Abstract
This article deals with practices related to cytotoxic drug dispersal, cytotoxic safety, and cytotoxic waste management and attempts at India-specific guidelines for their dispersal and disposal. The articles related to cytotoxic drug dispersal, cytotoxic safety, and cytotoxic waste management were reviewed from PubMed and their applicability in Indian health-care facilities (HCFs) was also reviewed. All HCFs dealing with cytotoxic drugs should consider cytotoxic policy, patient safety and health-care worker safety, and environmental monitoring program as per the available international guidelines customized as per Indian conditions. Utmost care in handling cytotoxic waste is quintessential. The formation of India-specific cytotoxic guidelines requires the inputs from all stakeholders. Cytotoxic waste, cytotoxic safety, and cytotoxic waste management should be the subject of a national strategy with an infrastructure, cradle-to-grave legislation, competent regulatory authority, and trained personnel.
Keywords: Cytotoxic drug dispersal, cytotoxic safety, cytotoxic waste
Introduction
Biomedical waste of genotoxic category is extremely hazardous to environment and human health owing to its mutagenic, teratogenic, or carcinogenic properties.[1] The disposal of genotoxic waste raises serious safety issues, both inside hospitals and after disposal, and should be given utmost attention. It includes cytotoxic drugs, vomit, urine, or feces from patients treated with cytotoxic drugs.[2,3,4,5] Technically, genotoxic means toxic to the deoxyribonucleic acid. Cytotoxic drugs are used in the therapy of various neoplastic conditions, organ transplantation, and collagen vascular disorders and are used in oncology, radiotherapy, transplant, and immunology units.[1,2,5] Their use in other hospital departments and outside the hospital in clinics and elsewhere is also increasing owing to increase in the incidence of carcinomas, transplants, and immunological diseases.[1,2,3,4,5,6]
The articles related to cytotoxic drug dispersal, cytotoxic safety, and cytotoxic waste management were reviewed from PubMed and their applicability in Indian health-care facilities (HCFs) was also reviewed. This article deals with practices related to cytotoxic waste, cytotoxic safety, and cytotoxic waste management and attempts at India-specific guidelines for their dispersal and disposal.
Sources of Cytotoxic Waste
Cytotoxic wastes are generated from protean sources such as manufacturing waste, home care waste, contaminated materials from drug preparation and administration (expired medicines, left-over drugs, returned drugs, syringes, needles, gauzes, vials, packaging; samples such as urine, feces, and vomit from patients), containing hazardous amounts of the cytotoxic drugs and their metabolites which are genotoxic for 48 h to 1 week after drug administration. In specialized oncological hospitals, genotoxic waste (containing cytostatic or radioactive substances) may constitute as much as 1% of the total health-care wastes.[2,3,4,6] These are classified by working groups of the International Agency for Research on Cancer.[1,3,5]
Hazards from Cytotoxic Waste
The severity of the hazards for health-care workers (HCWs) responsible for the handling or disposal of cytotoxic waste is mainly attributed to its toxicity and the extent and duration of exposure. Exposure to cytotoxic substances in HCFs occurs during the preparation of, or treatment with drugs or these drugs occurring through inhalation of dust or aerosols, absorption through the skin, ingestion of food contaminated with cytotoxic drugs, ingestion owing to unsafe practices, or from waste items. Exposure may also occur through contact with body fluids and secretions of patients undergoing chemotherapy. Experimental studies have shown that many antineoplastic drugs are carcinogenic and mutagenic and secondary neoplasia is well documented in literature.[1,2,3]
A study from Finland observed increased incidence of spontaneous abortions during pregnancy and malformations in children of females with a history of working with anticancer agents. Similar results were found in studies from Canada and the United States of America.[1,3,4,6] A prior study demonstrated that exposure of personal cleaning hospital urinals exceeded that of nurses and pharmacists owing to less awareness of risks by lower staff.[1]
Many cytotoxic drugs are extreme irritants and have harmful local effects after direct contact with skin or eye, dizziness, nausea, headache, or dermatitis. Any discharge of genotoxic waste into the environment has disastrous ecological consequences in the form of persistent land, air, and water pollution.[3]
Cytotoxic Waste Minimization
Cytotoxic waste should first be minimized by careful segregation, purchasing optimal drug quantities, using proper spill containment and clean-up procedures, and substituting environmentally persistent drugs with degradable drugs (green chemistry and green procurement), where possible.
Cytotoxic Waste Disposal Techniques
Cytotoxic waste is highly hazardous and should never be landfilled or discharged into the sewerage system. The disposal options include: Return to the original supplier or take-back program, incineration at high temperatures, and chemical degradation in accordance with manufacturer's instructions. Full destruction of all cytotoxic substances requires incineration temperatures up to 1200°C and a minimum gas residence time of 2 s in the secondary chamber with gas-cleaning equipment. Incineration at lower temperatures may release hazardous cytotoxic vapors into the atmosphere. Incineration in most incinerators, in single-chamber incinerators or by open-air burning, is hazardous for the disposal of cytotoxic waste.[1] Chemical degradation methods require expertise and are customized for drug residues and for cleaning contaminated urinals, spillages, and protective clothing.[4,6,7,8,9] They are not appropriate for treating contaminated body fluids. In settings where high-temperature incineration or chemical degradation is not feasible, encapsulation or inertization or alkaline hydrolysis can be considered. The novel technologies for cytotoxic waste disposal include global environmental facility technology reactor, alkaline hydrolysis, and thermophilic anaerobic digestion.[1]
Table 1 depicts cytotoxic waste disposal techniques.
Table 1.
Cytotoxic waste disposal techniques/methodology

Cytotoxic drugs and their waste should be properly identified with the capital “C” symbol and under it, the words “cytotoxic” in capital letters and disposed off in appropriate disposal bag or containers [Figure 1a and b].
Figure 1.
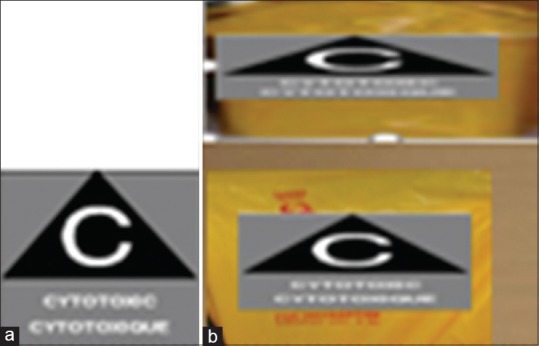
(a) Cytotoxic label (b) Cytotoxic container and cytotoxic bag for final disposal with cytotoxic label
Target Population
The target population for this guideline is HCWs, hospital administrators, drug industry workers, educators, and managers, occupational health and safety services and pharmacy workers.
Recommendations
Cytotoxic committee
All HCFs administering cytotoxic drugs should form a committee responsible for policy and procedures for cytotoxic drugs. This committee should include representatives from various departments and support services (occupational health and safety, joint health and safety committee, pharmacy, nursing, medical oncology [physician], environmental services, and risk management). This committee is accountable for drafting, developing, reviewing, and revising policies and procedures related to cytotoxic drugs. The committee undertakes continued medical education, training for implementation, and follow-up of the risk prevention management program related to the use of cytotoxic drugs.
Hierarchy of Controls
The U.S. Centers for Disease Control and Prevention documents that controlling exposures to occupational hazards is the fundamental method of protecting workers.[2]
The feasible and effective controls are elimination, substitution, engineering controls, administrative controls, and use of personal protective equipment (PPE). Engineering controls are used as a barrier between the worker and the hazard (biosafety cabinets and safety-engineered medical devices – safety-engineered needles). Administrative controls include policies and procedures and staff education and training. The appropriate and adequate selection and use of PPE for protection against exposure to cytotoxic drugs ensures health care worker safety. Appropriate PPE consists of gloves, splash proof gowns, and eye protection goggles, boots, etc., when essential [Figure 2a].[2,3,10]
Figure 2.

(a) A health-care worker wearing appropriate and adequate personal protective equipment for handling cytotoxic spill. (b) Cytotoxic spill kit: Cytotoxic container, cytotoxic bag, cytotoxic degrading agent, hypochlorite solution, water, personal protective equipment as shown, absorbent, forceps, scissors, tape, marker pen, dust pan, shovels, mops, securing signage, tape, appropriate disposal bag
Safe Handling of Cytotoxics
Continuing medical education program
Initial and ongoing hospital-approved education should be provided to all staff involved with cytotoxic drugs in HCFs, including education for safe handling and spill or leak management. There should be a record that initial and annual training for safe handling of cytotoxic drugs has occurred.[8,11]
Identification and safety
Each institution should maintain a log book of cytotoxic drugs. Cytotoxic drugs and their waste should be properly identified with the capital “C” symbol and under it, the word “Cytotoxic” in capital letters [Figure 1a].[11,12]
Purchasing cytotoxic drugs
When procuring cytotoxic drugs, HCFs should include in their rate tender contract vendors that include safe handling measures such as prewiped or protective containers, or smaller receptacles to decrease the volume of potential spills.
Spill kit
A spill management kit should be available in all areas of HCFs where cytotoxic drugs are stored, transported, handled, and administered. Figure 2 depicts the cytotoxic spill kit and its constituents.
Staff safety
All staff should be fully aware of the potential reproductive hazards of cytotoxic drugs. The HCFs should consider alternative duties for women who are pregnant or breastfeeding.[1]
Personal Protective Equipment
Workers should work in compliance with the Occupational Health and Safety Act of the country and regulations and use or wear the PPE, protective devices, or clothing that the employer requires to be used.[13,14,15,16]
The appropriate PPE for the task should be donned throughout the medication circuit [Figure 2a].[13] It is the HCFs’ responsibility to provide the necessary and adequate PPE and training on how to use the equipment. The gloves used to handle cytotoxic drugs should comply with ASTM standard D-6978-(05)-13 and be powder free. Gloves used are recommended to be made of nitrile, polyurethane, neoprene, or latex. Vinyl gloves should not be used.[14] The frequency of glove changes can be adjusted according to the level of exposure at each step in the drug dispersal circuit. For example, when administering reconstituted medications, workers should change gloves immediately if the gloves become torn, punctured, or visibly contaminated with a cytotoxic drug, and should ensure to follow routine practices.[14] Great care should be taken in the removal of gloves so as not to contaminate the skin. When two pairs of gloves are required, put on the first pair before putting on the gown. Gowns used for handling cytotoxic drugs should be disposable; should be made of lint free, low-permeability fabric; should have long sleeves with tightfitting cuffs; and should fasten in the back. Gowns have to be changed in the event of contamination, spillage, or tear, and at the completion of the procedure (changed halfway through a shift or every 3.5 h).[15] The supplier should be able to certify that the gown protects against cytotoxic drugs. Care must be taken to avoid contamination of the hands by avoiding touching the outside of the gown when removing it. For facial protection surgical masks are required when handling and preparing medications in a biological safety cabinet with high-efficiency particulate air (HEPA) filters [Figure 3] and are worn to prevent microbial contamination of the sterile field. Full-face protection mask should be worn whenever a risk of splashing is present. If goggles are used, they have to be worn along with a fluid-resistant mask.[16] Fit-tested respirators such as those certified N95 or N100 and should be used in accordance with the Canadian Standards Association standard or by the U.S. National Institute for Occupational Safety and Health guidelines or CE approved.[2,17] Caps are required only in the sterile preparation room and are worn to prevent microbial contamination of the sterile field. Disposable shoe covers are donned to prevent contamination and covers should be worn when in the sterile preparation room or in the event of a spill. Shoe covers should be removed immediately when leaving the sterile preparation room to avoid contamination of other areas.
Figure 3.

Biosafety hood with high-efficiency particulate air filter
Receiving and Transport
All receiving workers should receive training in the safe handling of cytotoxic drugs. The receiving workers should check the external packaging upon receipt; in the event of a damaged parcel likely to cause a spill, apply the spill protocol of the hospital. Delivery containers should be taken immediately to the pharmacy department by the receiving workers. The receiving or storeroom workers should not open the delivery containers. The delivery containers should be handled with care to avoid breakage of the cytotoxic drug containers. Only trained workers should proceed with the unpacking and subsequent steps. Damaged containers should be treated as cytotoxic spills and should be notified to pharmacy and manufacturer or distributor.
Unpacking and Storage
Packaging can have high levels of contamination. The pharmacy should have unpacking area to limit exposure risks. The unpacking area should be a separate dedicated room, separate from eating areas. There should be adequate ventilation in the area, negative pressure, and preferably vented to the outside and it should have a receptacle for the disposal of secondary packing.[17,18] Workers should wear a protective gown and two pairs of gloves when unpacking and cleaning cytotoxic drugs, from the opening of the external packaging to the placing of the secondary or primary packaging (or both) in their storage space. Workers should check the integrity of all packaging at each step of the unpacking process. The primary and secondary packaging should be cleaned before being placed in storage. A regular cleaning protocol should be in place either at this stage or before storage in the clean room. All drug containers should be cleaned to reduce external contamination and use premoistened towelettes. The cleaning procedure should not damage the containers or labels and also should not increase the risk of incidents or accidents resulting from damage to the cytotoxic drug container or label. Procedures should be in place to reduce the risk of contamination of surfaces during the cleaning of vials. All surfaces should be cleaned when the task is complete. Establish a dedicated negative pressure storage area for cytotoxic drugs that minimizes the risk of contamination.[18,19] When removing or transporting drugs out of the storage area, one pair of gloves and a gown should be worn.
Cytotoxic drug preparation
The oncology pharmacy should be in compliance with standard guidelines.[2,3,18,19] Special requirements for heating, ventilation, and air-conditioning systems in HCF should be taken into consideration. All mixing and preparation of administration sets with a cytotoxic drug should be performed in one centralized area in a specially designated class II, type B (preference for the type B2 to prevent recirculation) biological safety cabinet that is exhausted through a HEPA filter to the outside atmosphere to prevent recirculation into any inside area. It should have an exhauster fan and ventilation systems and should be equipped with a continuous monitoring device to permit confirmation of adequate airflow and cabinet performance with airlocks [Figure 3]. The plan of the room should allow for and facilitate the cleaning of all surfaces (walls, floors, ceilings, doors, diffusers, and windows). The furniture and other equipment should be minimum. The sterile preparation room and the pharmacy should have a visual link window for monitoring. Access to the sterile room should be limited to trained and authorized workers. Limit worker traffic, particularly near unpacking and storage areas and near preparation cabinets. It should have an emergency eyewash station (minutes of flushing to both eyes) and a shower facility.[13,18,20] Biological safety cabinets should remain in operation 24 h per day, 7 days per week, as recommended by the manufacturers. In the nonsterile drug preparation process (oral preparations), the same level of worker protection should be strictly followed.
Pharmacy policies
In drug preparation, the following recommendations apply to the preparation of all cytotoxic medications, parenteral, oral, and topical, and both sterile and nonsterile. Policies and procedures should include appropriate PPE; equipment for preparation, including appropriate ventilation; other automated equipment for packaging; and a dedicated work area. The pharmacists or pharmacy technicians should wear a cap, surgical or procedure mask, shoe covers, a protective gown, and two pairs of gloves to make sterile preparations of cytotoxic drugs in preparation cabinets. They should arrange the work to limit microbial and environmental contamination. For both sterile and nonsterile preparations, workers should cover the work surface with a disposable absorbent plastic-backed sterile pad to absorb any liquid contamination that may occur during handling. The pad should not cover the front and rear parts of the preparation cabinet. It should be changed after 3.5 h of continuous work or for a new batch of preparations or in case of a spill or contamination. A cytotoxic waste receptacle should be used for pad disposal.[21] Limit the quantity of supplies and cytotoxic drugs in the cabinet to avoid adversely affecting the laminar flow and to facilitate regular cleaning of the work surface; place the sterile products in the center and the nonsterile products along the sides of the cabinet. Remove the packaging, when applicable, and clean all the drug containers before taking them into the preparation cabinet. For sterile preparations, adhere to aseptic technique for sterility.
Handling techniques
Use handling techniques that limit the risk of injury or accidental exposure. The spiking of bags and priming of tubing should occur before the addition of the cytotoxic drug unless the clinical protocol requires otherwise. Preparation, priming, and removing air from tubing cytotoxic drugs should be reconstituted in the pharmacy environment as already described. The drug containers should not be overfilled to avoid compromising the integrity of the container. Air should never be removed from intravenous tubing with a solution containing the drug. Intravenous tubing should be primed and air should be removed in the pharmacy, before the cytotoxic drug or drugs are added to the infusion solution.
Labeling and Final Packaging
Cytotoxic drugs should be labeled to inform those handling these preparations of the nature of the drugs and the precautions to be taken. Cytotoxic drugs should display the “Cytotoxic” hazard symbol or the word “Cytotoxic.”[11,12,13] The outside surface of cytotoxic drug containers (for example, syringes, infusion bags, and tubing) in the preparation cabinet should be cleaned in the cabinet. Place each cytotoxic drug container (for example, syringe, bag) as well as the administration supplies (for example, tubing) in a clear, leakproof plastic bag (Ziploc) to facilitate identification by the nurse without having to remove the container from the bag. After final verification, the plastic bags containing the cytotoxic drugs should be placed in a rigid transport container (opaque), properly identified with the “Cytotoxic” hazard symbol.
Everything that comes out of the cabinet should be wiped clean. All contaminated waste should be disposed of in the chemotherapy waste stream.
On-Site Transport of Cytotoxic Drugs
Cytotoxic drugs should be placed in a closed, leakproof nonchlorinated plastic bag and the cytotoxic drug from the pharmacy should be transported to an area not adjacent to the preparation area in a rigid, leakproof nonchlorinated container made of a material that can be easily cleaned and decontaminated in the event of a drug leak. The bottom of the container should be covered with an absorbent nonchlorinated plastic-backed cloth. The transport container should be identified with the “Cytotoxic hazard symbol” [Figure 1] and should be cleaned regularly.[21] Pneumatic tubes are not recommended owing to potential spills. Prepared medications should be stored in an identified area before administration, and this area should be cleaned regularly.
Off-Site Shipping and Transport of Cytotoxic Drugs
Establish policies and procedures regarding the shipping of cytotoxic drugs.[22] In the event that cytotoxic drugs are shipped offsite, they should be packed separately from other drugs, according to the recommendations of the manufacturer. Pharmacy should be consulted about the packaging of cytotoxic drugs. Cytotoxic drugs should be packed in double plastic bags and placed in a box that is properly identified with the “Cytotoxic” hazard symbol [Figure 1]. If necessary, the drug should be immobilized with packing material.[23] Reusable delivery containers should be cleaned regularly.[21] In drug administration, safe handling and safe administration techniques should be used to minimize possible exposure to individuals and the environment.[11] Appropriate PPE should be made available to all HCWs and should be donned as prescribed by the employer. Luer-lock connectors and needleless administration systems should be used to administer intravenous medications. Disposable plastic-backed absorbent pads should be used over work surfaces and must be placed under tubing or bag connections and ports when attaching any tubing, bag, or syringe that has been exposed to a cytotoxic drug. Unless a closed system is used, never disconnect tubing from cytotoxic drug bags. Discard the bag with its attached tubing into an appropriate waste container as a single unit.[11,13] Safety-engineered needles should be used.[1] Do not purge air from the needle before administration. Oral cytotoxics should be handled in a manner that avoids skin contact, liberation of aerosols or powdered medicine into the air, and cross-contamination with other medicines.[24] Solid oral preparations of cytotoxic drugs should be crushed within the biological safety cabinet. If patients are unable to take in the solid format, the pharmacy should provide these drugs in an oral syringe, in a ready-to-use liquid oral form. Application of topical cytotoxic drugs should be done using appropriate PPE to avoid contamination of the environment. Between applications, the cytotoxic medication (tube or jar) should be kept in a safe container (Ziploc bag) and in a secure place that prevents contamination of the surrounding environment.
Administration of Cytotoxic Drugs in Home-Care Patients
For home care of patients who have received cytotoxic drugs, these preparations should be compounded in pharmacies meeting the requirements for cytotoxic drug preparation. Cytotoxic drugs should be transported, administered, and disposed of by individuals who have received appropriate training. Health-care providers should follow the same recommendations as recommended for admitted patients. Health-care providers who administer cytotoxic drugs in the home should wear PPE. Patients should be informed of and must be provided with written instructions for their safe handling. The institution should have a clear process to address the issue of cytotoxic waste from patients in their homes, in compliance with national biomedical waste rules.[12] HCFs should provide education to patients and caregivers involved in administering cytotoxic drugs in the home with a process for the appropriate disposal of cytotoxic waste, including leftover drugs.[13] A spill kit should be readily available in the home in case of accidental spills. Bodily fluid workers who handle the biologic fluids, excreta, contaminated bedding, and soiled equipment of patients who have received cytotoxic drugs should wear a pair of gloves and a protective gown, face protection mask should be worn when there is a risk of splashing.[13]
Cytotoxic Waste Disposal
Cytotoxic drug waste disposal and policies need to be established as per the existing national BWWM rules.[12] The term “cytotoxic waste” includes any material that comes in contact with cytotoxic drugs during their storage, handling, preparation, administration, and disposal (packaging material; protective equipment; preparation supplies such as syringes, tubing, drug bags, soiled disposable incontinence briefs of patients who have received cytotoxic drugs during the previous 48 h, hood prefilters, and HEPA filters). Cytotoxic waste should be placed in a waste container clearly identified with the “Cytotoxic” hazard symbol.[20]
Other waste (soft items such as tubing and protective equipment) should be also placed in leakproof and tear-resistant containers identified with the “Cytotoxic” hazard symbol. For final disposal outside the institution, all cytotoxic waste should be in rigid leakproof containers identified with the “Cytotoxic” hazard symbol and scheduled for transport outside the institution.[20] Any excess fluid from cytotoxic drugs should be disposed off in a sealed container and placed in a rigid container, the bottom of which is to be covered with an absorbent pad. This rigid container will be handled like other cytotoxic waste.[21] Disposable incontinence briefs soiled by patients who have received cytotoxic drugs should be placed in a cytotoxic waste container. Cytotoxic waste should be incinerated at a high temperature (equal to or above 1200°C).[7,12,20] Every area in which cytotoxic drugs are handled will have an appropriate cytotoxic waste receptacle as close as possible to the work area.[20] The lids of cytotoxic drug receptacles must remain closed, except when depositing waste. Bins with foot pedals and lids, which lock automatically when full, are recommended to minimize exposure. Workers should be careful to avoid contaminating the outside of the receptacle when depositing waste. The transport of cytotoxic waste receptacles should be assigned to trained workers. Workers who handle cytotoxic waste receptacles should wear one pair of disposable gloves and have a spill kit at their disposal. The waste should travel through as few care units, public areas, and areas containing food or linens as possible. The final storage areas for cytotoxic waste receptacles should be secure.[1,12,20]
Recommendations suggested for cytotoxic drug dispersal and cytotoxic waste disposal are summarized in Table 2.
Table 2.
Recommendations for safe handling of cytotoxic drugs

Accidental Exposure to Cytotoxic Drugs
HCFs have to frame policies and procedures regarding accidental worker exposure as per standard guidelines.[1,13] If a cytotoxic drug accidentally comes into contact with a worker's skin or clothing, the worker should immediately remove the contaminated clothing, thoroughly wash the skin of the affected area with soap and water, and continue to rinse for 15 min and take a shower, wherever applicable. All contaminated clothing should be discarded in the cytotoxic waste. If a cytotoxic drug comes into contact with a worker's eyes, the worker should flush his/her eyes at an eyewash station. An isotonic solution (sterile NaCl 0.9%) can be used to flush eyes for at least 15 min.[13] In the event of a needlestick or sharps injury, it should be treated as per HCF policy made as per standard guidelines.[1] A spill management kit should be readily available within the work area. Items used during cleanup of a spill should be placed into the cytotoxic waste receptacle.[21] Most spills (leaking intravenous tubing) can be contained and managed by a trained HCW. When a spill is not contained or easily managed (a large volume of fluid or powder that is a risk to the environment), an emergency code should be called.[21]
Environmental Cleaning
HCFs have to establish environmental cleaning policies and procedures for all surfaces where contact with cytotoxic drugs may occur. Cleaning of the biological safety cabinets should be performed by trained personnel following manufacturers’ guidelines.[25,26]
HCFs have to ensure that there is an appropriate policy to clean and inspect the equipment (like drug delivery pumps) and laundry between uses.[11]
Medical Surveillance
Medical surveillance methods used to investigate the potential health effects of exposure to cytotoxic drugs should meet several requirements: sensitive, specific, quantitative, rapid, and reproducible and noninvasive. However, there is currently no suitable test to meet these requirements. As a consequence, there is conflicting information and opinion about the value of routine biological monitoring for employees handling cytotoxic drugs. Further research is warranted on such tests which determine whether there are adverse health effects that result from exposure to cytotoxic drugs.
Adherence to agreed standard operating procedures, with sufficient initial and regular ongoing training in safe handling and administration, is paramount in reducing the potential for exposure and risk.[1,3,4,6,27] There are no other identified medical conditions known to result from chronic exposure of HCW to cytotoxic drugs, no exposure limits set for cytotoxic drugs, and no standards for interpretation of test results of exposed HCW to enable meaningful interpretation or action based on biological monitoring results. Environmental monitoring policy would include surface testing, which would audit contamination of the environment (for example, pharmacy counters, patient bedside tables) and provide a quality indicator of cleaning effectiveness and adherence to recommended work.[9]
All HCFs dealing with cytotoxic drugs should consider cytotoxic policy, patient safety, and HCW safety and environmental monitoring program as per the available international guidelines customized as per Indian conditions. The formation of India-specific cytotoxic guidelines requires the inputs from all stakeholders such as representatives from the Ministry of Environment Forest and Climate Change, Ministry of Health and Family Welfare, Apex HCFs from Central and State Institutes, Indian Council of Medical Research, Drug Controller General of India, nongovernmental organizations, Indian Medical Association, and Central and State Pollution Control Board. Cytotoxic waste, cytotoxic safety, and cytotoxic waste management should be the subject of a national strategy with an infrastructure, cradle-to-grave legislation, competent regulatory authority, and trained personnel.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chartier Y, Emmanuel J, Pieper U, Prüss A, Rushbrook P, Stringer R, et al., editors. Management of Wastes from Health-Care Activities’ WHO Blue Book. 2nd ed. Malta: World Health Organization; 2014. [Google Scholar]
- 2.NIOSH Publications. Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings. Publication Number 2004-165. Cincinnati, OH: NIOSH Publications; 2004. [Last assessed on 2017 Apr 25]. Available from: http://www.cdc.gov/niosh . [Google Scholar]
- 3.Cytotoxic Drugs and Their Waste: Risk Management Guide for South Australian Health Services. 2015. [Last assessed on 2017 Apr 25]. Available from: http://www.sahealth.sa.gov.su .
- 4.IARC (International Agency for Research on Cancer). Laboratory Decontamination and Destruction of Carcinogens in Laboratory Waste: Some Hydrazines. IARC Scientific Publications No. 54. Lyon: International Agency for Research on Cancer; 1983. [PubMed] [Google Scholar]
- 5.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. List of IARC Evaluations. Lyon: International Agency for Research on Cancer; 1994. [Google Scholar]
- 6.IARC. Laboratory Decontamination and Destruction of Carcinogens in Laboratory Waste: Some Antineoplastic Agents. IARC Scientific Publications No. 73. Lyon: International Agency for Research on Cancer; 1985. [PubMed] [Google Scholar]
- 7.Hansel S, Castegnaro M, Sportouch MH, De Méo M, Milhavet JC, Laget M, et al. Chemical degradation of wastes of antineoplastic agents: Cyclophosphamide, ifosfamide and melphalan. Int Arch Occup Environ Health. 1997;69:109–14. doi: 10.1007/s004200050124. [DOI] [PubMed] [Google Scholar]
- 8.Castegnaro M, De Méo M, Laget M, Michelon J, Garren L, Sportouch MH, et al. Chemical degradation of wastes of antineoplastic agents 2: Six anthracyclines: Idarubicin, doxorubicin, epirubicin, pirarubicin, aclarubicin, and daunorubicin. Int Arch Occup Environ Health. 1997;70:378–84. doi: 10.1007/s004200050232. [DOI] [PubMed] [Google Scholar]
- 9.Easty AC, Coakley N, Cheng R, Cividino M, Savage P, Tozer R, et al. Safe handling of cytotoxics: Guideline recommendations. Curr Oncol. 2015;22:e27–37. doi: 10.3747/co.21.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United States, Department of Health and Human Services, Centers for Disease Control and Prevention (CDC), National Institute for Occupational Health & Safety. Workplace Safety and Health Topics: Engineering Controls. Atlanta, GA: CDC; 2014. [Last cited on 2014 May 08]. Available from: http://www.cdc.gov/niosh/topics/engcontrols/ [Google Scholar]
- 11.Service Ontario, e-Laws. Occupational Health and Safety Act. Ontario Regulation 67/93: Health Care and Residential Facilities. Toronto, ON: Government of Ontario; 2013. [Last cited on 2014 May 08]. Available from: http://www.e-laws.gov.on.ca/html/regs/english/elaws_regs_930067_e.htm . [Google Scholar]
- 12.Bio-Medical Waste Management Rules, 2016. Published in the Gazette of India, Extraordinary, Part II, Section 3, Sub-section (i), Government of India Ministry of Environment, Forest and Climate Change. Notification; New Delhi; the 28th March, 2016 [Google Scholar]
- 13.Service Ontario, e-Laws. Occupational Health and Safety Act. RSO 1990, Ch. 1. Toronto, ON: Government of Ontario; 2011. [Last cited on 2013 Jul 31]. Available from: http://www.elaws.gov.on.ca/html/statutes/english/elaws_statutes_90o01_e.htm . [Google Scholar]
- 14.ASTM International. ASTM D6978-05: Standard Practice for Assessment of Resistance of Medical Gloves to Permeation by Chemotherapy Drugs. West Conshohocken, PA: ASTM International; 2013. [Google Scholar]
- 15.Ontario Agency for Health Protection and Promotion, Provincial Infectious Diseases Advisory Committee. Routine Practices and Additional Precautions. 3rd ed. Toronto, ON: Queen's Printer for Ontario; 2012. [Last cited on 2014 May 08]. Available from: http://www.publichealthontario.ca/en/eRepository/RPAP_All_HealthCare_Settings_Eng2012.pdf . [Google Scholar]
- 16.ASTM International. ASTM F739-12 Standard Test Method for Permeation of Liquids and Gases through Protective Clothing Materials under Conditions of Continuous Contact. West Conshohocken, PA: ASTM International; 2012. p. 13. [Google Scholar]
- 17.Accreditation Canada. Accreditation Canada. Medication Management Standards: For Surveys Starting After: January 01. Ver. 8. Ottawa, ON: Accreditation Canada; 2013. [Google Scholar]
- 18.CSA Group. Canadian Health Care Facilities. CSA Z8000-11. Toronto, ON: CSA Group; 2011. [Google Scholar]
- 19.Public Health Agency of Canada (PHAC). Laboratory Biosafety Guidelines. 3rd ed. Ottawa, ON: PHAC; 2004. [Google Scholar]
- 20.Service Ontario, e-Laws. Occupational Health and Safety Act. Ontario Regulation 474/07: Needle Safety. Toronto, ON: Government of Ontario; 2010. [Last cited on 2013 Jul 31]. Available from: http://www.e-laws.gov.on.ca/html/regs/english/elaws_regs_070474_e.htm . [Google Scholar]
- 21.Ontario, Ministry of the Environment. Guideline C-4: The Management of Biomedical Waste in Ontario. Toronto, ON: Government of Ontario; 2009. [Last cited on 2013 Jul 30]. Available from: https://www.dr6j45jk9xcmk.cloudfrontl.net/documents/1761/197-bio medical-waste-en.pdf . [Google Scholar]
- 22.Acts and Regulations can be Searched. Ottawa, ON: Transport Canada; 2014. [Last cited 2014 May 08]. Transport Canada. Transportation of Dangerous Goods. Available from: http://www.tc.gc.ca/eng/tdg/safety-menu.htm . [Google Scholar]
- 23.International Society of Oncology Pharmacy Practicioners Standards Committee. ISOPP standards of practice. Safe handling of cytotoxics. J Oncol Pharm Pract. 2007;13(Suppl 1):1–81. doi: 10.1177/1078155207082350. [DOI] [PubMed] [Google Scholar]
- 24.Roos I, Makela T. Human resource issues in cytotoxic drug dispensing. J Oncol Pharm Pract. 1997;3:200–18. [Google Scholar]
- 25.Canadian Association of Pharmacy in Oncology (CAPHO). Standards of Practice for Oncology Pharmacy in Canada. Ver. 2. North Vancouver, BC: CAPHO; 2009. [Google Scholar]
- 26.NSF International. Biosafety Cabinetry: Design, Construction, Performance, and Field Certification. NSF/ANSI 49-2008. Ann Arbor, MI: NSF International; 2008. [Last cited on 2014 May 08]. Available from: http://www.standards.nsf.org/apps/group_public/download.php/3604/NSF_49–08e-rep-watermarked.pdf . [Google Scholar]
- 27.Lawson CC, Rocheleau CM, Whelan EA, Lividoti Hibert EN, Grajewski B, Spiegelman D, et al. Occupational exposures among nurses and risk of spontaneous abortion. Am J Obstet Gynecol. 2012;206:327.e1–8. doi: 10.1016/j.ajog.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


