Abstract
Congenital heart disease (CHD) is the most common congenital disorder among live births. When associated with extracardiac abnormalities, it is characterized as a syndromic heart disease (syndromic CHD) and corresponds to 25% of all liveborn infants with a heart defect. The etiology in about 65% of the cases still remains unknown, and in about 35% of the patients, it is associated with genetic factors. In the present study, MLPA and SNP-array techniques were used to investigate a group of 47 patients with syndromic CHD. In total, 16 defects (34%) were identified, of which 12 (25.5%) were classified as pathogenic or probably pathogenic. The most frequent abnormalities were 22q11.2 deletion (22q11.2 deletion syndrome) and 7q11.23 deletion (Williams-Beuren syndrome). We also show that rarer malformations may be associated with syndromic CHD, such as 14q32.33 deletion as well as 17q25.3, 15q11.2 (BP1-BP2), 22q13.31, and 12p13.31 (SLC2A3) duplications. The present study demonstrates that CNVs are important causal factors and should be studied in patients with syndromic CHD. Furthermore, the use of MLPA as a first screening test was appropriate, as this less expensive technology detected 11 of the 12 pathogenic abnormalities (91.6%).
Keywords: Copy number variation, MLPA, SNP array, SLC2A3, Heart disease
Congenital heart disease (CHD) is the most common disorder among live births with an incidence of 10/1,000 newborns [Payne et al., 2012; Osoegawa et al., 2014]. It is considered a major cause of morbidity and mortality, especially when associated with extracardiac malformations [van Karnebeek and Hennekam, 1999]. CHD in combination with other malformations occurs in about 25% of the cases, many of them being part of a pattern of specific defects or genetic syndromes [Bernstein, 2004].
About 65% of the CHD cases are of unknown etiology and around 20–35% are attributed to genetic factors [Kerstjens-Frederikse and Hofstra, 2013; Cowan and Ware, 2015]. Chromosomal abnormalities, including CNVs are known to be important causes of CHD: about 10% are due to numerical chromosomal abnormalities and 15% to submicroscopic changes, occurring in approximately 3–25% of the syndromic cases and 3–10% of nonsyndromic cases [Kerstjens-Frederikse and Hofstra, 2013; Cowan and Ware, 2015]. Monogenic mutations are reported in 5 and 10% of syndromic and nonsyndromic cases, respectively [Kerstjens-Frederikse and Hofstra, 2013; Cowan and Ware, 2015], but this number will probably increase with the use of next-generation sequencing (NGS) for large-scale diagnosis [Landis and Ware, 2016].
Using standard cytogenetic techniques, only a subset of these patients are diagnosed because of the limited cytogenetic resolution, which is incapable of detecting changes smaller than 5–10 Mb [Richards and Garg, 2010]. The use of technologies based on microarrays increases the rate of detection of chromosomal aberrations in CHD patients approximately 16.6–30% [Thienpont et al., 2007; Hightower et al., 2015]. Some authors suggest that the array methodology should be used as the first test for newborn carriers of congenital defects, such as CHD, due to the high rate of diagnosis [Sørensen et al., 2012; Wang et al., 2013]. However, because of the high cost of this method, its large-scale use in developing countries is limited. Therefore, MLPA is a viable alternative genetic screening tool with a good cost-benefit ratio for these patients [Jehee et al., 2011].
Some researchers have investigated the contribution of chromosomal alterations in CHD patients using the MLPA technique; the results ranged from 3.2–33.33% and showed to be highly dependent on the selection of patients and the type of kit used [Sørensen et al., 2012; Wang et al., 2013; Campos et al., 2015].
It is undeniable that establishing an early genetic diagnosis is essential, with important implications for assistance in treatment, understanding of aspects of the disease, and genetic counseling regarding the risks of recurrence. Therefore, the aim of the present study was to identify chromosomal abnormalities in patients with syndromic CHD using MLPA as the initial screening method followed by SNP array.
Patients and Methods
Patient Selection, Characterization, and Sample Collection
Patients were recruited from several collaborating centers, pediatric clinics, pediatric and neonatal intensive care units as well as outpatient genetic centers. Inclusion criteria were the presence of any type of CHD associated with extracardiac malformations and/or psychomotor developmental delay, or intellectual disability. Patients who presented with phenotypic characteristics easily recognized by the referring pediatrician or cardiologist, such as Down syndrome, or who had a chromosomal abnormality predetermined by karyotyping, were excluded. At the time of sample collection for the present study, none of the patients had a karyotype analysis.
The medical records were analyzed and compiled into a clinical file, and the pedigree of each family was included.
Samples of peripheral blood were collected from the patients and their parents whenever possible. DNA was extracted using the salting-out method, Puregene Blood Core (Qiagen-Sciences) kit, according to the manufacturer's instructions.
Multiplex Ligation Dependent Probe Amplification
All patients were screened by MLPA (MRC Holland b.v., Amsterdam, Holland), using a combination of commercial kits that included the MLPA® P064B2/C1 kits, in order to identify the most common microdeletion and microduplication syndromes, such as: DS22q11.2, Williams-Beuren (del 7q11), Smith-Magenis (del 17p11), del 1p36, and Prader-Willi/Angelman (del 15q11). The MLPA, P070-B1/B2 and P036-E2 kits were used for detecting subtelomeric abnormalities.
Analyses were performed according to the manufacturer's instructions in an ABI Veriti (Applied Biosystems Veriti Thermal Cycler) thermal cycler. Fragments were separated by capillary electrophoresis using the Applied Biosystems® 3730 DNA Analyzer with a 36-cm array using POP7™ polymer. Normalization and analysis of the results were performed using the GeneMarker® (SoftGenetics LLC® genotyping software) program. Peak heights were used to calculate the number of copies of each target sequence. The number of copies considered normal corresponded to the intensity of relative height (peak ratio) between 0.7 and 1.3 and, in the case of deletions and duplications, to a value <0.7 and >1.3, respectively. When an aberration was detected, the reaction was repeated, also including the samples from the parents when available.
SNP Array
The platform used for SNP array was the CytoScan® 750K Array (Affymetrix) which contains 550,000 non-polymorphic probes for CNVs from coding and noncoding regions, and approximately 200,000 SNPs along the human genome. The procedures followed those described by the manufacturer (http://www.affymetrix.com/estore/catalog/prod930003/AFFY/CytoScan+750K+Array+Kit+and+Reagent+Kit+Bundle#1_1). Data analysis was performed using the Software Affymetrix Chromosome Analysis Suite (ChAS), versions 2.0.1.2 and 3.0.0.42.
CNV Analysis
The ChAS software was used to analyze the CNVs, selecting abnormalities that involved at least 15 probes for deletions and 30 probes for duplications. Following this filtering, abnormalities were checked in the following databases of polymorphic CNVs: Database of Genomic Variants (DGV) and CytoScan Array Database, provided by the ChAS software. In addition, it was verified whether these aberrations had already been described in other noncardiac patients from our group, and the frequency thereof, in order to discard possible nonpathogenic polymorphisms among the Brazilian population. Subsequently, the CNVs were compared with rearrangements described in the DECIPHER database and also in PUBMED.
According to this analysis, the CNVs were classified into 4 groups:
-
(1)
benign or probably benign: when described in more than 5 individuals in both above-mentioned databases of nonpathogenic polymorphic abnormalities, when there is no gene or only noncoding or intronic regions involved in the interval of the abnormality, or when the abnormality involves a gene related to a known autosomal recessive disorder not related to the clinical symptoms of the patient;
-
(2)
probably pathogenic: when there is evidence that the genes involved were related to the patient's clinical condition;
-
(3)
pathogenic: when the CNVs are well-known to be pathogenic, related to syndromes of microduplication or microdeletions already described;
-
(4)
variant of unknown significance: when it is not possible to classify the CNVs in any of the categories above; e.g., the genes involved do not present a well-established function or do not have an obvious relationship to the patient's clinical condition.
Additionally, the loss of heterozygosity (LOH) along the genome was investigated. All the regions that presented 2 identical alleles along 10 Mb were analyzed to verify recessive diseases and the possible effect of imprinting.
Quantitative Polymerase Chain Reaction
qPCR analysis was used to confirm the inheritance pattern of some CNVs. Thus, specific primers were designed for the affected genes in the CNVs, using the Primer3 (version 0.4.0) software. The SYBR-based duplex qPCR technique was used, which is based on the dissociation temperature difference of the PCR products generated. All reactions were performed in duplicate, using 10 disomic controls for the regions studied and standardized according to Torrezan et al. [2012].
Results
In total, 47 patients were included in the present study, 20 (42.6%) male and 27 (57.4%) female individuals. The ages of the patients ranged from 2 months to 19 years, with a mean of 5 years, 2 months. The cardiac phenotype of the group studied consisted of a broad spectrum of malformations, ranging from simple lesions such as septal defects to complex defects; e.g., tetralogy of Fallot or the transposition of large vessels. Cyanotic heart diseases were the most prevalent, observed in more than 90% of the patients. In those corresponding to heart diseases with a single lesion, the ventricular septal defect and atrial septal defect were the most predominant, representing 52% of the cases. About 55.3% of the patients presented with multiple cardiac lesions.
After MLPA analysis, 12 individuals (25.5%) showed defects. However, one of them was considered to be of uncertain significance (G1.1; Xp22.33 duplication, inherited from the father). Thus, a total of 11/47 pathogenic malformations (23.4%) was obtained (Table 1).
Table 1.
Malformations identified by MLPA in patients with congenital heart disease
| Patients | Heart disease | Extracardiac malformations | MLPA kits |
Inheritance | ||
|---|---|---|---|---|---|---|
| P064 syndromes | P036 subtelomeric | P070 subtelomeric | ||||
| G1.1* | AVSD | DD, facial dysmorphism | NC | dup Xp22.33 | dup Xp22.33 | paternal |
| G2.1 | ASD, aortic valve stenosis | facial dysmorphism | DS22q11.2 | NC | NC | de novo |
| G3.1 | VSD | DD, convulsion | DS22q11.2 | NC | NC | de novo |
| G4.1 | VSD, ASD, CoA, hypoplasia of the ventricles | facial dysmorphism | DS22q11.2 | NC | NC | maternal |
| G5.1 | CoA | DD | DS22q11.2 | NC | NC | maternal |
| G6.1 | supravalvular aortic stenosis | DD, facial dysmorphism | WBS (del 7q11.23) | NC | NC | de novo |
| G7.1 | supravalvular aortic stenosis | DD | WBS (del 7q11.23) | NC | NC | de novo |
| G8.1 | VSD, PDA, AVSD | DD, facial dysmorphism, convulsion | WHS (del 4p16.3) | del 4p16.3 dup 8q24.3 |
del 4p16.3 dup 8q24.3 |
undetermined** |
| G9.1 | VSD | DD, facial dysmorphism | NC | dup 18q dup 18p |
dup 18q dup 18p |
de novo |
| G10.1 | ASD | DD, palpebral ptosis, microcephaly | NC | del 14q32.33 | del 14q32.33 | de novo |
| G11.1 | ASD, hypoplastic left ventricle, aortic arch interruption | facial dysmorphism | NC | dup 17q25.3 | dup 17q25.3 | de novo |
| G12.1 | ASD | DD, facial dysmorphism | dup 4p | del 18q23 dup 4p16 |
del 18q23 dup 4p16 |
undetermined** |
ASD, atrial septal defect; AVSD, atrioventricular septal deviation; CoA, coarctation of the aorta; DD, developmental delay; DS, deletion syndrome; NC: no change; PDA, patent ductus arteriosus; VSD, ventricular septal defect; WBS, Williams-Beuren syndrome; WHS, Wolf-Hirschhorn syndrome.
Abnormality of uncertain significance.
Parental carrier status of balanced translocation not evaluated.
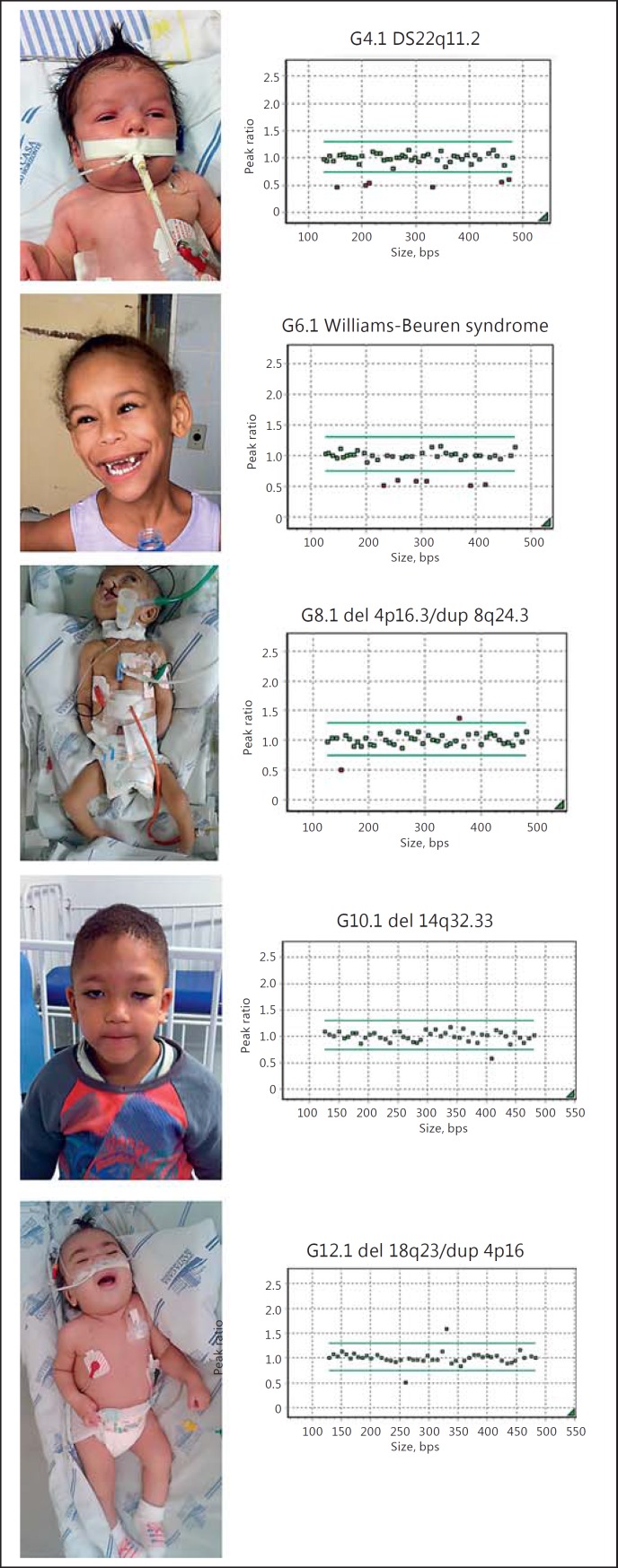
The most prevalent abnormalities were: 22q11.2 deletion syndrome (DS22q11.2) identified in 4 patients (G2.1-G5.1) and Williams-Beuren syndrome (deletion 7q11.23) identified in 2 patients (G6.1-G7.1). Other abnormalities included: an unbalanced translocation (del 4p16.3/dup 8q24.3) compatible with Wolf-Hirschhorn syndrome (G8.1), a trisomy of chromosome 18 which characterizes Edwards syndrome (G9.1), a deletion at 14q32.33 (G10.1), a duplication at 17q25.3 (G11.1), and an unbalanced translocation (del 18q23/dup 4p16) in patient G12.1. Five patients and their MLPA results are depicted in Figure 1.
Fig. 1.
Pictures of patients and respective MLPA panels depicting chromosomal abnormalities. Green dots show probes with a normal CNV count, and red dots show either deleted probes (below normal margin) or duplicated probes (above normal margin).
Seven de novo (58.3%) abnormalities occurred and 3 were inherited (25%). Two patients presented with unbalanced translocations; it was not possible to verify if one of the parents was a carrier of a balanced translocation so that the pattern of inheritance in these cases was not identified.
Among the 35 patients with no disorders detected after MLPA screening, 12 were randomly selected for the CNV study by SNP array. Of these, 8 patients (G17.1–G24.1; 66.7%) only had CNVs classified as group 1 (benign or probably benign; online suppl. Table 1, see www.karger.com/doi/10.1159/000477226 for online suppl. material). Of the other 4 patients (33.3%), 6 CNVs merited greater attention and examinations (Table 2).
Table 2.
Malformations identified by SNP array
| Patient | Heart disease | Extracardiac malformations | Chromosomal abnormalitiy | Genomic position | Location | Size, bp | Genes involved | Inheritance | Classification |
|---|---|---|---|---|---|---|---|---|---|
| G13. 1 | VSD, PDA, single lobby, interruption of the inferior vena cava, single valve with regurgitation | DD | duplication | arr[hg19] 15q13.1(29,057,675 – 29,531,305)×3 |
15q13.1 | 473.630 | LOC646278, GOLGA6L7P, APBA2, FAM189A1 | paternal | VOUS |
| deletion | arr[hg19] 5q14.3(89,511,085 – 90,121,361)×1 |
5q14.3 | 610.276 | CETN3, MBLAC2, POLR3G, LYSMD3, GPR98 | paternal | VOUS | |||
| G14. 1 | CoA | facial dysmorphism, genital abnormalities, renal dysfunction | deletion | arr[hg19] 22q11.22(22,311,348 – 22,579,064)×1 |
22q11.22 | 267.716 | TOP3B | not maternal* | VOUS |
| duplication | arr[hg19] 15q11.2 (22,770,421 – 23,214,339)×3 | 15q11.2 (BP1–BP2) |
443.918 | TUBGCP5, CYFIP1, NIPA2, NIPA1, LOC283683, WHAMMP3 | undetermined | probably pathogenic (susceptibility locus) | |||
| G15.1 | ASD, single lobby, AVSD | facial dysmorphism | duplication | arr[hg19] 7q35 (144,319,952 – 44,519,740)×3 | 7q35 | 199.788 | TPK1 | undetermined | VOUS |
| G16.1 | PDA | facial dysmorphism, DD | deletion | arr[hg19] 9q31.2(112,190,883 – 12,248,027)×1 |
9q31 | 57.144 | PTPN3 | undetermined | VOUS |
ASD, atrial septal defect; AVSD, atrioventricular septal deviation; CoA, coarctation of the aorta; DD, developmental delay; PDA, patent ductus arteriosus; VOUS, variant of unknown significance; VSD, ventricular septal defect.
Paternal sample not available.
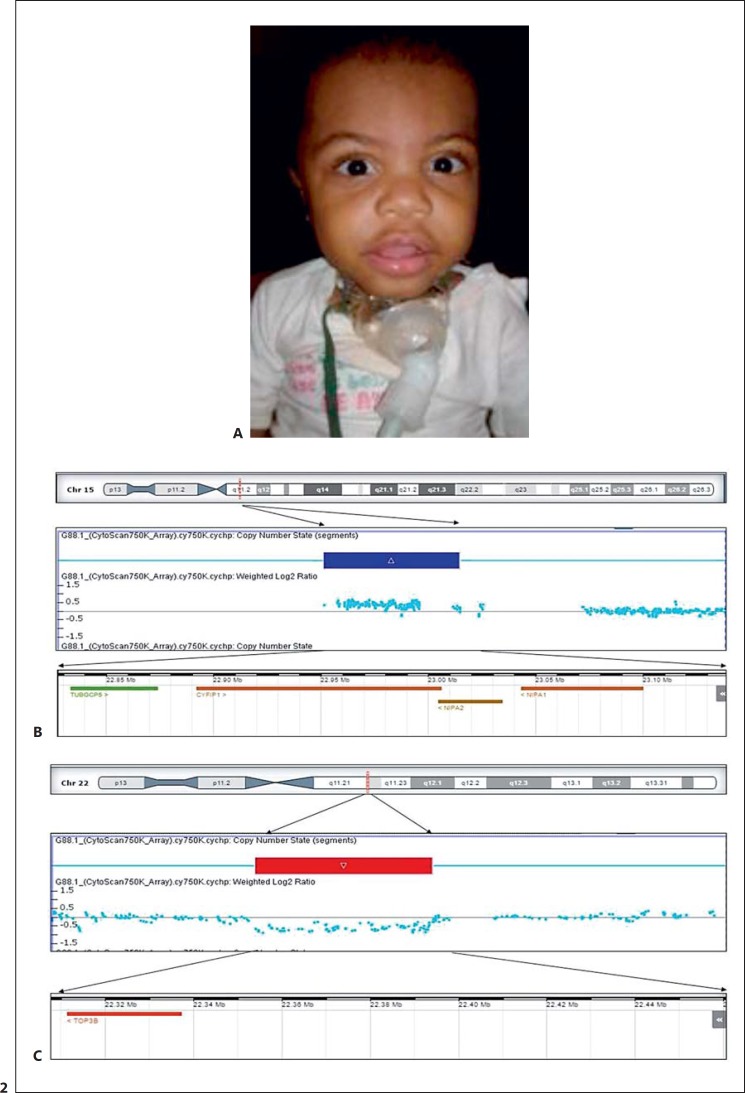
A 15q11.2 (BP1-BP2) duplication, which is considered to be a susceptibility locus for neurological dysfunction and congenital malformation, was found in patient G14.1. This CNV was classified as probably pathogenic (Fig. 2; Table 2) [Burnside et al., 2011; Vanlerberghe et al., 2015].
Fig. 2.
SNP-array data from patient G14.1. A Facial appearance of the patient. B Array panel showing a duplication at the 15q11.2 BP1-BP2 region. C A deletion at 22q11.2 involving the TOP3B gene. The duplicated and deleted regions are represented by a blue and red rectangle(s), respectively. The blue dots represent the probes distributed in these regions. The lower ideogram shows the genes mapped within the depicted region.
With the exception of patient G15.1, with a large percentage (25%) of LOH, all other regions of detected LOH were evaluated in relation to the presence of recessive disease genes or overlap of genomic imprinting regions, and no significant result was found. Through the calculation of the endogamy index, based on the percentage of LOH that each patient showed, we can infer that the 25% found in patient G15.1 is the result of a consanguineous marriage and that the patient has a greater risk of being a carrier of a recessive disease.
Discussion
Chromosomal screening for microdeletions and microduplications using MLPA detected abnormalities in 23.4% of the patients tested (11/47). DS22q11.2 was identified in 4 patients, 3 of them (G2.1, G3.1, and G4.1) with the most frequent 3-Mb deletion, and patient G5.1 with a 1.5-Mb deletion. Two patients (G6.1 and G7.1) were diagnosed with Williams-Beuren syndrome (del 7q11.23). Additionally, patient G8.1 presented with a malformation compatible with Wolf-Hirschhorn syndrome and patient G9.1 with Edwards syndrome. All syndromes described above and identified in our patients are commonly associated with CHD [Aracena, 2003].
It is known that DS22q11.2 is the second main cause of CHD [Ware and Jefferies, 2012], and this syndrome was the most frequent disorder in our sample (8.5%). This value is higher than those found by Sørensen et al. [2012], 0.49%, and Campos et al. [2015], 2.56%, and differs significantly with the results of Wang et al. [2013] and El Malti et al. [2016]; here, no patients were identified with DS22q11.2. These results are explained by the fact that the studies cited above included patients both with syndromic CHD and with isolated CHD, while our patients were all syndromic. Similarly, other syndromes commonly associated with CHD were observed in our patients, such as Wolf-Hirschhorn and Williams-Beuren, and these were not identified in the studies cited above.
The rates of diagnosis in the various studies are highly variable, from 3.2 to 33.3% [Sørensen et al., 2012; Wang et al., 2013; Campos et al., 2015], which is due to the technique used and the method of selecting patients. In our case, there is a bias towards more severe patients since all of them were syndromic. Furthermore, most patients were referred by clinical geneticists or assessed at the cardiac neonatal intensive care unit from Santa Casa Hospital in Belo Horizonte, which is known to be a reference for complex patients. This would explain our detection rate of 25.5% and the relatively high occurrence of DS22q11.2 and Williams-Beuren syndrome.
Most of the malformations found in our group of patients with CHD are common. However, some rarer defects were also detected, as seen in patient G10.1 who showed a rare de novo deletion at 14q32.33. Only 25 cases of distal 14q23.3 deletion are reported in the literature to date [Miller et al., 1992; van Karnebeek et al., 2002; Maurin et al., 2006; Zollino et al., 2009; Wu et al., 2010; Engels et al., 2012; Holder et al., 2012]. Individuals bearing this deletion show common clinical characteristics including cardiac defects, developmental delay, microcephaly, high forehead with lateral hypertrichosis, hypotonia, high palate, broad nasal bridge, thin upper lip, single palmar crease, micrognathia, and ophthalmological problems [Maurin et al., 2006; Zollino et al., 2009]. Patient G10.1 had delayed psychomotor development, microcephaly, and palpebral ptosis, in addition to an atrial septal defect. In the literature, CHD has been reported in 24% of the cases with 14q32.3 deletion (6/25), and the deletions of patients with CHD were generally larger (>3.5 Mb) than those observed in individuals without CHD [Engels et al., 2012]. Our patient had a deletion probably >5 Mb, since it was also detected by karyotyping.
Patient G11.1, in whom a de novo duplication was identified at 17q25.3, presented with multiple cardiac lesions including an atrial septal defect, and facial dysmorphisms. According to Lukusa and Fryns [2010], gains in this region are frequent and have been observed in association with cardiac septal defects, craniofacial dysmorphisms, and intellectual disability. In a study conducted by Probst et al. [2015], 8 patients with 17q25.3 abnormalities were studied: 5 deletions ranging from 0.08 to 1.42 Mb, all with CHD, and 3 duplications ranging from 0.14 to 8.06 Mb, but only one with CHD, were described. Although it is not possible to determine the extent of the duplication in our patient using the MLPA technique, we know that it is at least 0.6 Mb since the SECTM1 and TBCD genes are duplicated. The 17q25.3 region is extremely rich in genes and several, such as ACTG1, P4HB, ARHGDIA, NPLOC4, MRPL12, DCXR, CSNK1D, SLC16A3, STRA13, and UTS2R are expressed in cardiac tissues. The duplication identified in our patient reinforces the existence a susceptibility locus at 17q25.3.
The CNVs identified through SNP array ranged from 0.05 to 0.61 Mb, all smaller than the cytogenetic resolution (Table 2). Of the 12 patients tested, only 1 revealed probably pathogenic CNVs, 8.3% (1/12), while three, 25% (3/12), showed CNVs considered a variant of unknown significance. Patient G13.1 had 2 CNVs of paternal inheritance: a deletion of 610.276 bp at 5q14.3 and a duplication of 473.630 bp at 15q13.1. Despite numerous deletions at 5q14.3 described in the databases, the association of heart disease with deletions in this region is scant [Cardoso et al., 2009]. Engels et al. [2009] described one patient with heart disease out of 3 carrying a 5q14.3 deletion. The authors outline one area of 1.6 Mb as being the minimum critical region for the phenotype of the 5q14.3q15 microdeletion syndrome and propose the LYSMD3 gene as a possible candidate for developmental delay [Nakayama et al., 2002]. Although the deletion in patient G13.1 overlaps the minimum region of the 5q14.3q15 microdeletion syndrome, with the exception of severe developmental delay and apparent large ears, this patient did not appear to have the other characteristics of the syndrome. In addition, it is noteworthy that the father, also a carrier of the same deletion, is asymptomatic. These data suggest that the deletion in this region has incomplete penetrance or is not even related to the clinical signs of the 5q14.3q15 microdeletion syndrome. This is in line with the suggestion from Zweier and Rauch [2012] that the haploinsufficiency in the MEF2C gene, not deleted in our patient, is most responsible for the clinical signs of the 5q14.3q15 microdeletion syndrome.
Patient G13.1 also shows a duplication in the poorly described 15q13.1 region. None of the genes included in the duplication seem to be related to cardiac development or have notably elevated cardiac expression.
Two CNVs were identified in patient G14.1. First, a duplication of 443.918 bp in the 15q11.2 BP1-BP2 region, involving the genes TUBGCP5, NIPA1, NIPA2, CYFIP1, and WHAMMP3, was identified. This duplication is also found in healthy individuals. Therefore, until recently, it was considered a variant of uncertain significance [Abdelmoity et al., 2012]. However, latest studies show that abnormalities in the genes in the BP1-BP2 region may modify the regulation of multiple paths, leading to variable phenotypes [Glessner et al., 2014; Warburton et al., 2014]. Although there are reports that 9–17% of the individuals with 15q11.2 BP1-BP2 microdeletions are carriers of congenital cardiac defects [Cox and Butler, 2015; Vanlerberghe et al., 2015], this number has not yet been defined for the microduplication syndrome in this region. Some reports describe patients with 15q11.2 BP1-BP2 duplication and CHD, including some with coarctation of the aorta [Abdelmoity et al., 2012; Vanlerberghe et al., 2015], the same malformation as the patient in this study.
Second, a deletion of 267.716 bp, involving the TOP3B gene mapped at 22q11.2, overlapping the region affected in the distal deletion 22q11.2 syndrome was identified [Shaikh et al., 2007]. Heart disease patients with distal deletion 22q11.2 have a broad spectrum of cardiac defects such as septal defects, tetralogy of Fallot, and conotruncal defects [Breckpot et al., 2012]. The deletion involving only the TOP3B gene was reported in 2 patients in the study by Thorsson et al. [2015], and both presented CHD. However, the role of TOP3B in cardiac development still needs to be further studied because, even though TOP3B is also expressed in the heart, deletions or mutations of this gene are more frequently described in patients with neurological developmental delay [O'Roak et al., 2012; Stoll et al., 2013].
A duplication in the 7q35 region involving TPK1 was found in patient G15.1. It is known that mutations in this gene are inherited in a recessive mode. In general, affected individuals present with acute encephalopathy, resulting in progressive neurological dysfunction [Mayr et al., 2011]. Since this defect is rare, and this gene has low expression in the heart, it was not possible to determine a clear correlation between this duplication and CHD of the patient. Additionally, the patient bears 25% LOH, which infers that this is the result of a consanguineous relationship between siblings or father and daughter, and a recessive disease could be the cause of his phenotype.
Patient G16.1 presented a deletion involving the PTPN3 gene in the 9q31 region. Although it is already known that gain-of-function mutations in PTPN11 are responsible for Noonan syndrome, characterized by facial dysmorphisms and congenital heart disease [Digilio and Marino, 2001], no haploinsufficiency of this gene family has been associated with CHD to date.
Considering pathogenic and probably pathogenic chromosomal alterations, we detected 23.4% (11/47) of abnormalities through MLPA screening and 8.3% (1/12) using microarray, which sums up to a total detection rate of 25.5% (12/47). Most pathogenic and probably pathogenic CNVs were detected by MLPA (11/12, 91.6%) and represent the most common chromosomal abnormalities found in CHD, excluding Down syndrome.
The present study shows that our approach using MLPA as the first genetic test was highly effective in the detection of clinically significant chromosomal abnormalities in individuals with syndromic CHD. Moreover, MLPA was easier to perform and analyze in the diagnostic setting. We conclude that our approach of using the combination of the P064, P036, and P070 MLPA kits is a suitable initial screening tool in groups of patients with syndromic CHD, lowering costs particularly in developing countries.
Statement of Ethics
The parents or legal guardians of the patients consented to participate in the study and signed the necessary informed consent form. The present study was approved by the Ethics Committee of the Institute for Teaching and Research of Santa Casa, Belo Horizonte, CAAE 48581115.6.1001.5138.
Disclosure Statement
The authors have no conflicts of interest to declare.
Supplementary Material
Supplementary data
Acknowledgments
The authors would like to express their gratitude to all patients and their families for their participation in the study. This work was supported by the Fundação de Amparo a Pesquisa de Minas Gerais/FAPEMIG, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/CAPES, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq.
References
- Abdelmoity AT, LePichon JB, Nyp SS, Soden SE, Daniel CA, Yu S. 15q11. 2 proximal imbalances associated with a diverse array of neuropsychiatric disorders and mild dysmorphic features. J Dev Behav Pediatr. 2012;33:570–576. doi: 10.1097/DBP.0b013e31826052ae. [DOI] [PubMed] [Google Scholar]
- Aracena A. Cardiopatías congénitas y síndromes malformativos-genéticos. Rev Chil Pediatr. 2003;74:426–431. [Google Scholar]
- Bernstein D. Evaluation of the cardiovasacular system. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson Textbook of Pediatrics. Philadelphia: Saunders; 2004. pp. 1481–1488. [Google Scholar]
- Breckpot J, Thienpont B, Bauters M, Tranchevent LC, Gewillig M, et al. Congenital heart defects in a novel recurrent 22q11.2 deletion harboring the genes CRKL and MAPK1. Am J Med Genet A. 2012;158A:574–580. doi: 10.1002/ajmg.a.35217. [DOI] [PubMed] [Google Scholar]
- Burnside RD, Pasion R, Mikhail FM, Carroll AJ, Robin NH, et al. Microdeletion/microduplication of proximal 15q11. 2 between BP1 and BP2: a susceptibility region for neurological dysfunction including developmental and language delay. Hum Genet. 2011;130:517–528. doi: 10.1007/s00439-011-0970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CMR, Zanardo EA, Dutra RL, Kulikowski LD, Kim C. Investigation of copy number variation in children with conotruncal heart defects. Arq Bras Cardiol. 2015;104:24–31. doi: 10.5935/abc.20140169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C, Boys A, Parrini E, Mignon-Ravix C, McMahon JM, et al. Periventricular heterotopia, mental retardation, and epilepsy associated with 5q14.3-q15 deletion. Neurology. 2009;72:784–792. doi: 10.1212/01.wnl.0000336339.08878.2d. [DOI] [PubMed] [Google Scholar]
- Cowan JR, Ware SM. Genetics and genetic testing in congenital heart disease. Clin Pernatol. 2015;42:373–393. doi: 10.1016/j.clp.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Cox DM, Butler MG. The 15q11.2 BP1-BP2 microdeletion syndrome: a review. Int J Mol Sci. 2015;16:4068–4082. doi: 10.3390/ijms16024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digilio MC, Marino B. Clinical manifestations of Noonan syndrome. Images Paediatr Cardiol. 2001;3:19–30. [PMC free article] [PubMed] [Google Scholar]
- El Malti R, Liu H, Doray B, Thauvin C, Maltret A, et al. A systematic variant screening in familial cases of congenital heart defects demonstrates the usefulness of molecular genetics in this field. Eu J Hum Genet. 2016;24:228–236. doi: 10.1038/ejhg.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels H, Wohlleber E, Zink A, Hoyer J, Ludwig KU, et al. A novel microdeletion syndrome involving 5q14.3-q15: clinical and molecular cytogenetic characterization of three patients. Eur J Hum Genet. 2009;17:1592–1599. doi: 10.1038/ejhg.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels H, Schüler HM, Zink AM, Wohlleber E, Brockschmidt A, et al. A phenotype map for 14q32.3 terminal deletions. Am J Med Genet A. 2012;158A:695–706. doi: 10.1002/ajmg.a.35256. [DOI] [PubMed] [Google Scholar]
- Glessner J, Bick AG, Ito K, Homsy J, Rodriguez-Murillo L, et al. Increased frequency of de novo copy number variations in congenital heart disease by integrative analysis of SNP array and exome sequence data. Circ Res. 2014;115:884–896. doi: 10.1161/CIRCRESAHA.115.304458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower HB, Robin NH, Mikhail FM, Ambalavanan N. Array comparative genomic hybridisation testing in CHD. Cardiol Young. 2015;25:1155–1172. doi: 10.1017/S1047951114001838. [DOI] [PubMed] [Google Scholar]
- Holder JL, Jr, Lotze TE, Bacino C, Cheung SW. A child with an inherited 0.31 Mb microdeletion of chromosome 14q32.33: further delineation of a critical region for the 14q32 deletion syndrome. Am J Med Genet A. 2012;158A:1962–1966. doi: 10.1002/ajmg.a.35289. [DOI] [PubMed] [Google Scholar]
- Jehee FS, Takamori JT, Medeiros PFV, Pordeus ACB, Latini FRM, et al. Using a combination of MLPA kits to detect chromosomal imbalances in patients with multiple congenital anomalies and mental retardation is a valuable choice for developing countries. Eur J Med Genet. 2011;54:e425–432. doi: 10.1016/j.ejmg.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Kerstjens-Frederikse WS, Hofstra RMW. Genetische aspecten van aangeboren hartafwijkingen. In: Mulder BJM, Pieper PG, Meijboom FJ, Hamer JPM, editors. Aangeboren Hartafwijkingen BIJ Volwassenen. Houten: Bohn Stafleu van Loghum; 2013. pp. 233–245. [Google Scholar]
- Landis BJ, Ware SM. The current landscape of genetic testing in cardiovascular malformations: opportunities and challenges. Front Cardiovasc Med. 2016;3:22. doi: 10.3389/fcvm.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukusa T, Fryns JP. Pure de novo 17q25. 3 micro duplication characterized by micro array CGH in a dysmorphic infant with growth retardation, developmental delay and distal arthrogryposis. Genet Couns. 2010;21:25–34. [PubMed] [Google Scholar]
- Maurin ML, Brisset S, Le Lorc'h M, Poncet V, Trioche P, et al. Terminal 14q32.33 deletion: genotype-phenotype correlation. Am J Med Genet A. 2006;140:2324–2329. doi: 10.1002/ajmg.a.31438. [DOI] [PubMed] [Google Scholar]
- Mayr JA, Freisinger P, Schlachter K, Rolinski B, Zimmermann FA, et al. Thiamine pyrophosphokinase deficiency in encephalopathic children with defects in the pyruvate oxidation pathway. Am J Hum Genet. 2011;89:806–812. doi: 10.1016/j.ajhg.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BA, Jayakar P, Capó H. Child with multiple congenital anomalies and mosaicism 46,XX/46,XX,del(14)(q32. 3). Am J Med Genet. 1992;44:635–637. doi: 10.1002/ajmg.1320440521. [DOI] [PubMed] [Google Scholar]
- Nakayama J, Fu YH, Clark AM, Nakahara S, Hamano K, et al. A nonsense mutation of the MASS1 gene in a family with febrile and afebrile seizures. Ann Neurol. 2002;52:654–657. doi: 10.1002/ana.10347. [DOI] [PubMed] [Google Scholar]
- O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osoegawa K, Iovannisci DM, Lin B, Parodi C, Schultz K, et al. Identification of novel candidate gene loci and increased sex chromosome aneuploidy among infants with conotruncal heart defects. Am J Med Genet A. 2014;164:397–406. doi: 10.1002/ajmg.a.36291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AR, Chang SW, Koenig SN, Zinn AR, Garg V. Submicroscopic chromosomal copy number variations identified in children with hypoplastic left heart syndrome. Pediatr Cardiol. 2012;33:757–763. doi: 10.1007/s00246-012-0208-9. [DOI] [PubMed] [Google Scholar]
- Probst FJ, James RA, Burrage LC, Rosenfeld JA, Bohan TP, et al. De novo deletions and duplications of 17q25.3 cause susceptibility to cardiovascular malformations. Orphanet J Rare Dis. 2015;10:75. doi: 10.1186/s13023-015-0291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A, Garg V. Genetics of congenital heart disease. Current cardiology reviews Curr Cardiol Rev. 2010;6:91–97. doi: 10.2174/157340310791162703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, O'Connor RJ, Pierpont ME, McGrath J, Hacker AM, et al. Low copy repeats mediate distal chromosome 22q11.2 deletions: sequence analysis predicts breakpoint mechanisms. Genome Res. 2007;17:482–491. doi: 10.1101/gr.5986507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen KM, El-Segaier M, Fernlund E, Errami A, Bouvagnet P, et al. Screening of congenital heart disease patients using multiplex ligation-dependent probe amplification: early diagnosis of syndromic patients. Am J Med Genet A. 2012;158A:720–725. doi: 10.1002/ajmg.a.35214. [DOI] [PubMed] [Google Scholar]
- Stoll G, Pietiläinen OP, Linder B, Suvisaari J, Brosi C, et al. Deletion of TOP3β, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat Neurosci. 2013;16:1228–1237. doi: 10.1038/nn.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thienpont B, Mertens L, de Ravel T, Eyskens B, Boshoff D, et al. Submicroscopic chromosomal imbalances detected by array-CGH are a frequent cause of congenital heart defects in selected patients. Eur Heart J. 2007;28:2778–2784. doi: 10.1093/eurheartj/ehl560. [DOI] [PubMed] [Google Scholar]
- Thorsson T, Russell WW, El-Kashlan N, Soemedi R, Levine J, et al. Chromosomal imbalances in patients with congenital cardiac defects: a meta-analysis reveals novel potential critical regions involved in heart development. Congenit Heart Dis. 2015;10:193–208. doi: 10.1111/chd.12179. [DOI] [PubMed] [Google Scholar]
- Torrezan GT, da Silva FCC, Krepischi ACV, dos Santos ÉMM, Rossi BM, Carraro DM. A novel SYBR-based duplex qPCR for the detection of gene dosage: detection of an APC large deletion in a familial adenomatous polyposis patient with an unusual phenotype. BMC Med Genet. 2012;13:55. doi: 10.1186/1471-2350-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Karnebeek CD, Hennekam RC. Associations between chromosomal anomalies and congenital heart defects: a database search. Am J Med Genet. 1999;84:158–166. doi: 10.1002/(sici)1096-8628(19990521)84:2<158::aid-ajmg13>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- van Karnebeek CD, Quik S, Sluijter S, Hulsbeek MM, Hoovers J, Hennekam R. Further delineation of the chromosome 14q terminal deletion syndrome. Am J Med Genet. 2002;110:65–72. doi: 10.1002/ajmg.10207. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe C, Petit F, Malan V, Vincent-Delorme C, Bouquillon S, et al. 15q11.2 microdeletion (BP1-BP2) and developmental delay, behaviour issues, epilepsy and congenital heart disease: a series of 52 patients. Euro J Med Genet. 2015;58:140–147. doi: 10.1016/j.ejmg.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu Z, Liu H, Li N, Li S, et al. Rapid detection of aneuploidy and unbalanced chromosomal rearrangements by subtelomeric multiplex ligation-dependent probe amplification in fetuses with congenital heart disease. Fetal Diagn Ther. 2013;34:110–115. doi: 10.1159/000350272. [DOI] [PubMed] [Google Scholar]
- Warburton D, Ronemus M, Kline J, Jobanputra V, Williams I, et al. The contribution of de novo and rare inherited copy number changes to congenital heart disease in an unselected sample of children with conotruncal defects or hypoplastic left heart disease. Hum Genet. 2014;133:11–27. doi: 10.1007/s00439-013-1353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware SM, Jefferies JL. New genetic insights into congenital heart disease. J Clin Exp Cardiolog. 2012;S8:003. doi: 10.4172/2155-9880.S8-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Ji T, Wang J, Xiao J, Wang H, et al. Submicroscopic subtelomeric aberrations in Chinese patients with unexplained developmental delay/mental retardation. BMC Med Genet. 2010;11:72. doi: 10.1186/1471-2350-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollino M, Seminara L, Orteschi D, Gobbi G, Giovannini S, et al. The ring 14 syndrome: clinical and molecular definition. Am J Med Genet A. 2009;149A:1116–1124. doi: 10.1002/ajmg.a.32831. [DOI] [PubMed] [Google Scholar]
- Zweier M, Rauch A. The MEF2C-related and 5q14.3q15 microdeletion syndrome. Mol Syndromol. 2012;2:164–170. doi: 10.1159/000337496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data