Abstract
The mammalian Target of Rapamycin (mTOR) protein controls the machinery necessary for T-cell activation, differentiation, and memory formation, as a component of mTOR complex 1 (mTORC1) and mTORC2, which function both downstream and upstream of AKT. Invariant natural killer T (iNKT) cells are a unique T-cell subset that exists in a primed state, capable of rapid activation, and produces large quantities of cytokines. iNKT-cell effector differentiation is dependent on the mTORC1 complex; however, the requirements for mTORC2 in iNKT cells have been controversial. In this issue, Sklarz et al. [Eur. J. Immunol. 2017. 47: XXX-XXX] provide a careful analysis of the requirements for the mTORC2 component Rictor in iNKT cells, providing a new twist in this unfolding tale. The authors demonstrate that Rictor is required for iNKT-cell proliferation and survival during the key stage of intrathymic expansion and that Rictor supports the development of NKT17 cells, an effector subset which depends on the transcription factor RORγt and produces interleukin (IL)-17, in both the thymus and the lung. IL-4-producing NKT2 cells develop in the absence of Rictor but the cytotoxic potential of iNKT cells is Rictor-dependent.
Keywords: Natural killer T cell, thymus, development, cytotoxicity, differentiation, NKT17
CD1D-restricted natural killer T (NKT) cells comprise a unique T-cell lineage that displays characteristics of both adaptive and innate lymphocytes. The vast majority of these cells express a semi-invariant T-cell receptor (TCR) (Vα14-Jα18 paired with Vβ8, Vβ7 or Vβ2 in mice) [1] that recognizes lipid antigens (invariant NKT, iNKT). They branch from the conventional T-cell developmental pathway at the CD4+CD8+ (double positive, DP) stage through a positive selection process that involves homotypic DP-DP interactions, and signals from both the TCR and the Signaling Lymphocytic Activating Molecule (SLAM) family of receptors [2, 3]. This unique developmental pathway leads to the induction of the lineage-specifying transcription factor Promyelocytic Leukemia Zinc Finger (PLZF), and eventually to the generation of iNKT cells, with properties that overlap with activated T cells. Their poised effector state allows iNKT cells to produce a vast amount of various cytokines rapidly after activation, thereby influencing the functions of a number of innate and adaptive immune cells and orchestrating the early phases of an immune response [4]. Although rare, iNKT cells can modulate responses to a wide range of diseases, including microbial infection, hematopoietic malignancies, cancer, inflammation and autoimmunity, thus rendering them attractive targets in immune therapies and vaccination strategies (reviewed in [5]).
Unlike conventional T cells, which develop in a naïve state, iNKT cells acquire their effector functions concomitant with their thymic differentiation, which is accompanied by the polarization of their effector programs prior to foreign antigen encounter. At least three iNKT-cell effector subsets have been identified in the thymus [6]. Similar to conventional CD4+ T cells, these subsets are functionally defined based on the expression of the cytokines IFN-γ, IL-4 and IL-17. Consistent with their Th1-like properties, NKT1 cells express and are dependent on the Th1 signature transcription factor TBET. Th2-like NKT2 cells express high levels of GATA3, and the Th17-like NKT17 cells are positive for RORγt [6]. During their development, iNKT cells undergo a massive proliferative burst that helps to expand their numbers. Loss-of-function mutations of molecules regulating intrathymic proliferation of iNKT cells, including LEF1 [7], c-MYC [8, 9] and the IL-7 receptor a (IL7Ra) [10], have been shown to severely impair iNKT-cell development, without affecting the development of conventional T cells. This unique feature of iNKT cells contributes to their ability to function as innate-like effectors, which requires high cell numbers and rapid cytokine production; accordingly, T lymphocytes clonally proliferate only after activation, following antigen encounter. The mammalian Target of Rapamycin (mTOR) plays essential roles in multiple aspects of iNKT-cell development but there have been conflicting results on the role of mTOR complex 2 (mTORC2) [11, 12]. In the current issue of the European Journal of Immunology, Sklarz and colleagues provide insight into this controversy by demonstrating that mTORC2 regulates iNKT-cell number, cytolytic ability, and the development of NKT17 cells in the thymus and lung [13].
mTOR is a serine/threonine protein kinase that is conserved across species and integrates microenvironmental signals to multiple intracellular processes, including protein synthesis, cell growth, proliferation and metabolism [14]. mTOR exists in two protein complexes, mTORC1 and mTORC2, both of which are key effectors of the PI3K/Akt pathway. In the context of the immune system, both mTORC1 and mTORC2 are critical regulators of T-cell expansion during activation and differentiation. Naive T cells are quiescent and rely on oxidative phosphorylation and fatty acid oxidation to fulfill their energy needs. Upon activation and clonal expansion, mTORC1 and mTORC2 suppress quiescence and contribute to the upregulation of the metabolic machinery to support the increased metabolic demands of activated T cells [15]. Therefore, mTORC1 and mTORC2 are key complexes that link cellular metabolism with immune function. Given the parallels between iNKT cells and activated T cells, the authors hypothesized that mTORC activity is also required for the development and function of T cells with innate characteristics.
To test this hypothesis, the authors analyzed mice with a T-cell-specific deletion of Rictor, a scaffolding protein required for mTORC2 formation. Rictor-deficient mice lose mTORC2 activity, but retain normal mTORC1 activity. The authors show that loss of Rictor leads to a severe decrease in iNKT-cell numbers in the thymus [13], a finding that is consistent with previous studies [11, 12]. Analysis of the developmental stages showed that there is an overrepresentation of immature stage 0/1 cells at the expense of the more mature cells. Moreover, this phenotype is cell-intrinsic, as shown by mixed bone marrow chimeras. Taken together, these results suggest that positive selection of iNKT cells is intact but that post-selection mechanisms, in particular proliferation or survival, are affected by mTORC2 loss (Figure 1). Consistent with this, BrdU incorporation and Ki67 staining revealed impaired proliferation of stage 1 iNKT cells from Rictor-deficient mice. Importantly, Rictor-deficient iNKT cells could proliferate in response to cytokines but not to T-cell receptor (TCR) engagement, suggesting that mTORC2 is a downstream effector of TCR signaling. A putative target of mTORC2 that affects proliferation is p27kip1, a cyclin-dependent kinase inhibitor, which is increased in Rictor-/- iNKT cells [13], thus likely leading to cell cycle arrest. In addition to decreased proliferation, Rictor-deficient iNKT cells are more susceptible to apoptosis, both in vitro and in vivo, which could also contribute to the reduced iNKT-cell numbers.
Figure 1.
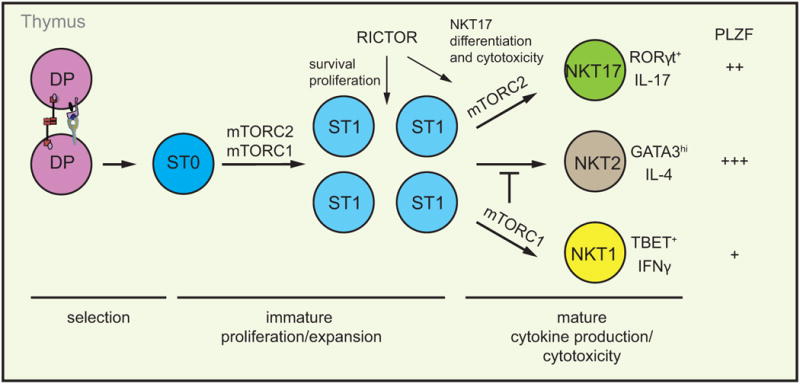
Multiple functions for mTOR in iNKT-cell development. RICTOR-deficient immature iNKT cells have a defect in proliferation and survival as measured by BrdU incorporation and binding or fluorescent caspases inhibitor. These data implicate mTORC2 in iNKT-cell expansion in addition to mTORC1. During iNKT-cell effector differentiation, mTORC1 and mTORC2 have discrete roles, with mTORC2 being necessary for the development of RORγt+PLZFint NKT17 cells and production of the signature cytokine IL-17 [13]. In addition, mTORC2 is essential for the cytolytic activity of iNKT cells, although it is not clear which subset mediates this function [13]. In contrast, mTORC1 is essential for development of TBET+PLZFlo NKT1 cells and inhibits GATA3hiPLZFhi NKT2-cell differentiation, but it is dispensable for NKT17 cells
Previous studies have produced contradictory results on the role of mTORC2 in iNKT-cell differentiation [11, 12]. While one report showed impaired IL-4, but normal IL-17 production in the absence of Rictor [12], another report demonstrated that mTORC2 is required for NKT17 lineage development, but not NKT1 or NKT2 [11]. The basis for this discrepancy is not clear, but one possibility is the genetic background of the mice, which has been shown to impact the composition of iNKT-cell effector fates [6]. The detailed analysis by Sklarz et al. is consistent with, and extends, the study of Rictor deficiency in mice purposefully backcrossed onto a C57Bl/6 background [11]. The authors showed that NKT17 development, as assessed by RORγt expression and IL-17 production, is severely compromised in the thymus and the lungs, a major site of peripheral NKT17 residency [13]. In contrast, the frequency of NKT2 cells, defined as PLZFhiGATA3hi, and NKT1 cells is similar between WT and Rictor-/- mice. Whereas IFN-γ production was normal in the thymus, IL-4 production was modestly reduced, suggesting that mTORC2 can contribute to thymic IL-4 production; however, both IL-4 and IFN-γ production was normal in peripheral tissues, including the liver and the lungs. A recent study showed that thymic NKT17 cells are able to co-produce IL-4 together with IL-17 [16]. Therefore, the loss of NKT17 cells in Rictor-/- thymi, observed in this study and by Prevot et al [12], may account for the partial loss of IL-4 production in the thymus. Precisely how mTORC2 influences the NKT17 fate is still not clear. NKT17 cells respond preferentially to IL-7 than to IL-15 during their development [17]. However, although Rictor-/- iNKT cells proliferate normally in response to IL-7, RORγt expression is still lacking, suggesting a developmental block rather than a proliferative defect in this lineage. Previous studies have shown that Th-POK [18, 19] and TET proteins [20] can regulate NKT17 development – whether these proteins act in the same pathway as mTORC2 remains to be resolved. Although cytokine production is a major function of iNKT cells, direct cytotoxicity, linked to their anti-tumor activity, has also been reported [21]. In addition to the proliferative defect and the skewing in iNKT-cell subsets, the authors showed that Rictor-deficient iNKT cells are less efficient in killing target cells after antigenic stimulation. Therefore, mTORC2 not only controls the size of the iNKT-cell pool, but also their function regarding cytokine production and cytolytic activity (Figure 1).
While differentiation of NKT17 cells requires mTORC2, it is not affected by loss of Raptor, a protein essential for formation of the mTORC1 complex [22]. In contrast, deletion of TSC1, a negative regulator of mTORC1 but a positive one for mTORC2 activity [23], promotes differentiation of NKT17 cells [24]. Moreover, while mTORC2 is essential for Th1 and Th2 differentiation [25] it appears to be dispensable for NKT1- or NKT2-cell development. Therefore, more work is warranted to decipher the molecular mechanisms that mTOR utilizes to exert its broad effects in the immune system.
Acknowledgments
M.V. is supported by a European Commission H2020-MSCA-IF grant No: 655271. B.K. is supported by grants from the National Institutes of Health (R01 AI123396, R01 AI106352, R21 AI115388, and R21 AI119894)
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180(3):1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egawa T, et al. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22(6):705–16. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Griewank K, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27(5):751–62. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11(2):131–42. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 5.Cerundolo V, et al. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9(1):28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 6.Lee YJ, et al. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14(11):1146–54. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr T, et al. The transcription factor lymphoid enhancer factor 1 controls invariant natural killer T cell expansion and Th2-type effector differentiation. J Exp Med. 2015;212(5):793–807. doi: 10.1084/jem.20141849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dose M, et al. Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proc Natl Acad Sci U S A. 2009;106(21):8641–6. doi: 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mycko MP, et al. Selective requirement for c-Myc at an early stage of V(alpha)14i NKT cell development. J Immunol. 2009;182(8):4641–8. doi: 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- 10.Tani-ichi S, et al. Interleukin-7 receptor controls development and maturation of late stages of thymocyte subpopulations. Proc Natl Acad Sci U S A. 2013;110(2):612–7. doi: 10.1073/pnas.1219242110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei J, Yang K, Chi H. Cutting edge: Discrete functions of mTOR signaling in invariant NKT cell development and NKT17 fate decision. J Immunol. 2014;193(9):4297–301. doi: 10.4049/jimmunol.1402042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prevot N, et al. Mammalian target of rapamycin complex 2 regulates invariant NKT cell development and function independent of promyelocytic leukemia zinc-finger. J Immunol. 2015;194(1):223–30. doi: 10.4049/jimmunol.1401985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sklarz T, et al. mTORC2 regulates multiple aspects of NKT-cell development and function. Eur J Immunol. 2017 doi: 10.1002/eji.201646343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell JD, et al. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev. 2012;249(1):43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgiev H, et al. Distinct gene expression patterns correlate with developmental and functional traits of iNKT subsets. Nat Commun. 2016;7:13116. doi: 10.1038/ncomms13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webster KE, et al. IL-17-producing NKT cells depend exclusively on IL-7 for homeostasis and survival. Mucosal Immunol. 2014;7(5):1058–67. doi: 10.1038/mi.2013.122. [DOI] [PubMed] [Google Scholar]
- 18.Engel I, et al. The transcription factor Th-POK negatively regulates Th17 differentiation in Vα14i NKT cells. Blood. 2012;120(23):4524–32. doi: 10.1182/blood-2012-01-406280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enders A, et al. ZBTB7B (Th-POK) regulates the development of IL-17-producing CD1d-restricted mouse NKT cells. J Immunol. 2012;189(11):5240–9. doi: 10.4049/jimmunol.1201486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsagaratou A, et al. TET proteins regulate the lineage specification and TCR-mediated expansion of iNKT cells. Nat Immunol. 2017;18(1):45–53. doi: 10.1038/ni.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui J, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278(5343):1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, et al. Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function. J Immunol. 2014;193(4):1759–65. doi: 10.4049/jimmunol.1400769. [DOI] [PubMed] [Google Scholar]
- 23.Yang K, et al. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12(9):888–97. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, et al. iNKT cells require TSC1 for terminal maturation and effector lineage fate decisions. J Clin Invest. 2014;124(4):1685–98. doi: 10.1172/JCI69780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–53. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


