Abstract
Recombinant adeno-associated virus (rAAV) is a commonly used gene therapy vector for the delivery of therapeutic transgenes in a variety of human diseases, but pre-existing serum antibodies to viral capsid proteins can greatly inhibit rAAV transduction of tissues. Serum was assayed from patients with Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), inclusion body myositis (IBM), and GNE myopathy (GNE). These were compared to serum from otherwise normal human subjects to determine the extent of pre-existing serum antibodies to rAAVrh74, rAAV1, rAAV2, rAAV6, rAAV8, and rAAV9. In almost all cases, patients with measurable titers to one rAAV serotype showed titers to all other serotypes tested, with average titers to rAAV2 being highest in all instances. Twenty-six percent of all young normal subjects (<18 years old) had measurable rAAV titers to all serotypes tested, and this percentage increased to almost 50% in adult normal subjects (>18 years old). Fifty percent of all IBM and GNE patients also had antibody titers to all rAAV serotypes, while only 18% of DMD and 0% of BMD patients did. In addition, serum-naïve macaques treated systemically with rAAVrh74 could develop cross-reactive antibodies to all other serotypes tested at 24 weeks post treatment. These data demonstrate that most DMD and BMD patients should be amenable to vascular rAAV-mediated treatment without the concern of treatment blockage by pre-existing serum rAAV antibodies, and that serum antibodies to rAAVrh74 are no more common than those for rAAV6, rAAV8, or rAAV9.
Keywords: : gene therapy, adeno-associated virus, muscular dystrophy, myopathy
Introduction
Use of recombinant adeno-associated virus (rAAV) as a vector for delivery of gene therapies has become increasingly standard in clinical studies.1 rAAV vectors have been used to deliver gene therapy to treat multiple diseases, including hemophilias, neurodegenerative diseases, neuropathies, cardiovascular diseases, and neuromuscular disorders.2–7 For treatment of many human diseases, systemic delivery of rAAV via the blood is required to transduce target tissues effectively. This is particularly true for the treatment of skeletal muscle, as muscle comprises a significant fraction of all human tissue and is distributed throughout the body plan. The authors have focused their studies on one vector that is well-suited for muscle transduction via blood delivery: rAAVrh74.8–10 rAAVrh74 was isolated from a macaque spleen and shown to share properties similar to rAAV8, with which it shares 93% identity in its capsid sequence. Unlike a number of other rAAV serotypes, however, little has been studied regarding rAAVrh74 antibodies in human patients, which is one goal of the current study.
One of the main impediments to clinical use of rAAV vectors is the suppression of tissue transduction by host humoral immune response to the viral capsid protein.11 This is the case not only for neutralizing rAAV antibodies, which can block rAAV infection of cells in vitro and/or in vivo, but also for non-neutralizing antibodies, which can impact tissue transduction through Fc-mediated capsid uptake into dendritic cells and macrophages and through increasing tissue inflammation.11 A number of strategies have been tested to attempt to bypass the block of tissue transduction by pre-existing rAAV serum antibodies. These include immune suppression through the use of the B-cell effectors such as rituximab,12–15 T-cell effectors such as rapamycin,16–21 plasmapheresis to remove serum antibodies,22,23 and direct tissue injection to bypass access of serum antibodies to delivered rAAV. In an isolated limb perfusion study where rAAV was delivered intra-arterially, it was shown that both rAAVrh74.MCK.GALGT2 and rAAVrh74.MCK.μDystrophin vectors were able to yield significant (near 50%) transduction of skeletal myofibers in the targeted gastrocnemius muscles if macaques had total serum antibody levels to rAAVrh74 that were positive below a 1:800 dilution.24 While this amount of total serum antibody is much higher than the standard cutoff typically used for neutralizing antibody assays, where a cutoff of 1:5 might be sufficient when an in vitro infectivity assay is used,11,25–27 these total serum assay measures provide an in vivo measure of antibody levels required to affect viral transduction of actual muscle tissue when rAAV is delivered via the vasculature. Such total serum antibody measures are also a generally accepted exclusion criterion for clinical trials. In macaques where serum rAAVrh74 titers were positive at a dilution of 1:800 or higher prior to treatment, muscle transduction rarely exceeded 10% of total muscle cells.24 Use of plasmapheresis prior to treatment in rAAV antibody-positive macaques, however, lowered serum antibody levels at the time of treatment to below the 1:800 threshold and allowed for muscle transduction at levels that were equivalent to those in macaques with no pre-existing serum rAAVrh74 antibodies.24 Thus, the presence of low levels of pre-existing rAAV serum antibody were not inhibitory to muscle tissue transduction, while higher levels of serum antibody were.
A number of studies have shown humans exposed to AAV develop serum rAAV antibodies that react with multiple of rAAV serotypes, including rAAV1, rAAV2, rAAV5, rAAV6, rAAV8, rAAV9, and rAAVrh10.11,12,26,28 In general, findings from these studies suggest that neutralizing antibodies to rAAV1 and rAAV2 are more commonly present than antibodies to other rAAV serotypes, and for rAAV2, neutralizing antibody and total serum antibody titers can be present in as many as 70% of all subjects tested.26 Serum rAAV antibodies for individual serotypes can also vary by geographic location and by age.25 It is unclear the extent to which chronic inflammatory conditions, for example the muscular dystrophies or inclusion body myositis, might influence such propensities. As a unique gene therapy, rAAVrh74.MCK.GALGT2, has been developed for the treatment of Duchenne muscular dystrophy (DMD),10,29–31 this study was undertaken to understand the prevalence and extent of rAAVrh74 serotype-specific serum antibodies in this patient population and the relationship of said prevalence to those of other rAAV serotypes. In addition, a cohort of young normal subjects was studied, who were age-matched to the DMD population, as well as adult normal subjects and patients with Becker muscular dystrophy (BMD), inclusion body myositis (IBM), and GNE myopathy (GNE).
DMD is the most common genetic form of muscular dystrophy.32 DMD arises from mutations in the dystrophin (DMD) gene, an X-linked gene where out-of-frame mutations give rise to loss of dystrophin protein expression in cardiac and skeletal muscle.33,34 BMD also arises from mutations in the DMD gene, but such mutations are typically in-frame and lead to expression of a partially functional dystrophin protein, giving rise to less clinically severe disease. For example, average life-span and the age at which ambulation is lost are significantly increased in BMD patients compared with DMD.35 IBM generally affects people older than 50 years of age and is characterized by inflammation of the muscles and the presence of intramuscular inclusion bodies with rimmed vacuoles.36 The cause of IBM is not known, but full-length dystrophin protein is expressed in skeletal muscle, suggesting IBM is mechanistically different from DMD or BMD. GNE is caused by recessive mutations in the UDP-GlcNAc epimerase/Man-6 kinase (GNE) gene, which encodes the enzymes that catalyze the committed steps in sialic acid biosynthesis.37 Patients with IBM and GNE are diagnosed as adults and can lose ambulation in the decades following diagnosis.36,38 Both IBM and GNE display common pathological findings in skeletal muscle, including muscle inclusion bodies with rimmed vacuoles. IBM patients, unlike GNE patients, additionally have myositis, or muscle inflammation, with the presence of large numbers of intramuscular white blood cells.
Gene therapy is being developed as a therapy for patients with DMD, BMD, IBM, and GNE. Follistatin (rAAV1.CMV.FS344) gene therapy to increase muscle mass has been tried in both BMD and IBM patients.3 Gene replacement with a partial-coding dystrophin cDNA, such as micro- or mini-dystrophin, has been tried in DMD patients.39 GNE gene replacement has been tested in a mouse model of GNE myopathy.40 Given the impediment of human serum rAAV antibodies to systemic gene therapy, a decision was made to study the repertoire and propensity of anti-rAAV serotype antibodies in these patient populations.
Materials and Methods
Human and macaque serum samples
De-identified human serum samples were obtained through the neuromuscular clinic at Nationwide Children's Hospital following informed consent obtained under a protocol approved by the Institutional Review Board at Nationwide Children's Hospital. A total of 16 samples were collected from patients with BMD, 22 samples were collected from patients with DMD, and 18 samples were collected from patients with IBM. Young normal serum samples (<18 years of age) were also collected through the neuromuscular clinic. De-identified adult normal human serum samples (>18 years of age) were purchased from Bioreclamation IVT (Westbury, NY), and de-identified GNE patient serum samples were obtained from Dr. Yadira Valles (HIBM Research Group, Chatsworth, CA). Macaque serum samples were collected from a previously described study under a protocol approved by the Institutional Animal Care and Use Committee at The Research Institute at Nationwide Children's Hospital.8
Enzyme-linked immunosorbent assay to identify serum rAAV antibodies
All rAAV viral vectors were obtained from the Viral Vector Core facility at Nationwide Children's Hospital. rAAV vectors were produced by the triple transfection method in HEK293 cells and highly purified using density centrifugation and anion exchange chromatography. The identity of various serotypes was confirmed using serotype-specific monoclonal antibodies. Plates were coated overnight at 4°C in coating solution with or without rAAV particles (0.2 M of bicarbonate buffer, pH 9.4, with or without or 2 × 109 viral particles/well of either rAAVrh74, rAAV8, rAAV1, rAAV2, rAAV6, or rAAV9). Plates were blocked for 2–3 h at 37°C in blocking buffer (5% milk, 1% goat serum in phosphate-buffered saline [PBS]). Initial screening of samples was done by diluting the serum 1:50 in blocking buffer and adding 100 μL to each of four wells. Each sample was done in duplicate in wells coated with viral particles or with bicarbonate buffer alone to adjust for background. Samples were incubated at 37°C for 1 h. Each plate was washed five times with wash buffer (PBS with 0.05% Tween-20). Horseradish peroxidase–conjugated secondary antibody was then added at a 1:10,000 dilution (Human IgG-Fc fragment antibody [Bethyl Laboratories, Montgomery, TX] for human serum or anti-monkey IgG, whole molecule [Sigma–Aldrich, St. Louis, MO] for macaque serum) in blocking buffer and incubated at room temperature for 30 min in the dark. Each plate was washed five times again with wash buffer. Substrate reagent (R&D Systems, Minneapolis, MN) was added to each well and allowed to develop in the dark for 15 min. The reaction was stopped with 1 N sulfuric acid, and the optical density (OD) of each well was read on a Synergy2 Plate Reader (BioTek, Winooski, VT) at 450 nm. Any samples that tested positive at 1:50 were retested by enzyme-linked immunosorbent assay (ELISA) with a dilution series from 1:100 until a dilution was identified where at least a twofold increase in signal was no longer obtained. For each sample, the mean OD was calculated by subtracting the background value (– viral particle wells) from the sample value (+ viral particle wells) and determining the signal/background ratio. The serum sample was called AAV-positive if the signal/background ratio was >2. Background signals remained constant throughout at an OD of 0.1–0.2, making for little or no variability in the quotient used to determine twofold or greater differences.
Statistics
Significant differences in lowest positive signal dilution were determined using a Kruskal–Wallis test followed by a multiple comparisons test, as done previously for non-linear serum dilution measures.41 Measures with a p-value of <0.05 were considered significant.
Results
Characterization of human serum rAAV antibodies
The study began by assessing antibody titers to rAAVrh74, rAAV8, rAAV1, rAAV2, rAAV6, and rAAV9 serotypes in the serum of normal human subjects (Table 1). Because some of the diseases to be studied occur in children, normal subjects were subdivided into two groups based on age: young normal (<18 years old) and adult normal (>18 years old). By chance, there were no 18-year-old patients in this study. Because rAAV8 and rAAVrh74 are most similar with regard to capsid sequence (93% identical), these comparisons were grouped next to one another. Sera that were not elevated twofold at a dilution of 1:50 were considered negative. Sera with positive signals at 1:50 were then assayed at subsequent 1:2 serial dilutions to identify the dilution at which the last positive titer signal could be identified. In each instance, the reciprocal of that dilution factor is presented. In 19 young normal samples, five were identified with positive rAAV ELISA signals. In each instance, a positive signal at a 1:50 dilution was identified for all six rAAV serotypes tested in rAAV antibody-positive subjects. In addition, the reciprocal dilution factor at which a positive signal was measured for rAAV2 was greater than or equal to the titer identified for all other serotypes. Of 21 adult normal samples, eight samples showed positive antibody titers to all rAAV serotypes tested. All rAAV serotypes except rAAV9 showed positive titers in ≥48% of these patient samples, with 76% having positive titers to rAAV2. There were three instances where no measurable titer to rAAV9 was observed but where measurable titers to all other rAAV serotypes were present. Adult normal samples were the only group where this was the case. The magnitude of dilution required for a positive signal was quite varied between antibody-positive individuals and between serotypes within the same subject. Dilution factors required for a negative rAAV2 signal were far and away the most variable, and positive titers for this serotype were the most common. Patient N0035, for example, had a minimal positive signal for rAAV2 at a serum dilution of 1:204,800, while other patients were positive for rAAV2 only at a 1:50 dilution.
Table 1.
Antibody titers to rAAV serotypes in young and adult normal human subjects
| Sample | Sex | Age (years) | rAAVrh74 | rAAV8 | rAAV1 | rAAV2 | rAAV6 | rAAV9 | |
|---|---|---|---|---|---|---|---|---|---|
| Young normal | HC14 | F | 2.4 | — | — | — | — | — | — |
| HC13 | F | 6.1 | — | — | — | — | — | — | |
| HC05 | M | 6.2 | 25,600 | 12,800 | 51,200 | 102,400 | 25,600 | 25,600 | |
| HC10 | M | 7.9 | — | — | — | — | — | — | |
| HC11 | M | 9.8 | 102,400 | 25,600 | 51,200 | 204,800 | 25,600 | 12,800 | |
| HC04 | M | 10.3 | — | — | — | — | — | — | |
| HC12 | M | 10.6 | — | — | — | — | — | — | |
| HC03 | M | 10.9 | — | — | — | — | — | — | |
| HC06 | M | 11.9 | — | — | — | — | — | — | |
| HC15 | F | 11.9 | 800 | 1,600 | 1,600 | 6,400 | 800 | 800 | |
| HC07 | M | 12.8 | — | — | — | — | — | — | |
| HC19 | M | 13.0 | — | — | — | — | — | — | |
| HC02 | M | 15.2 | 12,800 | 25,600 | 25,600 | 102,400 | 25,600 | 12,800 | |
| HC18 | M | 15.4 | — | — | — | — | — | — | |
| HC08 | M | 15.8 | — | — | — | — | — | — | |
| HC09 | M | 15.9 | — | — | — | — | — | — | |
| HC16 | M | 16.0 | — | — | — | — | — | — | |
| HC01 | M | 16.3 | 12,800 | 25,600 | 25,600 | 102,400 | 25,600 | 12,800 | |
| HC17 | M | 17.1 | — | — | — | — | — | — | |
| Adult normal | N0033 | F | 19 | — | — | — | 400 | — | — |
| N9049 | F | 19 | — | — | 200 | 25,600 | — | — | |
| HC20 | F | 19 | — | — | — | — | — | — | |
| N0041 | M | 20 | — | — | — | — | — | — | |
| N9047 | F | 21 | — | — | — | — | — | — | |
| N9051 | F | 22 | 6,400 | 3,200 | 6,400 | 51,200 | 6,400 | 1,600 | |
| N0031 | F | 24 | — | — | — | 1,600 | — | — | |
| N0035 | F | 24 | 12,800 | 12,800 | 12,800 | 204,800 | 25,600 | 12,800 | |
| N9041 | M | 25 | — | — | — | 400 | — | — | |
| N9045 | M | 27 | 200 | 1,600 | 3,200 | 25,600 | 1,600 | 1,600 | |
| N9044 | M | 28 | 400 | 400 | 1,600 | 6,400 | 800 | 400 | |
| N9048 | F | 29 | 800 | 1,600 | 3,200 | 12,800 | 3,200 | 800 | |
| N0038 | M | 31 | — | — | — | — | — | — | |
| N0034 | F | 32 | 800 | 1,600 | 3,200 | 12,800 | 3,200 | 1,600 | |
| N9050 | F | 33 | 50 | 200 | 200 | 800 | 100 | — | |
| N0032 | F | 36 | 50 | 50 | 400 | 6,400 | 400 | — | |
| N9043 | M | 37 | — | — | — | — | — | — | |
| N9042 | M | 39 | — | — | — | 3,200 | — | — | |
| N9052 | F | 45 | 100 | 400 | 800 | 6,400 | 400 | 100 | |
| N0036 | F | 46 | — | — | — | 800 | — | — | |
| N9046 | M | 48 | 12,800 | 3,200 | 25,600 | 25,600 | 6,400 | 1,600 |
Highest reciprocal dilutions allowing for positive signal for serum antibodies to rAAVrh74, rAAV8, rAAV1, rAAV2, rAAV6, or rAAV9 are shown in 19 young (<18 years old) and 21 adult (>18 years old) normal subjects.
f, female; m, male; rAAV, recombinant adeno-associated virus.
Next, the frequency of rAAV titers was assessed in patients with DMD or BMD (Table 2). In 22 DMD samples, only four patients were identified with positive titers to all rAAV serotypes tested, and three additional samples with positive titer only to rAAV2. Here, the positive reciprocal titer trended toward lower amounts of antibody than what was seen in young normal subjects. Surprisingly, in 16 BMD samples, no patients were identified with antibody titers to rAAVrh74, rAAV8, rAAV1, rAAV6, or rAAV9, and only one patient with a measurable titer to rAAV2. By contrast to BMD and DMD samples, both of which had concentrated samples from young patients, a far greater propensity was identified to rAAV titers in IBM and GNE patients (Table 3), who had average ages of 60 and 32 years, respectively. In both instances, titer frequencies in these patient groups matched those found in adult normal subjects, with 9/18 IBM samples and 2/4 GNE samples showing positive titers to all rAAV serotypes. Three additional IBM patients showed a positive titer to rAAV2, with two of these also showing positive titer to rAAV1.
Table 2.
Antibody titers to rAAV serotypes in patients with DMD and BMD
| Sample | Sex | Age (years) | rAAVrh74 | rAAV8 | rAAV1 | rAAV2 | rAAV6 | rAAV9 | |
|---|---|---|---|---|---|---|---|---|---|
| DMD | B010 | M | 5.2 | — | — | — | — | — | — |
| B022 | M | 5.3 | — | — | — | — | — | — | |
| B053 | M | 8.4 | 400 | 12,800 | 12,800 | 6,400 | 6,400 | 3,200 | |
| B014 | M | 8.4 | — | — | — | 50 | — | — | |
| B052 | M | 8.5 | — | — | — | — | — | — | |
| B012 | M | 9.4 | 400 | 800 | 1,600 | 12,800 | 1,600 | 400 | |
| B060 | M | 9.6 | — | — | — | 50 | — | — | |
| B007 | M | 10.5 | — | — | — | — | — | — | |
| B048 | M | 11.8 | — | — | — | — | — | — | |
| B051 | M | 11.8 | 6,400 | 6,400 | 12,800 | 51,200 | 6,400 | 3,200 | |
| B027 | M | 12.6 | — | — | — | — | — | — | |
| B002 | M | 12.8 | — | — | — | — | — | — | |
| B023 | M | 13.0 | — | — | — | — | — | — | |
| B008 | M | 13.5 | — | — | — | 400 | — | — | |
| B043 | M | 14.8 | — | — | — | — | — | — | |
| B057 | F | 16.7 | — | — | — | — | — | — | |
| B042 | M | 16.9 | — | — | — | — | — | — | |
| B059 | M | 17.6 | — | — | — | — | — | — | |
| B055 | M | 17.9 | 100 | 400 | 800 | 3,200 | 400 | 200 | |
| B047 | M | 19.1 | — | — | — | — | — | — | |
| B046 | M | 20.8 | — | — | — | — | — | — | |
| B061 | M | 28.8 | — | — | — | — | — | — | |
| BMD | B041 | M | 6.7 | — | — | — | — | — | — |
| B036 | M | 7.3 | — | — | — | — | — | — | |
| B040 | M | 8.2 | — | — | — | — | — | — | |
| B049 | M | 8.7 | — | — | — | — | — | — | |
| B001 | M | 11.5 | — | — | — | — | — | — | |
| B054 | M | 11.6 | — | — | — | — | — | — | |
| B044 | M | 17.6 | — | — | — | — | — | — | |
| B038 | M | 17.7 | — | — | — | 400 | — | — | |
| B039 | M | 18.3 | — | — | — | — | — | — | |
| B013 | M | 18.9 | — | — | — | — | — | — | |
| B016 | M | 19.5 | — | — | — | — | — | — | |
| B037 | M | 23.7 | — | — | — | — | — | — | |
| B045 | M | 24.8 | — | — | — | — | — | — | |
| B005 | M | 25.9 | — | — | — | — | — | — | |
| B006 | M | 29.2 | — | — | — | — | — | — | |
| B004 | M | 29.3 | — | — | — | — | — | — |
Highest reciprocal dilutions allowing for positive signal for serum antibodies to rAAVrh74, rAAV8, rAAV1, rAAV2, rAAV6, or rAAV9 are shown in 22 DMD patients and 16 BMD patients.
DMD, Duchenne muscular dystrophy; BMD, Becker muscular dystrophy.
Table 3.
Antibody titers to rAAV serotypes in patients with inclusion body myositis and GNE myopathy
| Sample | Sex | Age (years) | rAAVrh74 | rAAV8 | rAAV1 | rAAV2 | rAAV6 | rAAV9 | |
|---|---|---|---|---|---|---|---|---|---|
| IBM | B015 | M | 52.4 | — | — | — | — | — | — |
| B026 | M | 57.9 | — | — | — | — | — | — | |
| B020 | M | 58.9 | — | — | — | 400 | — | — | |
| B003 | M | 61.3 | — | — | — | — | — | — | |
| B035 | F | 61.7 | — | — | — | — | — | — | |
| B030 | F | 62.1 | — | — | — | — | — | — | |
| B017 | M | 64.0 | 12,800 | 12,800 | 25,600 | 51,200 | 12,800 | 3,200 | |
| B034 | M | 65.4 | 12,800 | 12,800 | 12,800 | 104,800 | 6,400 | 3,200 | |
| B011 | M | 65.5 | — | — | 50 | 800 | — | — | |
| B031 | M | 65.6 | 400 | 200 | 800 | 1,600 | 800 | 200 | |
| B021 | M | 67.1 | 800 | 3,200 | 12,800 | 51,200 | 1,600 | 800 | |
| B025 | F | 70.6 | — | — | — | — | — | — | |
| B018 | M | 71.9 | 1,600 | 3,200 | 800 | 400 | 400 | 6,400 | |
| B032 | F | 72.8 | — | — | 50 | 200 | — | — | |
| B033 | M | 74.6 | 1,600 | 3,200 | 6,400 | 25,600 | 3,200 | 800 | |
| B029 | M | 77.3 | 800 | 800 | 1,600 | 6,400 | 1,600 | 100 | |
| B019 | M | 79.0 | 6,400 | 6,400 | 6,400 | 25,600 | 6,400 | 3,200 | |
| B024 | M | 80.1 | 3,200 | 800 | 1,600 | 3,200 | 1,600 | 400 | |
| GNE | 1 | F | 32 | 400 | 400 | 1,600 | 6,400 | 800 | 50 |
| 2 | F | 34 | 100 | 800 | 800 | 6,400 | 800 | 200 | |
| 3 | M | 43 | — | — | — | — | — | — | |
| 4 | M | 45 | — | — | — | — | — | — |
Highest reciprocal dilutions allowing for positive signal for serum antibodies to rAAVrh74, rAAV8, rAAV1, rAAV2, rAAV6, or rAAV9 are shown in 18 IBM patients and four GNE patients.
IBM, inclusion body myositis; GNE, GNE myopathy.
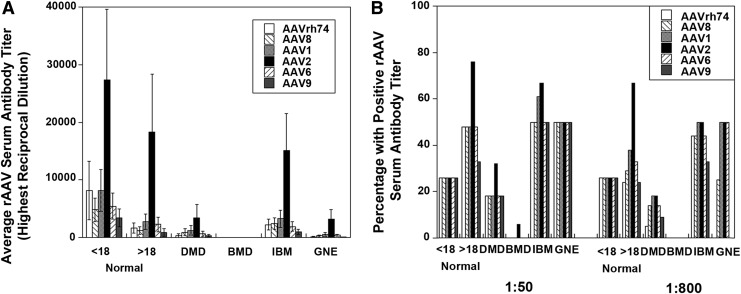
Next, the cumulative average dilution required for a positive antibody signal (Fig. 1A) and the frequency of positive signals (Fig. 1B) were compared in each patient group. Because the serial antibody dilution assay we used was not linear with respect to signal, a Kruskal–Wallis test followed by a multiple comparisons test were performed to assess significant differences between average dilution factors for the various serotypes.41 This analysis included all samples. In adult normal samples, positive titer signals for rAAV2 were significantly increased with respect to titers for rAAVrh74 and rAAV9 (p < 0.05). In general, while average titers may have declined slightly in adult normal subjects relative to young normal subjects, adult normal subjects had a higher likelihood of having pre-existing serum antibody titers to rAAV serotypes. In addition, it was found that antibody titers for rAAVrh74 were significantly higher for adult normal and IBM than for BMD, titers for rAAV8 and rAAV9 were significantly higher for IBM than for BMD, titers to rAAV1, rAAV2 and rAAV6 were significantly higher for adult normal and IBM than for BMD, and titers to rAAV2 were significantly higher for adult normal than for DMD (p < 0.05). None of these comparisons account for age, and so in some instances these are inappropriate comparisons. DMD ages, for example, only really match the young normal controls, while IBM patients in this analysis are older than patients in all other groups. A general increase in the frequency of subjects with rAAV serum antibodies with age was also observed when assessing all groups in a single pool. For example, young subjects (<18 years old) had a 26% cumulative incidence of rAAV titers, while this increased in 20–39 year olds to 58% and to 65% in 40–89 year olds. Additionally, there was a bias toward increased rAAV incidence in females compared with males (61% vs. 36%) when the data were pooled independent of disease groups, but this was due to the fact that DMD and BMD are X-linked diseases composed almost entirely of young male subjects and incidence in younger subjects was lower than incidence in older subjects. Last, antibodies to rAAVrh74 appeared to be in line, both in terms of propensity of patients with positive titers and in terms of amplitude of those titers, with rAAV8, rAAV6, and rAAV9.
Figure 1.
Average reciprocal dilution factor for positive signal and propensity of positive signals in young and adult normal patients compared to Duchenne muscular dystrophy (DMD), Becker muscular dystrophy (BMD), inclusion body myositis (IBM), and GNE myopathy (GNE) patients. Average reciprocal dilution factor required for a positive serum antibody signal to rAAVrh74, rAAV8, rAAV1, rAAV2, rAAV6, or rAAV9 (A) and the percentage of patients with positive signal at a 1:50 or 1:800 serum dilution (B) are shown. Errors in (A) are standard error of the mean for 19 (<18 normal), 21 (>18 normal), 22 (DMD), 16 (BMD), 18 (IBM) or 4 (GNE) patients.
Previously, the serum dilution of 1:800 was defined as being the dilution at which positive antibody signals to rAAVrh74 could significantly diminish muscle transgene transduction levels in rhesus macaques treated with either rAAVrh74.MCK.GALGT2 or rAAVrh74.MCK.μDystrophin.8,23 For these experiments, rAAV vector was delivered by intra-arterial delivery using an isolated limb perfusion method to treat the gastrocnemius muscle. Therefore, the frequency of positive signals to all rAAV serotypes was also assessed at a 1:800 dilution in addition to assessing the frequency at a 1:50 dilution (Fig. 1B). Young normal subjects showed no change in propensity of rAAV antibodies to any serotype between the 1:50 and 1:800 dilution. However, there were a few patients, particularly in the DMD group, who showed reduced rAAV antibodies at 1:800 compared with 1:50. For example, the percentage of DMD patients positive for antibodies to rAAVrh74 at 1:800 was only 5% compared with 20% at 1:50. Similarly, the frequency of DMD patients with positive titers to rAAV9 at 1:800 was only 10% compared with 20% at 1:50. By contrast, the frequency of positive antibody signals to rAAV1 and rAAV2 was identical at 1:50 and 1:800 for DMD patients. Thus, most DMD patients have pre-existing antibody levels to rAAVrh74 that are below the amount that would be expected to block muscle tissue transduction with vascular delivery. This was also the case for BMD patients, who showed no measurable rAAVrh74 serum antibodies.
Assessment of cross-reactive serotype antibodies after vascular rAAV treatment of rhesus macaques
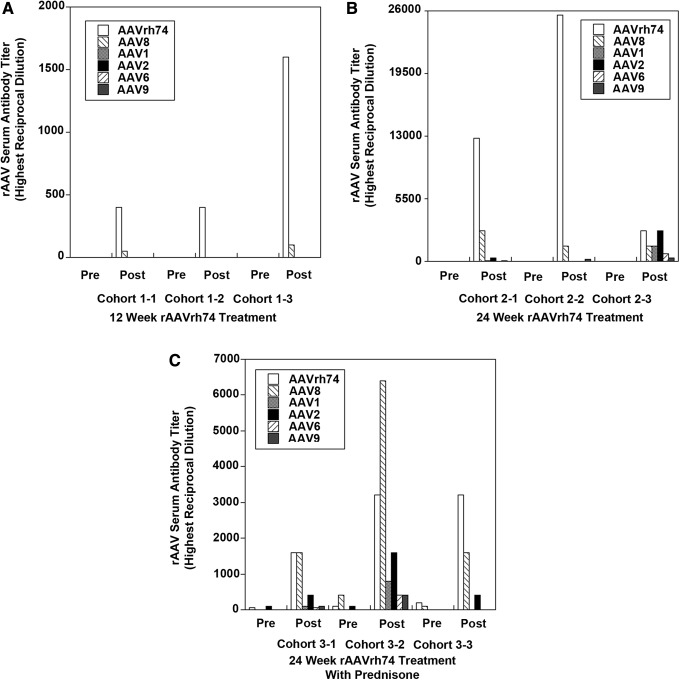
Given that the majority of human patients with positive rAAV titers, regardless of disease, showed rAAV antibodies to all serotypes tested, the study next explored whether immunization with one particular rAAV serotype would induce cross-reactive antibodies that recognize other rAAV serotypes. To do this, a previous study was called upon of systemic rAAVrh74.MCK.GALGT2 delivery in rhesus macaques.8 In this study, many macaques showed pre-existing antibodies to a variety of rAAV serotypes, but through pre-screening, it was possible to select small cohorts where no rAAV antibodies to the serotypes tested here were present prior to treatment. rAAV serum titers were assessed in three cohorts of three animals each prior to rAAV treatment and at 12 or 24 weeks after treatment (Fig. 2). Cohort 1 showed no positive rAAVrh74 antibody signal at a 1:50 dilution prior to treatment and was treated with 2 × 1012vg/kg rAAVrh74.MCK.GALGT2 in the femoral artery to induce GALGT2 transgene expression in 56 ± 12% of all myofibers in the gastrocnemius muscle at 12 weeks post treatment. Cohort 2 was treated identically to cohort 1 but was analyzed at 24 weeks post treatment, showing GALGT2 transgene expression in 35 ± 30% of myofibers. Cohort 3 had slight pre-existing serum antibody titers to rAAVrh74 and also rAAV8 and rAAV2, but these levels were all below the positive 1:800 signal required for blockage of rAAVrh74 muscle tissue transduction.8,23 Cohort 3 was treated with prednisone for 2 weeks prior to treatment and thereafter, and showed expression in 56 ± 6% of myofibers with GALGT2 transgene expression at 24 weeks post treatment. In each of the three cohorts, a very significant induction in rAAVrh74 serum antibodies occurred post treatment, but as titers were low at the time of treatment, rAAV transduction of muscle tissue was not inhibited.8 As with the human serum samples, pre- and post-treatment serum was screened in these three cohorts of macaques for macaque antibodies to rAAVrh74, rAAV8, rAAV1, rAAV2, rAAV6, and rAAV9. For cohort 1, only induction of serum antibodies was identified to rAAVrh74 (3/3 macaques) and rAAV8 (2/3 macaques) at 12 weeks post treatment (Fig. 2A). Antibodies to rAAV1, rAAV2, rAAV6, and rAAV9 were not present in any animal at a 1:50 dilution for this cohort. In cohort 2, however, cross-reactive antibodies to rAAV9 were identified in 3/3 macaques in addition to antibodies to rAAVrh74 and rAAV8 at 24 weeks post treatment (Fig. 2B). In addition, cross-reactive antibodies to rAAV1 and rAAV2 were found in 2/3 macaques and antibodies to rAAV6 in 1/3 macaques. Similar results were found in cohort 3. As before, post-treatment levels of rAAVrh74 and rAAV8 antibodies were higher than for other serotypes, but antibodies to rAAV1, rAAV2, rAAV6, and rAAV9 were all found in 2/3 macaques, and antibodies to rAAV2 were found in all three macaques (Fig. 2C). Thus, 3/6 macaques analyzed in cohorts 2 and 3, both of which were analyzed at 24 weeks post treatment, showed cross-reactive antibodies to all serotypes tested as a result of rAAVrh74.MCK.GALGT2 treatment, and 6/6 macaques showed cross-reactive antibodies to at least one serotype aside from rAAV8, which is the serotype most similar to rAAVrh74. Thus, it appears that the commonalities found with regard to serotype-specific antibody titers in human patients could arise from the prolonged presence of a single serotype in tissues after vascular delivery.
Figure 2.
Induction of cross-reactive macaque serum rAAV antibodies after systemic treatment with rAAVrh74.MCK.GALGT2. Highest reciprocal dilutions required for positive signal of serum antibodies to rAAVrh74, rAAV8, rAAV1, rAAV2, rAAV6, or rAAV9 are shown in patients in macaques treated for 12 weeks (A) or 24 weeks (B and C) with 2 × 1012vg/kg rAAVrh74.MCK.GALGT2 with (C) or without (A and B) prednisone pre- and co-treatment.
Discussion
This study shows that the majority of patients with DMD and BMD would be amenable to treatment with rAAV gene therapy vectors, including rAAVrh74, rAAV8, rAAV1, rAAV2, rAAV6, and rAAV9, as only a minority of DMD and BMD patients have pre-existing serum antibodies to these serotypes of rAAV that might inhibit transduction of tissues when they are delivered via the blood. Serum antibody titers were lowest in DMD patients for antibodies to rAAVrh74 and rAAV9 when assayed at a 1:800 dilution, the dilution previously found to block rAAVrh74 muscle tissue transduction during vascular delivery.24 The propensity of DMD and BMD patients to have such antibodies is equal to or lower than those found in age-matched normal subjects, in spite of the fact that these disorders are associated with increased tissue inflammation in skeletal muscles. In general, serum antibodies to rAAVrh74 appear to be on a par with antibodies to rAAV6, rAAV8, and rAAV9, which are three of the rAAV serotypes that commonly have the lowest incidence of serum antibodies in humans.11 Such findings are significant, as it is hoped that rAAVrh74 will be used in future DMD and BMD gene therapy studies, much as has recently been done in LGMD2D patients.6 They are also similar to a number of other studies showing shared antibodies to multiple serotypes of rAAV.11 For example, Halbert et al. found neutralizing antibodies (NAbs) to rAAV2 and rAAV6 in 25–30% of otherwise normal adults and cystic fibrosis patients,28 while Thwaite et al. found total serum antibodies in roughly 50–70% of subjects to rAAVrh10, rAAV9 and rAAV2.26 Interestingly, the later study also looked at NAb titers and found NAb titers to rAAV2 in about 70% of subjects, while NAb titers to rAAVrh10 and rAAV9 were present only about 20% of the time.26 While NAb titers have not been defined here, previous work in rAAVrh74-treated macaques has defined total serum antibody titers that prevent muscle expression, and this information is analogous to the information provided by in vitro NAb infectivity assays.23,24
While it is encouraging that few DMD and BMD patients show pre-existing anti-rAAV antibodies, it is curious that the frequency of rAAV antibodies in patients with these diseases appears to be even lower than their preponderance in otherwise normal children. This may be due to the relatively small sample size of each group, which can increase the chance for random effects, or it may reflect a disease-specific effect of AAV biology in these patient populations. One difference between normal subjects and DMD/BMD subjects is that many DMD/BMD subjects have been treated with corticosteroids for prolonged periods of time, as this can prolong ambulation by several years in DMD patients.42 Alternatively, there may be an as yet unidentified role for dystrophin in muscle or non-muscle cells that intersects with adenovirus or AAV replication, infectivity, or immunogenicity. Further work will be required to resolve this issue.
The flip side of the encouraging findings in DMD and BMD patients was that the preponderance of pre-existing antibodies to all rAAV serotypes tested increased with the age of patients. Thus, IBM and GNE patients, all of whom are adults at the time of diagnosis, have only a one in two chance of not possessing pre-existing rAAV antibodies to any given serotype. This high incidence of subjects with pre-existing serum rAAV antibodies is similar to the findings in normal adult patients. The likelihood of adult subjects having pre-existing antibodies to rAAV2 was higher still, with 8/10 adult normal subjects and 7/10 IBM subjects showing a positive signal at a 1:50 dilution, much as has been seen previously by others.11,26 Clearly, to reach all IBM or GNE patients effectively, some method to reduce pre-existing rAAV antibodies, such as immunosuppression or plasmapheresis, will be required.
An additional finding of this study was that the majority of patients with positive rAAV antibody titers to a particular serotype were highly likely to display positive serum antibodies to all of the other serotypes tested. This is not an uncommon result in human and nonhuman primate studies11,25,27 and is not surprising, as the capsid sequences of rAAV1, rAAV2, rAAV6, rAAV8, rAAV9, and rAAVrh74—the serotypes tested here—all share at least 80% sequence homology.43 rAAV4 and rAAV5, by contrast, show less capsid homology to these other serotypes, and studies of serum rAAV4 antibodies in fact show that they are far less common in human subjects.25 These results suggest that there would seem to be a significant chance of developing cross-reactive anti-capsid rAAV antibodies to related serotypes as the result of infection with a single rAAV vector, and this is also supported by numerous studies in humans, nonhuman primates, and mice.11,26,27,44,45 For example, a recent study by Calcedo and Wilson followed naturally occurring anti-rAAV serum antibody levels in a cohort of chimpanzees over a period of 10 years.27 They found that chimpanzees that chronically had neutralizing serum rAAV8 antibodies, an AAV serotype that is fairly common in non-human primates, generally showed high levels of cross-reactive antibodies to rAAV1, rAAVrh10, rAAV5, and rAAV9, while chimpanzees that did not develop rAAV8 antibodies over that same 10-year period did not develop neutralizing antibodies to the other serotypes.27 Such findings are consistent with the macaque studies, where cross-reactive antibodies developed in multiple animals after 24 weeks of rAAVrh74 treatment. Interestingly, cross-reactive antibodies to rAAV8 were only observed at 12 weeks post treatment, which is the serotype most similar to rAAVrh74, while antibodies to other serotypes took another 12 weeks to develop. This suggests that cross-reactive antibody responses may proceed and expand over a prolonged incubation period, and that patients treated with one rAAV serotype are likely ultimately to develop antibodies against many other serotypes over the ensuing months after treatment. Moreover, shorter analysis times after experimental rAAV treatment might lead to misleadingly specific cross-reactive antibody data.
Acknowledgments
We would like to thank Dr. Rui Xu for technical support and Dr. Scott Loiler and the Viral Vector Core at Nationwide Children's Hospital for purified forms of the rAAV serotypes used. Thanks also to Darren Murrey (NCH) for assistance with the serum antibody ELISA protocol. This work was funded by NIH grant R01 AR049722 to P.T.M. and grant P50 AR070604 to K.M.F.
Author Disclosure
rAAVrh74.MCK.GALGT2 is a licensed product for which P.T.M. is the inventor. None of the other authors have any conflicts to disclose.
References
- 1.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 2011;12:341–355 [DOI] [PubMed] [Google Scholar]
- 2.Spencer HT, Riley BE, Doering CB. State of the art: gene therapy of haemophilia. Haemophilia 2016;22 Suppl 5:66–71 [DOI] [PubMed] [Google Scholar]
- 3.Mendell JR, Sahenk Z, Malik V, et al. A Phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol Ther 2015;23:192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoyng SA, de Winter F, Tannemaat MR, et al. Gene therapy and peripheral nerve repair: a perspective. Front Mol Neurosci 2015;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalia LV, Kalia SK, Lang AE. Disease-modifying strategies for Parkinson's disease. Mov Disord 2015;30:1442–1450 [DOI] [PubMed] [Google Scholar]
- 6.Mendell JR, Rodino-Klapac LR, Rosales XQ, et al. . Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol 2010;68:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rincon MY, VandenDriessche T, Chuah MK. Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation. Cardiovasc Res 2015;108:4–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chicoine LG, Rodino-Klapac LR, Shao G, et al. . Vascular delivery of rAAVrh74.MCK.GALGT2 to the gastrocnemius muscle of the rhesus macaque stimulates the expression of dystrophin and laminin alpha2 surrogates. Mol Ther 2014;22:713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodino-Klapac LR, Montgomery CL, Bremer WG, et al. . Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther 2010;18:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin PT, Xu R, Rodino-Klapac LR, et al. . Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. Am J Physiol Cell Physiol 2009;296:C476–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 2013;4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mingozzi F, Chen Y, Edmonson SC, et al. . Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther 2013;20:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corti M, Elder ME, Falk DJ, et al. . B-cell ablation is protective against anti-AAV capsid immune response: a human subject case study. Mol Ther 2014;22:S303–S303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sack BK, Merchant S, Markusic DM, et al. . Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One 2012;7:e37671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furst DE. Serum immunoglobulins and risk of infection: how low can you go? Semin Arthritis Rheum 2009;39:18–29 [DOI] [PubMed] [Google Scholar]
- 16.Nayak S, Sarkar D, Perrin GQ, et al. . Prevention and reversal of antibody responses against factor IX in gene therapy for hemophilia B. Front Microbiol 2011;2:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghimi B, Sack BK, Nayak S, et al. . Induction of tolerance to factor VIII by transient co-administration with rapamycin. J Thromb Haemost 2011;9:1524–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera VM, Gao GP, Grant RL, et al. . Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 2005;105:1424–1430 [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Voutetakis A, Papa M, et al. . Rapamycin control of transgene expression from a single AAV vector in mouse salivary glands. Gene Ther 2006;13:187–190 [DOI] [PubMed] [Google Scholar]
- 20.Teachey DT, Obzut DA, Axsom K, et al. . Rapamycin improves lymphoproliferative disease in murine autoimmune lymphoproliferative syndrome (ALPS). Blood 2006;108:1965–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther 2010;17:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteilhet V, Saheb S, Boutin S, et al. . A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther 2011;19:2084–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chicoine LG, Montgomery CL, Bremer WG, et al. . Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol Ther 2014;22:338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chicoine LG, Rodino-Klapac LR, Shao G, et al. . Vascular delivery of rAAVrh74.MCK.GALGT2 to the gastrocnemius muscle of the rhesus macaque stimulates the expression of dystrophin and laminin alpha2 surrogates. Mol Ther 2014;22:713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calcedo R, Vandenberghe LH, Gao G, et al. . Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thwaite R, Pages G, Chillon M, et al. . AAVrh.10 immunogenicity in mice and humans. Relevance of antibody cross-reactivity in human gene therapy. Gene Ther 2015;22:196–201 [DOI] [PubMed] [Google Scholar]
- 27.Calcedo R, Wilson JM. AAV natural infection induces broad cross-neutralizing antibody responses to multiple AAV serotypes in chimpanzees. Hum Gene Ther Clin Dev 2016;27L79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halbert CL, Miller AD, McNamara S, et al. . Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther 2006;17:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu R, DeVries S, Camboni M, et al. . Overexpression of Galgt2 reduces dystrophic pathology in the skeletal muscles of alpha sarcoglycan-deficient mice. Am J Pathol 2009;175:235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu R, Chandrasekharan K, Yoon JH, et al. . Overexpression of the cytotoxic T cell (CT) carbohydrate inhibits muscular dystrophy in the dyW mouse model of congenital muscular dystrophy 1A. Am J Pathol 2007;171:181–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu R, Camboni M, Martin PT. Postnatal overexpression of the CT GalNAc transferase inhibits muscular dystrophy in mdx mice without altering muscle growth or neuromuscular development: evidence for a utrophin-independent mechanism. Neuromuscul Disord 2007;17:209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emery AE. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Disord 1991;1:19–29 [DOI] [PubMed] [Google Scholar]
- 33.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987;51:919–928 [DOI] [PubMed] [Google Scholar]
- 34.Koenig M, Hoffman EP, Bertelson CJ, et al. . Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987;50:509–517 [DOI] [PubMed] [Google Scholar]
- 35.Wein N, Alfano L, Flanigan KM. Genetics and emerging treatments for Duchenne and Becker muscular dystrophy. Pediatr Clin North Am 2015;62:723–742 [DOI] [PubMed] [Google Scholar]
- 36.Needham M, Mastaglia FL. Sporadic inclusion body myositis: a continuing puzzle. Neuromuscul Disord 2008;18:6–16 [DOI] [PubMed] [Google Scholar]
- 37.Eisenberg I, Avidan N, Potikha T, et al. . The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet 2001;29:83–87 [DOI] [PubMed] [Google Scholar]
- 38.Broccolini A, Gidaro T, Morosetti R, et al. . Hereditary inclusion-body myopathy with sparing of the quadriceps: the many tiles of an incomplete puzzle. Acta Myol 2011;30:91–95 [PMC free article] [PubMed] [Google Scholar]
- 39.Bowles DE, McPhee SW, Li C, et al. . Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther 2012;20:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitrani-Rosenbaum S, Yakovlev L, Becker Cohen M, et al. . Sustained expression and safety of human GNE in normal mice after gene transfer based on AAV8 systemic delivery. Neuromuscul Disord 2012;22:1015–1024 [DOI] [PubMed] [Google Scholar]
- 41.Reverberi R. The statistical analysis of immunohaematological data. Blood Transfus 2008;6:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendell JR, Moxley RT, Griggs RC, et al. . Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med 1989;320:1592–1597 [DOI] [PubMed] [Google Scholar]
- 43.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev 2008;21:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flotte TR, Trapnell BC, Humphries M, et al. . Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gene Ther 2011;22:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riviere C, Danos O, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther 2006;13:1300–1308 [DOI] [PubMed] [Google Scholar]