Abstract
Background
This study aimed to explore the effects of HMGA2 on cell proliferation and metastases in lung cancer and its underlying mechanism.
Methods
HMGA2 expression in lung cancer tissues and its association with overall survival were analyzed based on data from a public database. The roles of HMGA2 were validated via loss‐of‐function and gain‐of‐function experiments in vitro. HMGA2 regulation by microRNA‐195 (miR‐195) was validated by real time‐PCR, Western blotting, and luciferase reporter assays.
Results
HMGA2 was upregulated and associated with reduced overall survival in patients with lung adenocarcinoma. HMGA2 knockdown suppressed the proliferation and motility of H1299 cells, while HMGA2 ectopic expression in A549 cells increased cell proliferation and migration. HMGA2 affected cell apoptosis through caspase 3/9 and Bcl‐2, and regulated epithelial‐to‐mesenchymal transition by targeting Twist 1. Moreover, miR‐195 was found to directly target the 3′ untranslated region of HMGA2 messenger RNA and suppress its expression in lung cancer.
Conclusion
This study demonstrated that HMGA2, regulated by miR‐195, played important roles in proliferation, metastases, and epithelial‐to‐mesenchymal transition in lung cancer. HMGA2 might serve as a biomarker and potential therapeutic target for lung cancer treatment.
Keywords: HMGA2, lung cancer, metastasis, miR‐195, proliferation
Introduction
Lung cancer is the leading cause of cancer death in both men and women in the United States, accounting for approximately 30% of cancer‐associated deaths in 2014.1, 2 Lung cancer is also one of the leading causes of cancer death worldwide.1, 2 Sustained proliferation is one of the hallmarks of cancer and it is also the fundamental basis of cancer dissemination and metastasis.3 Cancer recurrence and metastasis is the main cause of treatment failure in most cancer cases.
Recent studies have shown that the phenotype of epithelial‐to‐mesenchymal transition (EMT) can be observed in cancer progression.4, 5, 6, 7, 8 Cancer cells that have undergone EMT could acquire certain mesenchymal characteristics, including increased cell motility and resistance to apoptosis and therapy.7 In the past several decades, HMGA2 has been shown to be associated with EMT.9, 10, 11, 12 HMGA2 is a member of the high motility group (HMG) protein family and is widely expressed in undifferentiated mesenchymal tissues.13 It contains AT‐hook basic domains, and assigns them the ability to bind the DNA minor groove at sequences rich with A and T nucleotides, and to assemble transcriptional or enhancer complexes on chromatin.14 Apart from working as a transcription co‐regulator, HMGA2 could also directly regulate gene transcription activation.15, 16 HMGA2 could function as a regulator of cell proliferation and its expression is increased in many types of human tumor tissues.17, 18, 19 HMGA2 is also suggested to be involved in EMT regulation in many epithelial carcinomas.9, 10, 11, 12, 20, 21 Intense HMGA2 expression is strongly associated with metastases and poor prognosis in lung cancer.22 HMGA2 is a functional target of several EMT‐associated noncoding RNAs.23, 24 Further studies have shown that HMGA2 could modify the expression of Bcl‐2, EMT‐associated proteins, and caspase activity, indicating that HMGA2 plays a direct role in regulating cell apoptosis and EMT.
In spite of these findings, the exact effects of HMGA2 on lung cancer proliferation and metastasis are not fully understood. Therefore, we explored the effects of HMGA2 on cell proliferation and metastases in lung cancer and its underlying mechanism.
Methods
Database analysis of HMGA2 in lung cancer
Data on HMGA2 expression in LAC was obtained from Oncomine. Lung adenocarcinoma (LAC) survival data was obtained from Kaplan–Meier plotter and SurvExpress.25, 26
Patients and specimens
Fifty‐six LAC and 20 normal tissue samples were collected from patients who underwent lung cancer surgery at the Affiliated Hospital of Guangdong Medical University between January 2010 and December 2013. All of the patients were diagnosed by pathological examination and none had received preoperative chemotherapy or radiotherapy. Patients were followed‐up after surgery by telephone. The Ethics Committee of Guangdong Medical University approved this experiment and all patients provided informed consent.
RNA isolation and quantitative real‐time‐PCR
Total RNA of all tissues and cells was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Real‐time (RT)‐PCR was carried out applying SYBR Premix Ex Taq II (TaKaRa, Dalian, China) and detected using a LightCycler 480 system (Roche, Basel, Switzerland) according to the manufacturer's instructions. HMGA2 primer (TaKaRa) is shown in Table S1. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as an internal control and the fold change was calculated by 2−ΔΔCt.
Cell culture
Cell lines A549 and H1299 were maintained in RPMI‐1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified incubator with 5% CO2.
Plasmid construction and transfection
HMGA2 plasmid was obtained from GeneCopoeia (Rockville, MD, USA). Small interfering RNAs (siRNAs) for HMGA2, and miR‐195 mimics and inhibitors were purchased from RiboBio Co. Ltd. (Guangzhou, Guangdong, China). Target cells were transfected with oligonucleotides using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions.
Wound healing assay
For wound‐healing assays, 1 × 105 cells were seeded into 12‐well plates. Following 48 hours incubation, a scratch was drawn across the center of the well using a plastic pipette tip to produce a sharp wound area. The cells were washed with fresh, FBS‐free medium three times and incubated in medium containing 5% FBS for 14 hours. Cell movement into the wound area was examined using a phase‐contrast microscope (Olympus, Tokyo, Japan).
In vitro migration and metastasis assays
The 1 × 105 cells were seeded in FBS‐free media into the upper chamber of each transwell, which was pre‐coated with Matrigel. Medium with 20% FBS was placed in the lower chamber. Twenty‐four hours after seeding, non‐invasive cells in the upper chamber were removed with a cotton swab, and the cells on the lower surface of the membrane were fixed and stained with 0.1% crystal violet and photographed. Photographs of three random fields from triplicate wells were recorded and the number of cells was counted. For the migration assays, all procedures were the same as in invasion assays except that 5 × 104 cells were seeded in each chamber without Matrigel coating.
BrdU assay
BrdU assay was performed using a BrdU Cell Proliferation Chemiluminescent Assay Kit (Cell Signaling Technology, Danvers, MA, USA) according to the manufacturer's instructions.
Colony‐forming assay
Single cell suspensions were prepared by trypsinization. Cells were placed into 10 cm plates at 103 per well, 10 mL medium with 10% FBS was added, and the cells were incubated for seven days. After incubation, the cells were washed with PBS and fixed with methanol for 10 minutes at RT. The cells were then stained with 0.1% crystal violet for 10 minutes at RT and washed with PBS. The colony formation was counted.
Western blot analysis
Protein samples were prepared using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) containing protease inhibitor cocktail tablet (Roche Applied Science, Mannheim, Germany). Proteins were subject to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane. After blocking with 5% fat‐free milk for 1 hour at RT, the membrane was incubated with anti‐HMGA2, anti‐BCL2, anti‐E‐cadherin, anti‐Vimentin, and anti‐actin separately at 4°C overnight. The membrane was then incubated with a goat anti‐mouse/rabbit secondary antibody (Boster, Wuhan, China) for 1 hour at RT, and enhanced chemiluminescence was used to visualize the protein bands in a Bio‐Rad ChemiDoc XRS Imaging system (Hercules, CA, USA).
Antibodies included anti‐HMGA2, anti‐Bcl2, anti‐E‐cadherin, anti‐Vimentin (Cell Signaling Technology), and anti‐actin (Sigma‐Aldrich, St. Louis, MO, USA).
Caspase activity determination
Caspase‐3/9 activity was assessed using commercial assay kits (Beyotime Biotechnology) according to the manufacturer's instructions.
Luciferase reporter assay
Wild‐type 3’ untranslated region (UTR) of HMGA2 and mutant constructs, which were produced via mutations in the binding site of miR‐195 in 3’UTR of HMGA2, were cloned downstream of the luciferase gene in the psiCHECK‐2 Luciferase vector (Promega, Madison, WI, USA). Luciferase reporter vectors and miR‐195 mimic were transfected into HEK293T and H1299 cells using Lipofectamine 2000, and luciferase activity was measured using the Dual‐Luciferase Reporter Assay System (Promega).
Statistics
Data is represented as mean ± standard deviation, and all statistical analyses were conducted using GraphPad PRISM software (La Jolla, CA, USA). Statistical tests were one or two‐sided, and the differences between groups were assessed using student's t‐tests. Overall survival (OS) curves were analyzed using the Kaplan–Meier method and compared by log‐rank test. P values < 0.05 were considered to indicate a statistically significant difference.
Results
Increased HMGA2 was a marker for poor prognosis in lung adenocarcinoma
To clarify the expression of HMGA2 in LAC, we analyzed data from Oncomine. HMGA2 was significantly upregulated in LACs compared to normal lung tissues in six independent cohorts (Fig 1a). Further, patients with higher HMGA2 expression exhibited reduced OS in a cohort containing 673 LAC cases (Fig 1b), and similar results were also found in another four independent cohorts (Fig 1c). We detected HMGA2 messenger RNA (mRNA) expression in 56 LAC and 20 normal lung tissues, and also found that HMGA2 was upregulated in LAC and associated with OS (Fig 1d, e). These findings indicate that HMGA2 may server as a biomarker and is associated with poor prognosis in LAC.
Figure 1.
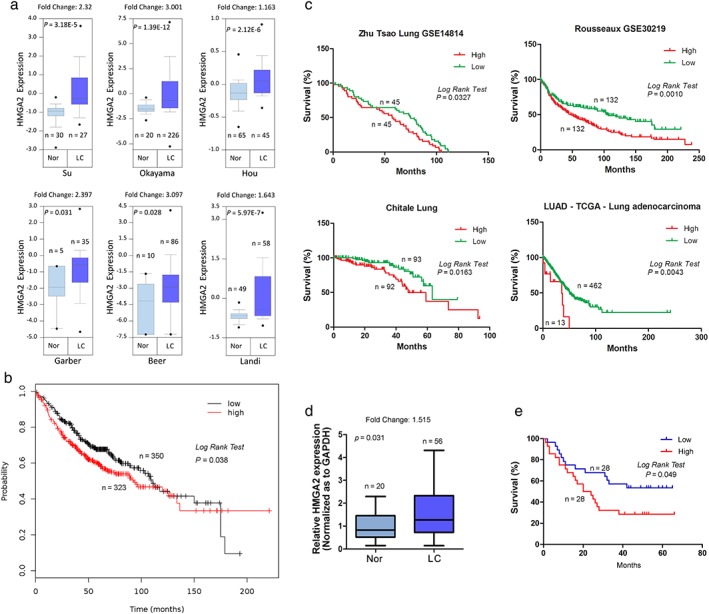
HMGA2 expression in lung cancer and its association with overall survival. (a) A comparison of HMGA2 expression between cancerous and normal lung tissues. (b ‐ c). Association of HMGA2 with overall survival in lung cancer. (b) Data from Kaplan–Meier plotter; (c) data from SurvExpress. (d) A comparison of HMGA2 expression between 56 cancerous and 20 normal lung tissues. (e) The association of HMGA2 with overall survival in 56 cases of lung cancer. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; LC, lung cancer; Nor, normal lung tissues.
HMGA2 regulated lung cancer cell proliferation
To demonstrate the effects of HMGA2 on cell proliferation, colony formation assay was performed. Compared to H1299 cells transfected with an empty vector, a significantly reduced number of cell colonies were observed in plates where H1299 cells with HMGA2 knockdown were seeded (Fig 2a,b). In addition, A549 cells with HMGA2 transfection exhibited a stronger capacity to form colonies (Fig 2a,b). DNA replication is the key step during cell mitosis; therefore we also used BrdU to evaluate cell proliferation. HMGA2 knockdown decreased BrdU intensity, while ectopic HMGA2 increased BrdU intensity in lung cancer cells (Fig 2c).
Figure 2.
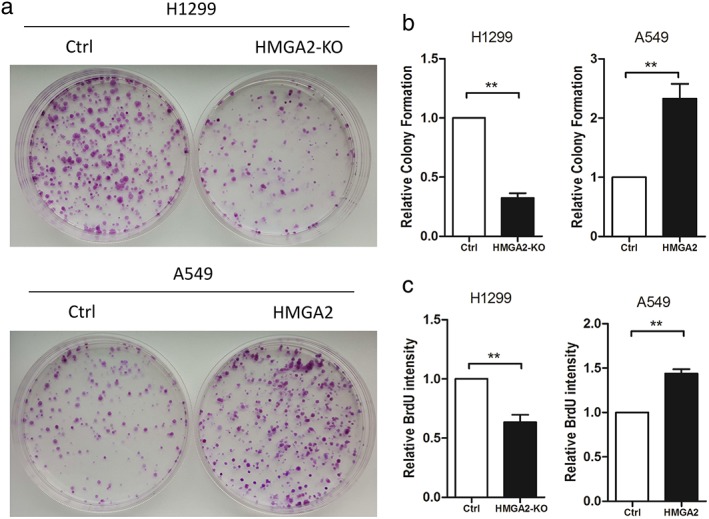
HMGA2 regulated cell proliferation and DNA replication of lung cancer cells.(a,b) Colony formation assays in H1299 and A549 cells with downregulated or upregulated HMGA2. (c) BrdU assays from H1299 cells with HMGA2 knockdown and A549 cells with ectopic HMGA2. ** P < 0.01. Ctrl, control.
HMGA2 regulated lung cancer cell motility in vitro
We used wound healing and transwell assays to detect lung cancer cell motility in vitro. HMGA2 knockdown in H1299 cells (with strong endogenous HMGA2) significantly inhibited wound closure, and cell migration and invasion in vitro (Fig 3a,b). Conversely, HMGA2 transfection into A549 cells (with weak endogenous HMGA2) promoted wound closure and increased cell migration and invasion (Fig 3a,b).
Figure 3.
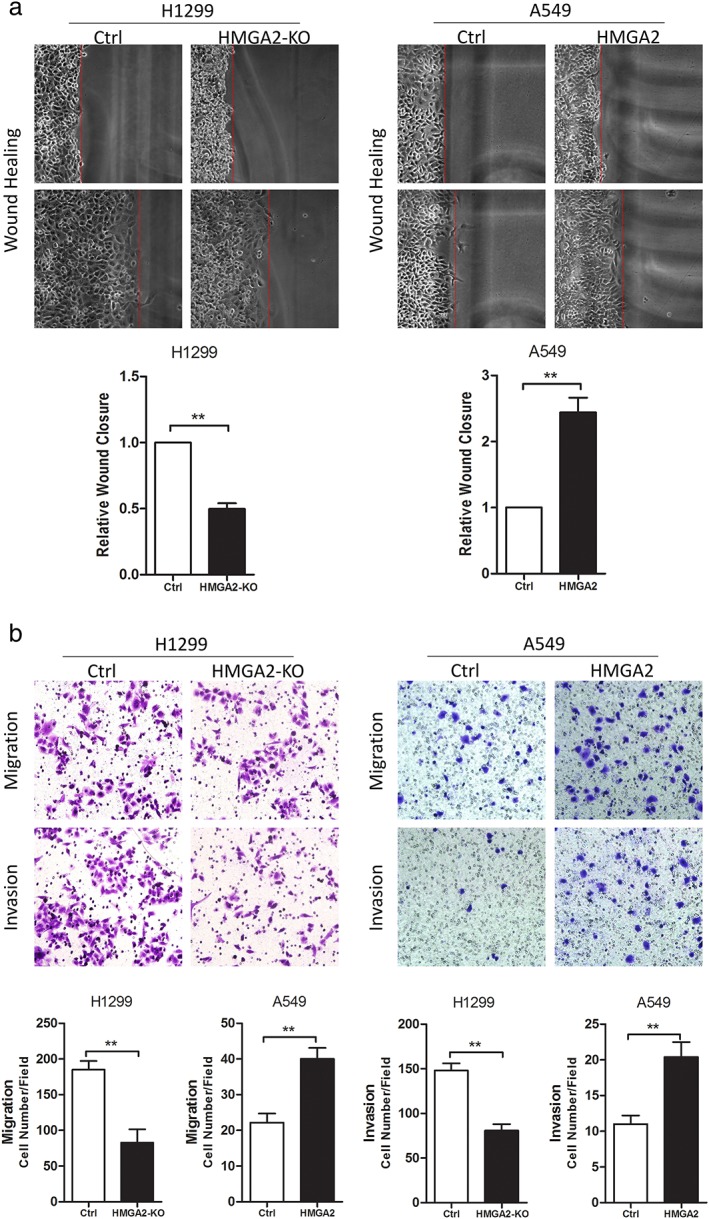
HMGA2 regulated cell migration and invasion of lung cancer cells in vitro. Representative images of (a) wound healing and statistical analyses of wound closure, and (b) transwell chamber assays and statistical analyses of cell migration and invasion. ** P < 0.01. Ctrl, control.
HMGA2 affected apoptosis and epithelial‐to‐mesenchymal transition in lung cancer cells
Colony formation was a combined consequence of cell mitosis and apoptosis; therefore we hypothesized whether HMGA2 could exert an effect on cell apoptosis. We detected Bcl‐2 expression, an important anti‐apoptosis executor, when HMGA2 was modified. As speculated, HMGA2 inhibition suppressed Bcl‐2 while HMGA2 transfection induced Bcl‐2 in lung cancer cells (Fig 4a). We also detected the activities of caspase‐3/9 in lung cancer cells. HMGA2 knockdown increased caspase activity in lung cancer cells, while HMGA2 upregulation led to a reduction in caspase activity (Fig 4b). These findings were consistent and indicate that HMGA2 could affect cell apoptosis.
Figure 4.
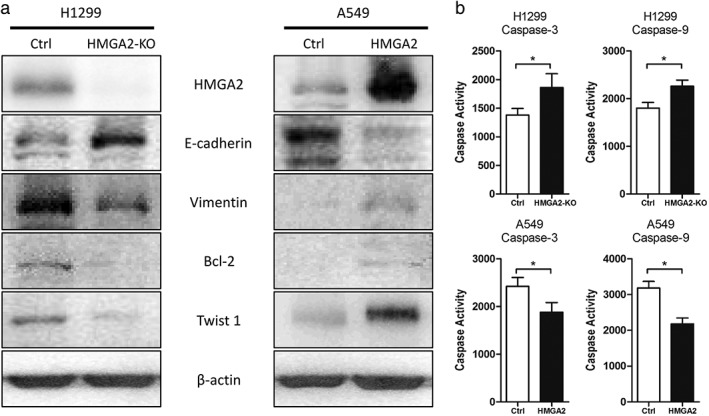
The effects of HMGA2 on (a) epithelial‐to‐mesenchymal transition ‐associated proteins and Bcl‐2 and (b) Caspase‐3/9 activity. * P < 0.05.
Epithelial‐to‐mesenchymal transition is known to initiate cancer cell metastasis; therefore we observed several markers of EMT following the modification of HMGA2. HMGA2 led to decreased levels of E‐cadherin and increased Vimentin, indicating that EMT was induced in lung cancer cells (Fig 4a). However, HMGA2 knockdown induced reverse alterations of these proteins (Fig 4a). In addition, we found that HMGA2 could directly affect Twist 1, which functioned as an EMT regulator by directly repressing E‐cadherin (Fig 4a).
HMGA2 was directly targeted and downregulated by miR‐195 in lung cancer
MicroRNAs (miRNAs) are small, evolutionarily conserved, non‐coding RNAs, 18–25 nucleotides in length, which bind to the 3’ UTR of target genes, causing either target mRNA degradation or reduced protein translation.27 To investigate the regulatory mechanism of HMGA2 at miRNA level, bioinformatic methods were used to identify the potential miRNAs. According to the results from three different bioinformatic programs (miRanda, Targetscan, and Pictar), miR‐195, which acted as a tumor suppressor in lung cancer,28, 29 was predicted to be a potential microRNA that targeted HMGA2 (Fig 5a). Luciferase reporter assays indicated that miR‐195 decreased luciferase activity in the vector containing wild type HMGA2 3’ UTR, but not in the vector containing the mutant site, suggesting a direct interaction of miR‐195 and HMGA2 3’ UTR (Fig 5b). In addition, both RT‐PCR and Western blot assays showed that miR‐195 overexpression reduced HMGA2 expression in H1299 cells, while miR‐195 downregulation increased HMGA2 expression in A549 cells (Fig 5c,d). These results reveal that HMGA2 is directly targeted and downregulated by miR‐195, inducing lung cancer proliferation and metastasis.
Figure 5.
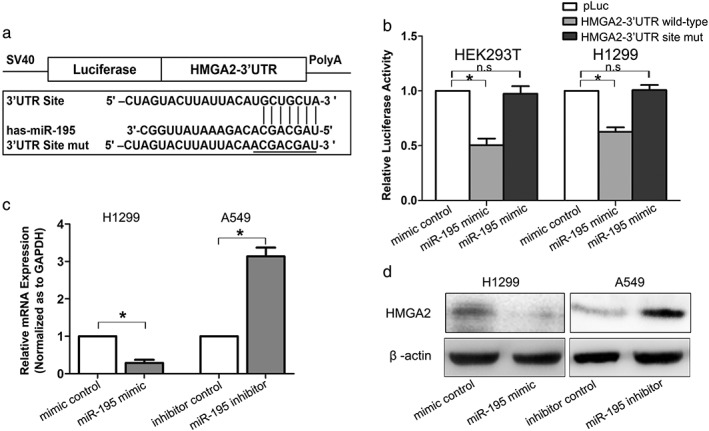
HMGA2 was directly targeted and downregulated by miR‐195. (a) Diagram of HMGA2 3’ untranslated region (UTR)‐containing reporter construct. (b) Relative luciferase activity after the wild type or mutant reporter plasmids were co‐transfected with miR‐195 mimic or control. n.s., no significance. (c) Real time‐PCR analysis of HMGA2 messenger RNA (mRNA) expression and (d) Western blot analysis of HMGA2 protein expression in lung cancer cells transfected with miR‐195 mimic or inhibitor. * P < 0.05. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.
Discussion
In the present study, we analyzed the expression of HMGA2 in LAC and showed that HMGA2 was upregulated and associated with LAC prognosis. Using a series of functional tests, we revealed that HMGA2 exerted direct effects on cell movement and proliferation, and this phenomenon was associated with cell apoptosis and EMT. These findings suggested that HMGA2 plays critical roles in regulating multiple malignant phenotypes of lung cancer and might serve as a potential biomarker for LAC.
HMGA2 is reportedly increased in LAC.22, 30, 31 Meyer et al. detected HMGA2 mRNA in 17 pairs of adenocarcinoma and normal tissues, and found that HMGA2 was significantly upregulated in cancerous tissues.22, 23, 24, 25, 26, 30, 31 HMGA2 was also generally increased in another cohort of LAC.22 In recent years, the emerging public sequencing database has enabled researchers to analyze gene expression in multiple cohorts of various cancers. Using this database, we found that HMGA2 was upregulated in six independent cohorts of LAC. A total of 477 LAC samples were included in our analyses. We revealed that increased HMGA2 was associated with reduced survival of LAC, consistent with the results of previous studies. All of these findings suggest that HMGA2 might serve as a biomarker for the diagnosis and evaluation of prognosis of LAC.
The association between HMGA2 and cancer proliferation has been reported previously.18, 19, 31, 32, 33, 34 In general, HMGA2 knockdown could inhibit cell growth in several types of cancer, which was similar to our findings in LAC. HMGA2 has also been shown as a direct target of ANG, a transcription factor involved in the proliferation of lung squamous carcinoma cells.35 From these results, we conclude that HMGA2 plays important roles in regulating lung cancer cell growth. Apart from the impact on DNA replication, we have also shown here that HMGA2 affected cell apoptosis by exerting opposite influences on Bcl‐2 and Caspase‐3/9. The key for cancer cell growth is the balance between cell mitosis and death. Our findings show that HMGA2 could regulate cancer growth by modifying the mitosis/apoptosis ratio of cancer cells, suggesting that HMGA2 plays a central role in lung cancer growth.
The typical characteristics of EMT are the loss of epithelial markers and the acquisition of mesenchymal markers. EMT plays important roles in embryo development, and cancer cells might become more invasive and drug‐resistant via EMT.7, 36 We observed decreased E‐cadherin and increased Vimentin when ectopic HMGA2 was introduced into A549 cells, indicating that HMGA2 might induce EMT in lung cancer cells. Besides the direct effects of HMGA2 on EMT shown here, HMGA2 has also been implicated to be associated with EMT in lung cancer by serving as the direct target of a wide range of EMT‐associated non‐coding RNAs.37, 38 All of these facts indicate that HMGA2 might play a central role in regulating EMT of lung cancer cells.
Reduced E‐cadherin expression is one of the hallmarks of EMT, therefore, the direct repressors of E‐cadherin, such as Snail, Twist, and Zeb, are frequently found to be the direct targets of EMT‐associated stimuli.5 Using RT‐PCR, we showed that Twist 1 may be a target of HMGA2 because Twist 1 is upregulated by ectopic HMGA2 but downregulated when HMGA2 is knocked down. HMGA2 directly binds to the promoter regions of its target proteins or functions by recruiting other proteins to form a transcription complex.15, 16 In pancreatic cancer, HMGA2 has been shown to bind and increase the binding of Smad3 to the Snail 1 promoter, and could also directly interact with Twist.39, 40 In gastric cancer, potential binding of HMGA2 to the Twist 1 promoter has been demonstrated, indicating that Twist might be a target of HMGA2.41, 42 All of these molecular events could induce the expression of E‐cadherin suppressors and ultimately lead to EMT. The mechanisms of Twist regulation by HMGA2 remain unclear in lung cancer, but we speculate that HMGA2 might function in a similar manner as reported.
miRNAs have emerged as important regulators of gene expression at the post‐transcriptional level, contributing to the pathogenesis of most human cancers.27 HMGA2 has also been found to be a functional target of several tumor‐related miRNAs, with the let‐7 family of miRNAs being the most frequent.43, 44 In addition, Lin et al. reported that miR‐33b dramatically downregulated the expression of HMGA2 in breast cancer cells to suppress cell self‐renewal, migration, and invasion.23 In the present study, we showed that miR‐195, a tumor suppressive miRNA in lung cancer,28, 29 directly targeted HMGA2 to regulate lung cancer progression.
Several limitations to our study need to be addressed. Firstly, all available function studies of HMGA2 were conducted in vitro, thus in vivo research is required. Secondly, because of the limited information of DFS, we were unable to classify the role of HMGA2 in DFS prediction.
In conclusion, we found that HMGA2 is upregulated in LAC and increased HMGA2 expression is associated with reduced OS in patients with lung cancer. HMGA2, regulated by miR‐195, could directly regulate cell motility and proliferation in lung cancer cells. HMGA2 could induce EMT by targeting Twist and might serve as a potential biomarker and therapeutic target of lung cancer.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. Primers for real time (RT)‐PCR.
Acknowledgment
We thank all of the patients who participated and the people who assisted with this study.
Contributor Information
Xiaotian Gao, Email: gxtian2006@sohu.com.
Zeqing Song, Email: 1347240737@qq.com.
References
- 1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends‐‐An update. Cancer Epidemiol Biomarkers Prev 2016; 25: 16–27. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016; 893: 1–19. [DOI] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 4. Diepenbruck M, Christofori G. Epithelial‐mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr Opin Cell Biol 2016; 43: 7–13. [DOI] [PubMed] [Google Scholar]
- 5. Lee JY, Kong G. Roles and epigenetic regulation of epithelial‐mesenchymal transition and its transcription factors in cancer initiation and progression. Cell Mol Life Sci 2016; 73: 4643–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aoi T. Biology of lung cancer: Genetic mutation, epithelial‐mesenchymal transition, and cancer stem cells. Gen Thorac Cardiovasc Surg 2016; 64: 517–23. [DOI] [PubMed] [Google Scholar]
- 7. Shang Y, Cai X, Fan D. Roles of epithelial‐mesenchymal transition in cancer drug resistance. Curr Cancer Drug Targets 2013; 13: 915–29. [DOI] [PubMed] [Google Scholar]
- 8. Mittal V. Epithelial mesenchymal transition in aggressive lung cancers. Adv Exp Med Biol 2016; 890: 37–56. [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Zhao Z, Xu C, Zhou Z, Zhu Z, You T. HMGA2 induces transcription factor Slug expression to promote epithelial‐to‐mesenchymal transition and contributes to colon cancer progression. Cancer Lett 2014; 355: 130–40. [DOI] [PubMed] [Google Scholar]
- 10. Morishita A, Zaidi MR, Mitoro A et al. HMGA2 is a driver of tumor metastasis. Cancer Res 2013; 73: 4289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu J, Liu Z, Shao C et al. HMGA2 overexpression‐induced ovarian surface epithelial transformation is mediated through regulation of EMT genes. Cancer Res 2011; 71: 349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watanabe S, Ueda Y, Akaboshi S, Hino Y, Sekita Y, Nakao M. HMGA2 maintains oncogenic RAS‐induced epithelial‐mesenchymal transition in human pancreatic cancer cells. Am J Pathol 2009; 174: 854–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hammond SM, Sharpless NE. HMGA2, microRNAs, and stem cell aging. Cell 2008; 135: 1013–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pfannkuche K, Summer H, Li O, Hescheler J, Dröge P. The high mobility group protein HMGA2: A co‐regulator of chromatin structure and pluripotency in stem cells? Stem Cell Rev 2009; 5: 224–30. [DOI] [PubMed] [Google Scholar]
- 15. Fedele M, Visone R, De Martino I et al. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell 2006; 9: 459–71. [DOI] [PubMed] [Google Scholar]
- 16. Wu J, Wang Y, Xu X et al. Transcriptional activation of FN1 and IL11 by HMGA2 promotes the malignant behavior of colorectal cancer. Carcinogenesis 2016; 37: 511–21. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Liu X, Li AY et al. Overexpression of HMGA2 promotes metastasis and impacts survival of colorectal cancers. Clin Cancer Res 2011; 17: 2570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shi Z, Li X, Wu D et al. Silencing of HMGA2 suppresses cellular proliferation, migration, invasion, and epithelial‐mesenchymal transition in bladder cancer. Tumour Biol 2016; 37: 7515–23. [DOI] [PubMed] [Google Scholar]
- 19. Tan L, Wei X, Zheng L et al. Amplified HMGA2 promotes cell growth by regulating Akt pathway in AML. J Cancer Res Clin Oncol 2016; 142: 389–99. [DOI] [PubMed] [Google Scholar]
- 20. Zhao XP, Zhang H, Jiao JY, Tang DX, Wu YL, Pan CB. Overexpression of HMGA2 promotes tongue cancer metastasis through EMT pathway. J Transl Med 2016; 14: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A. Transforming growth factor‐beta employs HMGA2 to elicit epithelial‐mesenchymal transition. J Cell Biol 2006; 174: 175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarhadi VK, Wikman H, Salmenkivi K et al. Increased expression of high mobility group A proteins in lung cancer. J Pathol 2006; 209: 206–12. [DOI] [PubMed] [Google Scholar]
- 23. Lin Y, Liu AY, Fan C et al. MicroRNA‐33b inhibits breast cancer metastasis by targeting HMGA2, SALL4 and Twist1. Sci Rep 2015; 5: 9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He QY, Wang GC, Zhang H et al. miR‐106a‐5p suppresses the proliferation, migration, and invasion of osteosarcoma cells by targeting HMGA2. DNA Cell Biol 2016; 35: 506–20. [DOI] [PubMed] [Google Scholar]
- 25. Szász AM, Lánczky A, Nagy Á et al. Cross‐validation of survival associated biomarkers in gastric cancer using transcriptomic data of 1,065 patients. Oncotarget 2016; 7: 49322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aguirre‐Gamboa R, Gomez‐Rueda H, Martínez‐Ledesma E et al. SurvExpress: An online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS ONE 2013; 8: e74250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bartel D. MicroRNAs: Target recognition and regulatory functions. Cell 2009; 136: 215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou Y, Tian L, Wang X et al. MicroRNA‐195 inhibits non‐small cell lung cancer cell proliferation, migration and invasion by targeting MYB. Cancer Lett 2014; 347: 65–74. [DOI] [PubMed] [Google Scholar]
- 29. Liu B, Qu J, Xu F et al. MiR‐195 suppresses non‐small cell lung cancer by targeting CHEK1. Oncotarget 2015; 6: 9445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meyer B, Loeschke S, Schultze A et al. HMGA2 overexpression in non‐small cell lung cancer. Mol Carcinog 2007; 46: 503–11. [DOI] [PubMed] [Google Scholar]
- 31. Pallante P, Sepe R, Puca F, Fusco A. High mobility group A proteins as tumor markers. Front Med (Lausanne) 2015; 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ikeda K, Ogawa K, Takeishi Y. The role of HMGA2 in the proliferation and expansion of a hematopoietic cell in myeloproliferative neoplasms. Fukushima J Med Sci 2012; 58: 91–100. [DOI] [PubMed] [Google Scholar]
- 33. Mansoori B, Mohammadi A, Shirjang S, Baradaran B. HMGI‐C suppressing induces P53/caspase9 axis to regulate apoptosis in breast adenocarcinoma cells. Cell Cycle 2016; 15: 2585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi Z, Wu D, Tang R et al. Silencing of HMGA2 promotes apoptosis and inhibits migration and invasion of prostate cancer cells. J Biosci 2016; 41: 229–36. [DOI] [PubMed] [Google Scholar]
- 35. Xu L, Liao WL, Lu QJ et al. ANG promotes proliferation and invasion of the cell of lung squamous carcinoma by directly up‐regulating HMGA2. J Cancer 2016; 7: 862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen T, You Y, Jiang H, Wang ZZ. Epithelial‐mesenchymal transition (EMT): A biological process in the development, stem cell differentiation, and tumorigenesis. J Cell Physiol 2017. https://doi.org/10.1002/jcp.25797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alam M, Ahmad R, Rajabi H, Kufe D. MUC1‐C induces the LIN28B‐LET‐7‐HMGA2 axis to regulate self‐renewal in NSCLC. Mol Cancer Res 2015; 13: 449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee YS, Dutta A. The tumor suppressor microRNA let‐7 represses the HMGA2 oncogene. Genes Dev 2007; 21: 1025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A. HMGA2 and Smads co‐regulate SNAIL1 expression during induction of epithelial‐to‐mesenchymal transition. J Biol Chem 2008; 283: 33437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan EJ, Thuault S, Caja L, Carletti T, Heldin CH, Moustakas A. Regulation of transcription factor Twist expression by the DNA architectural protein high mobility group A2 during epithelial‐to‐mesenchymal transition. J Biol Chem 2012; 287: 7134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zha L, Wang Z, Tang W, Zhang N, Liao G, Huang Z. Genome‐wide analysis of HMGA2 transcription factor binding sites by ChIP on chip in gastric carcinoma cells. Mol Cell Biochem 2012; 364: 243–51. [DOI] [PubMed] [Google Scholar]
- 42. Li W, Wang Z, Zha L, Kong D, Liao G, Li H. HMGA2 regulates epithelial‐mesenchymal transition and the acquisition of tumor stem cell properties through TWIST1 in gastric cancer. Oncol Rep 2017; 37: 185–92. [DOI] [PubMed] [Google Scholar]
- 43. Madison B, Jeganathan A, Mizuno R et al. Let‐7 represses carcinogenesis and a stem cell phenotype in the intestine via regulation of HMGA2. PLoS Genet 2015; 11: e1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang K, Gao H, Wu X et al. Frequent overexpression of HMGA2 in human atypical teratoid/rhabdoid tumor and its correlation with let‐7a3/let‐7b miRNA. (Published erratum appears in Clin Cancer Res 2017; 23: 3225.). Clin Cancer Res 2014; 20: 1179–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers for real time (RT)‐PCR.


