Abstract
Primary angiosarcoma of the breast is a highly aggressive but rare malignant neoplasm. Palliative chemotherapy with different agents and combinations has been tried in the metastatic setting with poor results. We present the case of a young woman with this disease describing her aggressive course. We used a metronomic combination of oral drugs due to her poor general condition and achieved disease stabilization although not durable.
Keywords: Angiosarcoma, metronomic, propranolol
Introduction
Angiosarcoma of the breast may be primary or secondary to lymphedema or radiation. Pregnancy-related angiosarcoma is rarer. This disease follows an aggressive malignant course, and the relapsing metastatic disease is a nightmare for a medical oncologist. We present here the case of a young female with pregnancy-associated angiosarcoma who progressed while on adjuvant treatment and could be stabilized on oral metronomic chemotherapy in combination with a nonspecific beta-adrenergic blocker propranolol although the response did not remain durable.
Case Report
A 36-year-old female presented during the third trimester of her pregnancy with a progressive lump in her left breast. A trucut biopsy showed features of angiosarcoma of the breast. She delivered a healthy baby and underwent a modified radical mastectomy. It was a 10 cm mass with 1/15 LN+. Adjuvant postoperative radiotherapy was given 60 Gy/30#/6 weeks. In view of its high-risk behavior, adjuvant paclitaxel was planned in weekly fashion.
During her adjuvant chemotherapy, on d15, she presented with progressive abdominal distension, bipedal edema, jaundice, and dyspnea on exertion.
Clinically, she was pale, icteric, had bilateral pedal edema and dullness on percussion at the left base. Her Eastern Cooperative Oncology Group performance status (PS) was 3. She had a hemoglobin of 4.3 g/dl, total leukocyte count 14,100/mm3, platelet 135,000/mm3. Serum creatinine was 0.5 mg/dl. Serum bilirubin was 4.46 mg/dl (conjugated = 2.49 mg/dl). Serum albumin = 2.54 g/dl, aspartate aminotransferase/alanine aminotransferase = 128/82 U/L, serum alkaline phosphatase = 759 U/L. On evaluation, her positron emission tomography computed tomography (CT) scan showed left-sided pleural effusion with passive collapse of left lower lobe, no ascites, but multiple hypodense nodules (largest 10 cm) in an enlarged liver [Figure 1]. There were no significant uptake on FDG -PET Scan or DOTANOC -PET Scan. There were no intrahepatic biliary radicle dilatation. There were ill-defined lytic lesions in multiple visualized bones.
Figure 1.
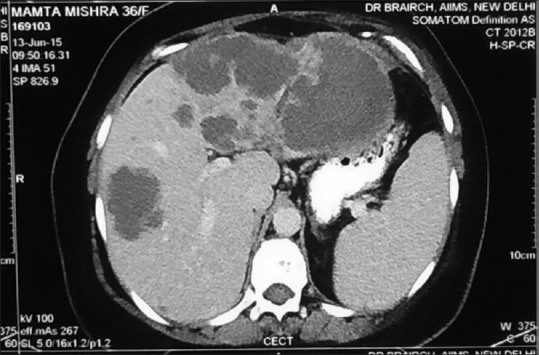
Computed tomography scan of abdomen of the patient in June 2015, showing multiple large hypodense nodules of metastatic angiosarcoma in both lobes of liver
Biopsy from the liver nodules showed features of angiosarcoma. However, pleural fluid cytology was negative for malignancy.
On evaluating the cause of her anemia, her iron profile was normal (serum transferrin = 283, total iron-binding capacity = 333, transferrin saturation = 18.62%, serum ferritin = 1636.57). Peripheral smear showed a leukoerythroblastic picture with myelocytes, nucleated red blood cell (RBC), and tear drop RBCs (N75 L9M5Myelo5 nRBC7). The bone marrow biopsy showed infiltration with tumor cells, CD31+ve.
Hence, the final diagnosis made was metastatic angiosarcoma of the breast (liver, bone marrow, pleural fluid) with anemia, conjugated hyperbilirubinemia, and hypoalbuminemia.
In view of her poor PS and disseminated disease, and progression on paclitaxel chemotherapy, her family was counseled and after discussing the benefits and risks, a decision of palliative oral metronomic chemotherapy was taken along with supportive care.
She was started on oral thalidomide 200 mg daily, capsule celecoxib 400 mg twice a day, and alternating cycles of oral etoposide (50 mg daily 3 weeks on 3 weeks off) and oral cyclophosphamide (100 mg daily 3 weeks on 3 weeks off). Added to this regimen was oral propranolol sustained release tablet 40 mg BD. She also received zoledronic acid 4 mg intravenous monthly.
We thought of the above regimen as a rational one because this regimen has been used as an antiangiogenic metronomic regimen in different studies as well as in our institution. Raina et al. had reported the successful use of thalidomide in the treatment of radiation-induced angiosarcoma.[1] Oral propranolol was recently reported by Banavali et al. to be an effective agent along with metronomic chemotherapy for advanced stage angiosarcoma.[2]
After 1 month of therapy, a contrast-enhanced CT (CECT) chest, abdomen, and pelvis was done for response assessment, which showed a stable disease as compared to the baseline CT.
Her transfusion requirement decreased significantly over the next 2 months. Her PS improved from 3 to 1, dyspnea resolved, and she was now ambulatory.
She developed Grade II neutropenia at the 4th week for which she received two injections of granulocyte-colony stimulating factor, and her dose of etoposide and cyclophosphamide was reduced by 50%.
She was on close follow-up in the outpatient department. At 2-month follow-up, she complained of some puffiness over her face and fatigue. Her hemoglobin was 8.9 g/dl, and she had not required transfusion for a month. A CECT was repeated which now showed extensive hepatic and skeletal metastases with the appearance of three new pulmonary nodular metastases and new hepatic nodules and ascites. There was a new metastatic lesion involving bilateral sphenoid sinuses with extradural component in the middle cranial fossa. This suggested a progressive disease on the metronomic oral chemotherapy.
We planned a palliative radiotherapy to the sphenoid lesion and changed her therapy to doxorubicin 30 mg/m2 q3 weekly and continued thalidomide and propranolol. However, her condition deteriorated with progressive disease, and she succumbed to her illness on September 29, 2015.
Discussion
This patient with pregnancy-associated angiosarcoma of the breast had a rapid progression while on adjuvant therapy. Her disease was extensive involving liver, bones, and bone marrow. She derived some significant clinical improvement from the oral metronomic regimen but progressed after 2 months with new sites of disease.
This case report captures the aggressive course of this rare malignancy. Metronomic chemotherapy can significantly improve these patients’ clinical status and quality of life without much toxicity, but responses are not as durable.
Breast angiosarcoma can be observed as a primary neoplasm or, more commonly, is described in upper limb lymphedema as a result of mastectomy and radiotherapy for breast carcinoma.[3] Primary angiosarcoma of the breast constitutes 0.04% of all malignant breast neoplasms. Both primary and secondary breast angiosarcomas have a prognosis inferior to mammary carcinoma.[4] The first documented case of breast angiosarcoma was presented by Borrman in 1907.[5]
Different from breast carcinomas, primary angiosarcoma of the breast occurs sporadically in young women, usually during the third and fourth decades of life. Between 6% and 12% of primary breast, angiosarcomas are diagnosed during pregnancy or shortly after, suggesting hormones involvement, but hormone receptor positivity is rare making it impossible to assign a link between estrogen dependency and angiosarcoma. In most published cases, breast angiosarcoma is presented as a palpable mass, without pain and with a fast growing rate.[6,7] Bluish skin discoloration occurs in up to a third of patients and is thought to be attributable to the vascular nature of the tumor. Radiographically, breast angiosarcomas exhibit no pathognomonic features. Tumors smaller than 5 cm are usually associated to a better prognosis, even in the presence of worsening factors. Pathologically, these tumors are subdivided into three grades according to the classification proposed by Donnell et al.[8] The degree of differentiation has a significant prognostic value, with regard to both local failure and metastases. The endothelial cells show reactivity for several markers, including CD31, CD34, and von Willebrand factor (factor VIII). Among them, CD31 is considered the most sensitive and most specific endothelial cell marker.[9] Differential diagnosis of this rare tumor include benign hemangioma, phyllodes sarcoma, stromal sarcoma, metaplastic carcinoma, fibrosarcoma, liposarcoma, squamous cell carcinoma with sarcomatoid features, myoepithelioma, fibromatosis, reactive spindle cell proliferative lesion, and high-grade mammary carcinoma, especially in small biopsy specimens containing only solid areas.
Surgery is the principal mode of treatment for primary angiosarcoma of the breast and generally consists of a total mastectomy.[10] Hematogenous dissemination is the rule, making axillary lymph node dissection unnecessary. Adjuvant radiation therapy should be administered especially when the margins of resection are microscopically involved after definitive surgical treatment. There was no statistical correlation of adjuvant radiation therapy with survival in the study, due to the small number of patients and the retrospective nature of the study. However, patients at high risk of recurrence (with large, high-grade tumors) may benefit from adjuvant treatment with improved local control and disease-free survival.[10] Studies examining the efficacy of adjuvant chemotherapy are lacking, due in part to the low incidence of breast angiosarcomas. One retrospective study revealed that 36% of patients with primary angiosarcoma received chemotherapy in an adjuvant or neoadjuvant setting.[11]
Sher et al. reported that adjuvant chemotherapy using an anthracycline and ifosfamide or gemcitabine and a taxane did not significantly improve recurrence-free survival compared with patients who did not receive chemotherapy (38 vs. 31 patients; hazard ratio, 0.47; P = 0.11). However, administration of chemotherapy at the time of recurrence resulted in a 48% response rate.[12] In the case of secondary angiosarcomas induced by radiation treatment, docetaxel showed promise in patients that were refractory to anthracycline-based chemotherapy.[13] Bevacizumab, the anti-vascular endothelial growth factor antibody, has been used as treatment for angiosarcomas, but the results have been variable.
Relapsed metastatic angiosarcoma is a challenge for medical oncologists. Neither standard maximum tolerated dose chemotherapy nor targeted agents has yielded good and durable responses. Options for chemotherapy in metastatic angiosarcoma include doxorubicin, liposomal doxorubicin, paclitaxel, docetaxel, and bevacizumab.
Several case reports[14,15] and small case series[16,17] have documented durable benefits of metronomic chemotherapy in relapsing angiosarcoma. Vogt et al. in their pilot study reported the use of a combination of trophosphamide, pioglitazone, and rofecoxib in advanced vascular malignancies. They reported two complete responses, one partial response, and three stable diseases with a median progression-free survival of 7.7 months.
Recently, beta-blockers have been reported to have anticancer effects in many tumors.[18] The anticancer effects of beta-blockers on angiosarcoma in vivo and in vitro have also been published.[19] Significant expression of beta-adrenergic receptors have also been reported in several types of vascular tumors.[20] Pasquier et al.[21,22] have reported synergy between chemotherapy and beta-blockers in breast cancer and neuroblastoma. Recently, Banavali et al. have reported a durable complete remission of a relapsing metastatic angiosarcoma with a three-drug metronomic regimen and propranolol.[2] All these evidence suggest that propranolol may also have contributed to the response we observed in this patient.
Conclusion
Pregnancy-associated primary angiosarcoma of the breast is an extremely rare and aggressive soft-tissue malignant tumor. The findings of this case report are of extreme significance. With this case, the combination of metronomic chemotherapy and propranolol in angiosarcoma warrants additional studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Raina V, Sengar M, Shukla NK, Deo SS, Mohanty BK, Sharma D, et al. Complete response from thalidomide in angiosarcoma after treatment of breast cancer. J Clin Oncol. 2007;25:900–1. doi: 10.1200/JCO.2006.09.7261. [DOI] [PubMed] [Google Scholar]
- 2.Banavali S, Pasquier E, Andre N. Targeted therapy with propranolol and metronomic chemotherapy combination: Sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience. 2015;9:499. doi: 10.3332/ecancer.2015.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armah HB, Rao UN, Parwani AV. Primary angiosarcoma of the testis: Report of a rare entity and review of the literature. Diagn Pathol. 2007;2:23. doi: 10.1186/1746-1596-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babarovic E, Zamolo G, Mustac E, Strcic M. High grade angiosarcoma arising in fibroadenoma. Diagn Pathol. 2011;6:125. doi: 10.1186/1746-1596-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrman R. Metastasis in histologic benign tumors: Case of metastasizing angioma. Beitr Pathol Anat. 1907;40:372–93. [Google Scholar]
- 6.Johnson CM, Garguilo GA. Angiosarcoma of the breast: A case report and literature review. Curr Surg. 2002;59:490–4. doi: 10.1016/s0149-7944(02)00629-3. [DOI] [PubMed] [Google Scholar]
- 7.Georgiannos SN, Sheaff M. Angiosarcoma of the breast: A 30 year perspective with an optimistic outlook. Br J Plast Surg. 2003;56:129–34. doi: 10.1016/s0007-1226(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 8.Donnell RM, Rosen PP, Lieberman PH, Kaufman RJ, Kay S, Braun DW, Jr, et al. Angiosarcoma and other vascular tumors of the breast. Am J Surg Pathol. 1981;5:629–42. doi: 10.1097/00000478-198110000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bennani A, Chbani L, Lamchahab M, Wahbi M, Alaoui FF, Badioui I, et al. Primary angiosarcoma of the breast: A case report. Diagn Pathol. 2013;8:66. doi: 10.1186/1746-1596-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaklamanos IG, Birbas K, Syrigos KN, Vlachodimitropoulos D, Goutas N, Bonatsos G. Breast angiosarcoma that is not related to radiation exposure: A comprehensive review of the literature. Surg Today. 2011;41:163–8. doi: 10.1007/s00595-010-4341-x. [DOI] [PubMed] [Google Scholar]
- 11.Rosen PP, Kimmel M, Ernsberger D. Mammary angiosarcoma. The prognostic significance of tumor differentiation. Cancer. 1988;62:2145–51. doi: 10.1002/1097-0142(19881115)62:10<2145::aid-cncr2820621014>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Sher T, Hennessy BT, Valero V, Broglio K, Woodward WA, Trent J, et al. Primary angiosarcomas of the breast. Cancer. 2007;110:173–8. doi: 10.1002/cncr.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mano MS, Fraser G, Kerr J, Gray M, Evans V, Kazmi A, et al. Radiation-induced angiosarcoma of the breast shows major response to docetaxel after failure of anthracycline-based chemotherapy. Breast. 2006;15:117–8. doi: 10.1016/j.breast.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Sumikawa Y, Inui S, Nishimura Y, Morita E, Kurachi K. Long-term metronomic docetaxel chemotherapy for inoperative angiosarcoma of the scalp. J Dermatol. 2011;38:393–5. doi: 10.1111/j.1346-8138.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- 15.Kopp HG, Kanz L, Hartmann JT. Complete remission of relapsing high-grade angiosarcoma with single-agent metronomic trofosfamide. Anticancer Drugs. 2006;17:997–8. doi: 10.1097/01.cad.0000224453.39200.d7. [DOI] [PubMed] [Google Scholar]
- 16.Vogt T, Hafner C, Bross K, Bataille F, Jauch KW, Berand A, et al. Antiangiogenetic therapy with pioglitazone, rofecoxib, and metronomic trofosfamide in patients with advanced malignant vascular tumors. Cancer. 2003;98:2251–6. doi: 10.1002/cncr.11775. [DOI] [PubMed] [Google Scholar]
- 17.Mir O, Domont J, Cioffi A, Bonvalot S, Boulet B, Le Pechoux C, et al. Feasibility of metronomic oral cyclophosphamide plus prednisolone in elderly patients with inoperable or metastatic soft tissue sarcoma. Eur J Cancer. 2011;47:515–9. doi: 10.1016/j.ejca.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Tang J, Li Z, Lu L, Cho CH. β-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol. 2013;23(6 Pt B):533–42. doi: 10.1016/j.semcancer.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Stiles JM, Amaya C, Rains S, Diaz D, Pham R, Battiste J, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS One. 2013;8:e60021. doi: 10.1371/journal.pone.0060021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chisholm KM, Chang KW, Truong MT, Kwok S, West RB, Heerema-McKenney AE. β-Adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446–51. doi: 10.1038/modpathol.2012.108. [DOI] [PubMed] [Google Scholar]
- 21.Pasquier E, Street J, Pouchy C, Carre M, Gifford AJ, Murray J, et al. β-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer. 2013;108:2485–94. doi: 10.1038/bjc.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasquier E, Ciccolini J, Carre M, Giacometti S, Fanciullino R, Pouchy C, et al. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: Implication in breast cancer treatment. Oncotarget. 2011;2:797–809. doi: 10.18632/oncotarget.343. [DOI] [PMC free article] [PubMed] [Google Scholar]


