katsura Y, et al. Effects of exercise training on gut hormone levels after a single bout of exercise in middle-aged Japanese women. Springerplus. 2013;2:83. doi: 10.1186/2193-1801-2-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–69. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 21.Teixeira-Lemos E, Nunes S, Teixeira F, Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. 2011;10:12. doi: 10.1186/1475-2840-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saremi A, Shavandi N, Vafapour H. Eight-week resistance training with vitamin D supplementation in postmenopausal women: Effects on skeletal muscle. Pejouhandeh. 2013;18:57–63. [Google Scholar]
- 23.Moosavi J, Habibian M, Farzanegi P. The effect of regular aerobic exercise on plasma levels of 25- hydroxy Vitamin D and insulin resistance in hypertensive postmenopausal women with type 2 diabetes. RJMS. 2016;22:80–90. [Google Scholar]
- 24.Baziar N, DJafarian K, Shadman Z, Qorbani M, Khoshniat Nikoo M, Razi F. Effect of Vitamin D supplementation on improving Vitamin D level and insulin resistance in Vitamin D insufficient or defficient type 2diabetics. Iran J Diabetes Metab. 2014;13:425–33. [Google Scholar]
- 25.Rapti E, Karras S, Grammatiki M, Mousiolis A, Tsekmekidou X, Potolidis E, et al. Combined treatment with sitagliptin and vitamin D in a patient with latent autoimmune diabetes in adults. Endocrinology, Diabetes and Metabolism. 2016;ID:15–0136. doi: 10.1530/EDM-15-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr. 2009;48:349–54. doi: 10.1007/s00394-009-0020-3. [DOI] [PubMed] [Google Scholar]
- 27.Breslavsky A, Frand J, Matas Z, Boaz M, Barnea Z, Shargorodsky M. Effect of high doses of Vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr. 2013;32:970–5. doi: 10.1016/j.clnu.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Maestro B, Molero S, Bajo S, Dávila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D (3) Cell Biochem Funct. 2002;20:227–32. doi: 10.1002/cbf.951. [DOI] [PubMed] [Google Scholar]
- 29.Nadi M, Marandi SM, Esfarjani F, Saleki M, Mohammadi M. The Comparison between effects of 12 weeks combined training and Vitamin D supplement on improvement of sensory-motor neuropathy in type 2 diabetic women. Adv Biomed Res. 2017;6:55. doi: 10.4103/2277-9175.205528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reis JP, von Mühlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–55. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 31.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of Vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aly YE, Abdou AS, Rashad MM, Nassef MM. Effect of exercise on serum Vitamin D and tissue Vitamin D receptors in experimentally induced type 2 Diabetes Mellitus. J Adv Res. 2016;7:671–9. doi: 10.1016/j.jare.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


