Abstract
Objective: Theacrine, a methylurate class purine alkaloid, triggers diverse pharmacologic responses, including psychostimulatory activity by modulation of adenosinergic and dopaminergic pathways. In a double-blind, placebo-controlled study, theacrine increased energy, concentration, and mood, while reducing fatigue. Because caffeine, a methylxanthine purine alkaloid, is frequently coadministered with theacrine, we sought to determine if a pharmacokinetic and/or pharmacodynamic interaction existed between theacrine and caffeine.
Methods: Eight healthy adults received theacrine, as TeaCrine® (25 or 125 mg), caffeine (150 mg), or a combination of theacrine (125 mg) and caffeine (150 mg) in a randomized, double-blind crossover study. Blood samples were collected over a 24-hour period and analyzed by Liquid chromatrography-mass spectrometry/mass spectrometry (LC-MS/MS) for theacrine, caffeine, and paraxanthine. Pharmacodynamic response markers, heart rate and blood pressure, were recorded.
Results: Theacrine pharmacokinetics was similar following administration of theacrine alone. Caffeine coadministration increased maximum plasma concentration and area under the curve of theacrine without altering theacrine half-life. Theacrine had no impact on caffeine or paraxanthine pharmacokinetics. There was no difference between treatment groups with regard to heart rate or systolic/diastolic blood pressure.
Conclusions: Coadministration of theacrine and caffeine results in a clinically significant pharmacokinetic interaction, viz., increased theacrine exposure. Enhanced oral bioavailability is the most likely mechanism by which caffeine alters theacrine exposure. However, further studies examining the contribution of presystemic elimination mechanisms, for example, efflux transport and/or gut metabolism, to theacrine bioavailability are needed to confirm the exact mechanism(s). Hemodynamic parameters were unaltered despite the pharmacokinetic interaction, suggesting that coadministration of caffeine and theacrine is safe at the doses administered.
Keywords: : theacrine, caffeine, pharmacokinetics
Introduction
Theacrine (1,3,7,9-tetramethyluric acid) is a methylurate class purine alkaloid with structural similarity to the methylxanthine class purine alkaloid, caffeine. Theacrine, originally discovered as a minor ingredient in normal tea (Camellia sinensis L.) leaves,1 is also found in human diet in fruits and seeds of Theobroma grandiflorum (cupuacu) and the expanding buds and young leaves of the Chinese tea plant kucha (Camellia assamica var. kucha).2
Pulse-chase experiments using kucha leaves demonstrated that theacrine is synthesized by a multistep reaction involving adenosine and caffeine.3 Consequently, theacrine content is highest in the expanding buds and young leaves of kucha (∼2.8% of dry weight) with diminishing amounts found in mature leaves (∼1.3%).3–5 Caffeine is the most abundant purine alkaloid found in C. sinensis varieties, the source of many teas, including green, oolong, and black tea.6 However, C. assamica var. kucha and C. sinensis var. puanensis (Puan tea) contain theacrine as the predominant purine alkaloid.6
Theacrine is reported to possess diverse pharmacologic activity, including antioxidant,7 anti-inflammatory/analgesic,8 antidepressive,9 locomotor,10 and sedative/hypnotic properties.11 In rats, the administration of theacrine reversed the motor depression induced by adenosine receptor (AR) agonists, whereas theacrine-induced hyperlocomotion was reversed by dopamine receptor antagonists suggesting that theacrine's psychostimulatory mechanism of action involves modulation of the adenosinergic and dopaminergic pathways.10
Repeat administration of psychostimulants, for example, amphetamine and caffeine, can lead to either sensitization,12 such as a heightened response for a given dose, or pharmacodynamic tolerance,13 including a diminished response for a given dose. The altered response appears to be related to the dosing regimen. For example, chronic administration of large caffeine doses leads to tolerance,14 whereas intermittent administration induced sensitization.15 Interestingly, a consistent locomotor response was observed in rats following intraperitoneal administration of theacrine (48 mg/kg, once daily, 7 days) suggesting, at least over the dosing period, that neither tolerance nor sensitization occurred.10
A recent study in spontaneously hypertensive rats (SHR) also supports the notion that caffeine and theacrine elicit a different pharmacologic response despite their structural similarity. Intragastric administration of extracts from C. sinensis, C. ptilophylla, and C. assamica var. kucha, which contain as their predominant purine alkaloid, caffeine, theobromine, and theacrine, respectively, had different hemodynamic effects in SHR.16 C. sinensis extracts acutely increased systolic and diastolic blood pressure (DBP), as well as heart rate. In contrast, no pressor effects were noted following administration of either C. ptilophylla or C. assamica var. kucha extracts leading to the suggestion that the variety and content of purine alkaloids found in teas contribute significantly to the difference in the pressor response.
Kucha, a tea that grows in the highlands of southwest China in provinces such as Jinan, is consumed as a “healthy beverage.” It is estimated that a cup of kucha tea delivers ∼50–75 mg (≈1 mg/kg) of theacrine.17 Recently, a 90-day oral toxicology study in rats identified the no-observed-adverse-effect-level (NOAEL) for theacrine as 180 mg/kg/day.17 However, limited theacrine pharmacokinetic data exist in rats making it difficult to determine exposure levels associated with the rat NOAEL.18
Theacrine (as TeaCrine®) appears to be safe in humans when administered once daily for 8 weeks at doses up to 300 mg.19 However, theacrine pharmacokinetics in humans has not been characterized, thus complicating attempts to scale theacrine exposure from rats to humans. The purpose of this study was to determine theacrine pharmacokinetics and dose-linearity following oral administration in humans. Moreover, since theacrine and caffeine are co-ingested, whether as kucha tea or through dietary supplements such as TeaCrine, we also sought to determine whether or not caffeine altered theacrine pharmacokinetics and/or pharmacodynamics.
Experimental
Subjects
Eight healthy nonsmokers were recruited by word-of-mouth conversations, formal presentations discussing study participation, online postings, and recruitment flyers posted on and off campus. A total of four men and four women were recruited to complete this study. Subjects were regular consumers of stimulants (i.e., caffeine, 50–400 mg/day) within beverages or nutritional supplements, who did not report a history of adverse reactions to caffeine or other stimulants.
Health history, medication and dietary supplement usage, and physical activity questionnaires were completed by all subjects and reviewed in detail by an investigator to determine eligibility. Before participation, each subject was informed of all procedures, potential risks, and benefits associated with the study through both verbal and written form. The study procedures were approved by the University of Memphis Institutional Review Board for Human Subjects Research.
Study design and procedures
This study has a randomized, double-blind, four-arm crossover design with each subject receiving four treatments consisting of theacrine (25 mg), theacrine (125 mg), caffeine (150 mg), and theacrine (125 mg) plus caffeine (150 mg). Theacrine, administered as TeaCrine, was provided by Compound Solutions (Carlsbad, CA). Caffeine, administered as caffeine anhydrous, was obtained from Nutravative Ingredients (Allen, TX). Treatment sequence was randomized using a 4 × 4 Latin square. There was an ∼1-week washout period between treatments for all subjects.
Test visit procedures
Each study day, subjects reported to the laboratory between 6:00 and 7:00 am after a 10-hour fast and abstinence from beverages, drugs, or supplements containing alcohol or caffeine (48-hours) and strenuous physical exercise (24-hours). A catheter was inserted into the forearm vein for blood sampling. Duplicate measurements of resting heart rate and blood pressure were taken predose and before each timed blood sample. In addition, respiratory rate was counted in 1 min and body temperature was measured using an ear scanning thermometer (dual readings taken at each time). At ∼8:00 am, subjects received a single oral dose of test article accompanied by water. Blood samples (5 mL) were obtained at baseline (predose), and at 15, 30, 60, and 90 minutes, and 2, 4, 6, 8, and 24 hours postdose. Collected samples were processed and stored in multiple aliquots (∼500 μL, −70°C) until analyzed for theacrine, caffeine, and paraxanthine using LC-MS/MS.20
All subjects were instructed to consume their usual diet throughout the study period, with the exception of the actual days of testing. During the 2 days before each test day, subjects recorded all food and drink consumed and attempted to mimic this intake for the 2-day period before subsequent visits. Diet records were analyzed using nutrient analysis software (Food Processor SQL, version 9.9; ESHA Research, Salem, OR).
For the actual test days, standardized meals (meal replacement food bars [Clif “Builder's 20 g Protein Bar”] and ready-to-drink shakes [Orgain Organic Nutrition™]) were provided to subjects after sample collection at hour 2 and 6 (one shake and one-half bar at each time). Subjects were also provided with adequate meal replacement bars and shakes to consume following the 8-hour sample collection (during their time outside the laboratory). Each bar contained 280 calories, 10 g of fat, 29 g of carbohydrate, and 20 g of protein. Each shake contained 250 calories, 7 g of fat, 32 g of carbohydrate, and 16 g of protein.
No food other than what was provided to subjects was allowed during each study day, including both time spent in the laboratory and outside the laboratory. The only beverage that subjects were allowed to consume was water and the volume consumed while in the laboratory was matched for each test day (men: 94 ± 25 ounces; women: 78 ± 17 ounces). Subjects returned the following morning for the 24-hour blood collection, again in a 10-hour fasted state.
The same volume of meal replacement bars or shakes were consumed by each subject during each visit (both in laboratory and outside the laboratory). All subjects with the exception of one female subject consumed three shakes and three bars during the period of time outside the laboratory. The one female subject consumed two bars and two shakes. Physical activity was to remain similar for subjects throughout the study period, with the exception of refraining from strenuous physical activity during the 24 hours before each test day (and for the actual test day itself).
Pharmacokinetic study
Plasma concentration–time data were evaluated using noncompartmental methods in Phoenix WinNonlin (version 7.0; Pharsight Corporation, Mountain View, CA) with adjustment for lag time after oral administration.
The maximum concentration (Cmax), lag time (tlag), and time of maximum concentration (tmax) were determined from the plasma concentration versus time data. The area under the plasma concentration–time curve from time 0 to infinity was calculated using the trapezoidal rule extrapolated to time infinity. The terminal half-life (t1/2) was calculated using 0.693/k, with k as the terminal rate elimination constant estimated from the slope of the linear portion of the log plasma concentration versus time curve. The oral clearance (CL/F) was calculated by dividing the oral dose by area under the curve (AUC)0-∞. The apparent volume of distribution during the terminal elimination phase (Vz/F) was calculated by dividing CL/F by k.
Statistical analysis
Differences between treatment group values were determined for systolic blood pressure, diastolic blood pressure (DBP), rate pressure product, and heart rate. Data were analyzed using a 4 (condition) × 10 (time) analysis of variance, with Tukey post hoc testing as appropriate. No difference was detected for any variable (p > 0.05). The probability of interaction magnitude between theacrine and caffeine was determined using 90% confidence intervals about the geometric mean ratio of the observed pharmacokinetic parameters.21
Results
Subject characteristics
Eight physically active and healthy men (n = 4; aged 34.5 ± 7.0 years; 94.3 ± 13.1 kg) and women (n = 4; aged 22.5 ± 3.9 years; 66.4 ± 10.1 kg) completed this study. Men ingested a daily amount of caffeine equal to 143.8 ± 168.7 mg, while women ingested 144.3 ± 139.7 mg. All subjects tolerated the treatments well and no adverse event was noted. Dietary intake was not different across treatment conditions for calories, macronutrients, or micronutrients (p > 0.05).
Pharmacokinetics
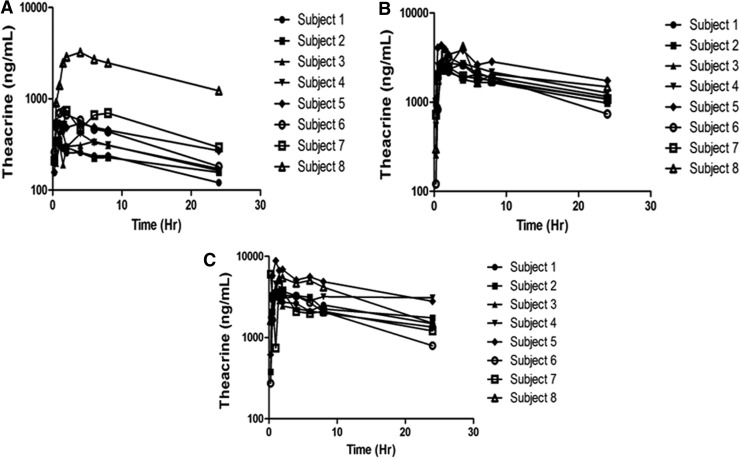
Mean plasma concentration–time profiles for theacrine, caffeine, and paraxanthine are shown in Figures 1–3. Theacrine was well absorbed following oral administration of theacrine alone reaching maximal concentration within ∼2 hours. Dose-adjusted theacrine pharmacokinetic parameters were not significantly different (Table 1).
FIG. 1.
Individual plasma concentrations of theacrine after a single oral dose of (A) theacrine 25 mg, (B) theacrine 125 mg, and (C) theacrine 125 mg plus caffeine 150 mg.
FIG. 2.
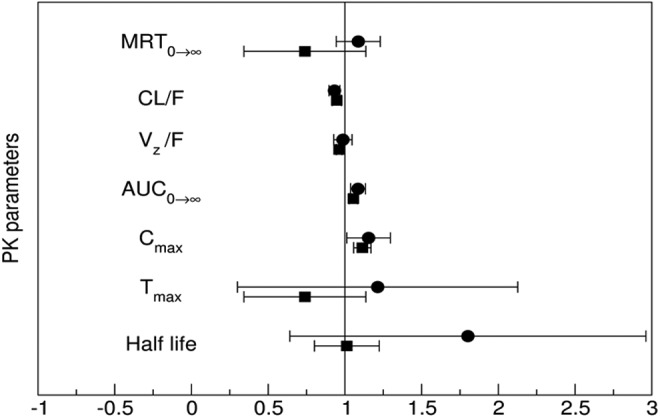
Forest plot illustrating the probability of interaction magnitude between theacrine and caffeine using 90% confidence intervals about the geometric mean ratio of the observed pharmacokinetic parameters following a single theacrine dose (●–25mg, ■–125 mg) alone or in combination with caffeine (150 mg). Abbreviations: MRT0→∞, mean residence time zero to infinity; CL/F, oral clearance; Vz/F, oral volume of distribution; AUC0→∞, area under the curve from zero to time infinity (dose normalized); Cmax, maximum plasma concentration (dose normalized); Tmax, time to reach maximum plasma concentration.
FIG. 3.
Individual plasma concentrations of caffeine after single oral dose of (A) caffeine 150 mg and (B) theacrine 125 mg plus caffeine 150 mg.
Table 1.
Theacrine Pharmacokinetics
| Parametera | Condition 1b | Condition 2c | Condition 4d |
|---|---|---|---|
| Cmax (ng/mL) | 34.1 ± 38.9 | 25.6 ± 5.5 | 38.6 ± 16.6 |
| Tmax (hours) | 1.8 (0.5–6.0) | 1.8 (1.0–4.0) | 1.0 (0.3–2.0) |
| t1/2 (hours) | 16.5 ± 2.4 | 26.1 ± 13.7 | 29.2 ± 25.3 |
| AUC (h × ng/mL/mg) | 809 ± 923 | 736 ± 312 | 1242 ± 1129 |
| CL/F (L/h) | 2.0 ± 0.9 | 1.6 ± 0.5 | 1.2 ± 0.6 |
| Vd/F (L) | 48.1 ± 23.5 | 51 ± 8.5 | 35.4 ± 12.4 |
| MRT (hours) | 24.9 ± 3.5 | 36.8 ± 18.9 | 41.7 ± 38.8 |
Tmax values are expressed as median (range). All other values are expressed as mean ± SD and represent dose-adjusted pharmacokinetic parameters.
Theacrine 25 mg.
Theacrine 125 mg.
Theacrine 125 mg + Caffeine 150 mg.
AUC, area under the curve; SD, standard deviation.
Theacrine absorption rate (Tmax) and half-life (t1/2) were unaffected by caffeine coadministration. However, caffeine coadministration significantly increased both mean theacrine exposure parameters Cmax (38.6 ± 16.6 vs. 25.6 ± 5.5 ng/mL) and AUC (1.2 ± 1.1 vs. 0.74 ± 0.31 h × μg/mL/mg) (Table 1), as well as geometric mean ratios (1.1 ± 0.06 and 1.1 ± 0.03) (Figure 2). Moreover, caffeine decreased both theacrine oral clearance (CL/F, 1.6 ± 0.49 vs. 1.2 ± 0.56 L/h) and oral volume of distribution (V/F, 50.5 ± 0.49 vs. 35.4 ± 12.4 L) by ∼30%. Of note, theacrine exposure (AUC) was consistently higher in Subject 8 than all other subjects in all treatment arms. However, caffeine pharmacokinetics in Subject 8 was similar to the other seven subjects.
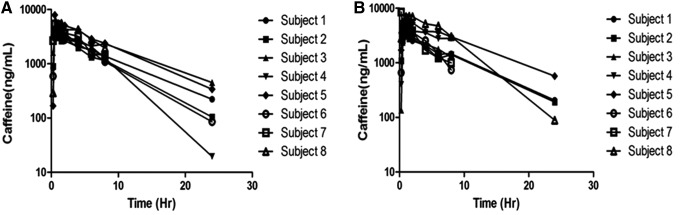
Caffeine pharmacokinetics was similar following caffeine alone or caffeine plus theacrine co-ingestion (Figs. 3 and 4 and Table 2). Likewise, theacrine co-ingestion did not alter paraxanthine exposure parameters, suggesting that caffeine metabolism was unaffected by theacrine (Table 3).
FIG. 4.
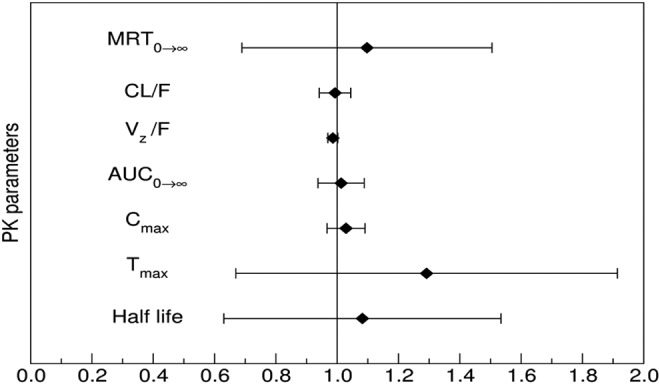
Forest plot illustrating the probability of interaction magnitude between caffeine and theacrine using 90% confidence intervals about the geometric mean ratio of the observed pharmacokinetic parameters following a single caffeine dose (150 mg) alone or in combination with theacrine (125 mg). Abbreviations: MRT0→∞, mean residence time zero to infinity; CL/F, oral clearance; Vz/F, oral volume of distribution; AUC0→∞, area under the curve from zero to time infinity (dose normalized); Cmax, maximum plasma concentration (dose normalized); Tmax, time to reach maximum plasma concentration.
Table 2.
Caffeine Pharmacokinetics
| Parametera | Condition 3b | Condition 4c |
|---|---|---|
| Cmax (ng/mL) | 33.4 ± 9.50 | 37.4 ± 11.8 |
| Tmax (hours) | 0.8 (0.5–1.5) | 1.0 (0.3–1.5) |
| t1/2 (hours) | 6.2 ± 3.8 | 5.5 ± 2.2 |
| AUC (h × ng/mL/mg) | 262 ± 74.1 | 323 ± 209 |
| CL/F (L/h) | 4.1 ± 1.1 | 4.3 ± 2.0 |
| Vd/F (L) | 33.5 ± 13.7 | 30.2 ± 12.4 |
| MRT (hours) | 8.4 ± 4.3 | 8.0 ± 3.2 |
Tmax values are expressed as median (range). All other values are expressed as mean ± SD and represent dose-adjusted pharmacokinetic parameters.
Caffeine 150 mg.
Theacrine 125 mg + Caffeine 150 mg.
Table 3.
Paraxanthine Pharmacokinetics
| Parametera | Condition 3b | Condition 4c |
|---|---|---|
| Cmax (ng/mL) | 7.3 ± 1.5 | 8.4 ± 3.5 |
| Tmax (hours) | 5.0 (4.0–8.0) | 7.0 (1.5–8.0) |
| t1/2 (hours) | 12.5 ± 12.7 | 14.8 ± 17.7 |
| AUC (h × ng/mL/mg) | 174 ± 152 | 209 ± 202 |
| MRT (hours) | 19.1 ± 18.6 | 22.7 ± 26.2 |
Tmax values are expressed as median (range). All other values are expressed as mean ± SD and represent dose-adjusted.
Caffeine 150 mg.
Theacrine 125 mg + Caffeine 150 mg.
Pharmacodynamics
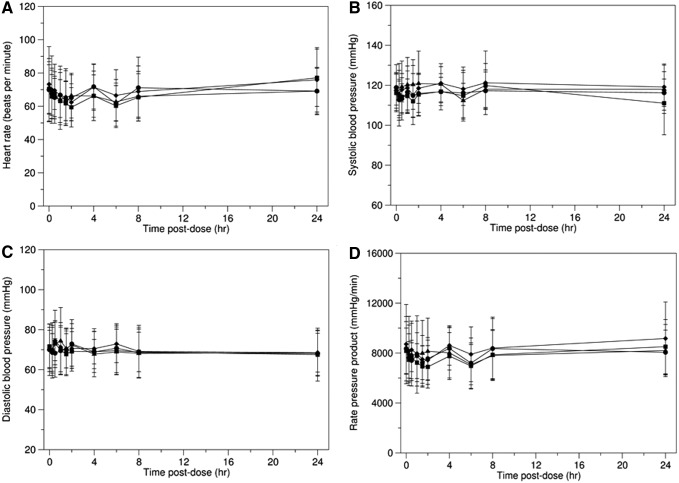
Hemodynamic parameters such as blood pressure and heart rate are elevated following coadministration of caffeine and other stimulants such as ephedrine.22 To determine the potential for a pharmacodynamic interaction between theacrine and caffeine, we evaluated systolic and diastolic blood pressure, heart rate, and rate-pressure product following administration of both theacrine (25 and 125 mg) and caffeine (150 mg) alone and in combination (theacrine 125 mg plus caffeine 150 mg). Heart rate decreased slightly over the first 2 hours following administration for each of the four conditions returning to baseline by 24 hours postingestion (Fig. 5A). systolic and diastolic blood pressure as well as rate-pressure product remained relatively constant across the 24-hour evaluation period for each of the four conditions (Fig. 5B–D).
FIG. 5.
Mean values in heart rate (A), systolic blood pressure (B), diastolic blood pressure (C), and rate-pressure product (D) after single-dose theacrine 25 mg (●), theacrine 125 mg (■), caffeine 150 mg (♦), or theacrine 125 mg plus caffeine 150 mg (▲). Data are presented as mean ± standard deviation.
Discussion
Recently, there has been intense interest in the use of theacrine as a dietary ingredient to enhance subjective “energy” levels in humans.23 A randomized, open-label dose-response (200 vs. 400 mg daily for seven consecutive days) study was conducted to evaluate the effect of theacrine (as TeaCrine) on subjective changes in cognitive, psychometric, and exercise attributes in healthy human subjects.24 Theacrine was found to be well tolerated with robust increases in energy, focus, and concentration compared to baseline values. Interestingly, no temporal response differences were reported, leading the authors to suggest that theacrine, unlike caffeine, does not appear to induce pharmacodynamic tolerance with chronic use, an intriguing suggestion requiring further study.
Anecdotal reports suggest that theacrine is frequently co-ingested with caffeine to enhance subjective feelings. Caffeine has often been described as eliciting pharmacokinetic and pharmacodynamic interactions with prescription as well as dietary supplement preparations.22,25,26 Despite anecdotal evidence, however, there has been no study examining the potential for caffeine to induce either a pharmacokinetic or pharmacodynamic interaction with theacrine.
In this study, we found that the pharmacokinetics of theacrine, when ingested alone, was similar between the two doses tested. However, following co-ingestion with caffeine, theacrine disposition was significantly altered. Specifically, caffeine decreased theacrine's oral clearance (CL/F), which correlated with enhanced theacrine exposure parameters, area under the plasma concentration–time curve (AUC), and maximum concentration (Cmax).
It is impossible to determine with certainty the exact mechanism for enhanced theacrine exposure, either decreased CL and/or increased oral bioavailability (F), in the absence of intravenous data. However, the finding that theacrine's elimination half-life (t1/2 α Vd/CL) was unaffected by caffeine supports the conclusion that caffeine enhanced theacrine's oral bioavailability (F), which is also consistent with decreased oral volume of distribution (Vd/F) of theacrine. Theacrine had no impact on the pharmacokinetics of caffeine or paraxanthine, which is the primary caffeine metabolite in humans formed by CYP1A2-mediated 3-N-demethylation.27,28
Caffeine is completely absorbed following oral administration.29 Thus, it is expected that theacrine would not have a reciprocal effect on caffeine bioavailability. Determination of whether or not theacrine is a CYP1A2 substrate will provide further insight into caffeine's effect on theacrine disposition, that is, enhanced fraction absorbed and/or reduced first-pass hepatic metabolism.
Intriguingly, one study subject was found to have exaggerated theacrine exposure in all treatment arms. The basis for this finding, whether genetic and/or environmental, is unclear. The presence of a 5-methyl substituent and a carbamide at the sixth position distinguish theacrine from caffeine. Because theacrine contains a 3-methyl substituent, the primary site of caffeine metabolism by CYP1A2-mediated demethylation, it is possible that theacrine is also susceptible to CYP1A2-mediated metabolism.
Caffeine exposure (AUC0→∞) is controlled by both environmental, as well as genetic factors.30 In particular, the CYP1A2 polymorphism (rs2470893), located in the common promoter region between CYP1A1 and CYP1A2, significantly associated with caffeine exposure in nonsmokers, but not in smokers. Nonsmokers heterozygous or homozygous for the CYP1A1/CYP1A2 A allele had a significantly lower caffeine exposure compared to nullizygous individuals.30
Additional environmental factors, including oral contraceptive use, mask the effect of genetics on caffeine metabolism.31 The role of pharmacogenetics in theacrine pharmacokinetics and pharmacodynamics is of potential importance, should CYP1A2 prove to be an important theacrine elimination pathway.
At the doses tested, we noted no difference in heart rate and blood pressure in subjects receiving theacrine or caffeine administered alone or in combination. Our data are consistent with other studies demonstrating that theacrine supplementation (up to 400 mg/day for 8 weeks) appears to be safe in humans with no adverse effects on hemodynamic parameters.19,24 Perhaps the most intriguing finding in repeat dose theacrine studies19,24 is the absence of either sensitization or pharmacodynamic tolerance. Caffeine is a mixed A1/A2 AR antagonist.
It is believed that the acute psychostimulatory activity of caffeine is related to its ability to antagonize the A1 AR, which removes inhibition of the A2A AR leading to NMDA-dependent release of glutamate and dopamine.32–34 Following chronic caffeine administration, however, caffeine's central effects shift from A1-dependent to A2A-dependent antagonism in tolerant individuals due to A1 AR desensitization.33
Administration of a cocktail containing both A1 and A2A AR antagonists blocks theacrine stimulating activity in rats.10 Unfortunately, simultaneous administration of A1 and A2A AR antagonists prevents determination of the individual contribution of A1 and A2A AR to the pharmacologic effects of theacrine. These data present the intriguing hypothesis that theacrine has different A1 and A2A binding affinities than caffeine, which permits discrimination between the A1 and A2A receptors at physiologically relevant concentrations.
Theacrine's preferential reliance on A2A AR antagonism would provide a mechanistic basis for lack of pharmacodynamic tolerance. AR binding affinities, as well as molecular dynamic simulations probing theacrine's interaction with AR subtypes would potentially prove helpful in unraveling this complex receptor/ligand interaction. Another pharmacodynamic consideration is that caffeine exhibits a biphasic dose–response curve with moderate doses enhancing the locomotor activity, while low and high doses suppress the locomotor activity.
In conclusion, coadministration of theacrine and caffeine results in a clinically significant pharmacokinetic interaction manifested as increased theacrine exposure. Enhanced oral bioavailability is the most likely mechanism by which caffeine alters theacrine exposure. However, further studies examining the contribution of presystemic elimination mechanisms, for example, efflux transport and/or gut metabolism, to theacrine bioavailability are needed to confirm the exact mechanism(s).
Despite the pharmacokinetic interaction between caffeine and theacrine, no difference was noted in hemodynamic parameters between groups, suggesting that coadministration of caffeine and theacrine is safe at the doses administered. Further studies designed to assess the potential for a pharmacodynamic interaction are needed to more thoroughly characterize the safety profile of combined ingestion of theacrine and caffeine.
Author Contributions
All authors contributed to the study in the following manner: H.H., D.M., and C.R.Y. were responsible for assay development, analysis of blood samples, and statistical analysis and modeling. L.B.C. and M.B. were responsible for subject recruitment, screening, and data collection. R.J.B. and C.R.Y. were equally responsible for the conception and design of the study, as well as article preparation. B.M. contributed to data interpretation and article preparation. All authors contributed to and approved the final article.
Acknowledgments
Funding for this work was provided by Compound Solutions, Inc., and The University of Memphis. In addition, this work was supported by a Grant (S10OD16226) from the Office of the Director, National Institutes of Health.
Author Disclosure Statement
R.J.B. and C.R.Y. have received research funding from Compound Solutions, Inc., including this study. These contracts paid for direct and indirect costs, as well as salary. This study was funded by Compound Solutions, Inc., who was consulted in the design of the study. All other authors have no competing interests.
References
- 1.Johnson TB. Purines in the plant kingdom: The discovery of a new purine in tea. J Am Chem Soc 1937;59:1261–1263 [Google Scholar]
- 2.Ye C, Lin Y, Su J, Song X, Zhang H. Purine alkaloids in Camellia assamica var. kucha Changet Wang. Acta Sci Nat Univ Sunyatseni 1999;38:82–86 [Google Scholar]
- 3.Zheng X-Q, Ye C-X, Kato M, Crozier A, Ashihara H. Theacrine (1,3,7,9-tetramethyluric acid) synthesis in leaves of a Chinese tea, kucha (Camellia assamica var. kucha). Phytochemistry 2002;60:129–134 [DOI] [PubMed] [Google Scholar]
- 4.Ashihara H, Kato M, Crozier A. Distribution, biosynthesis and catabolism of methylxanthines in plants. Handb Exp Pharmacol 2011;11–31 [DOI] [PubMed] [Google Scholar]
- 5.Li K, Shi X, Yang X, Wang Y, Ye C, Yang Z. Antioxidative activities and the chemical constituents of two Chinese teas, Camellia kucha and C. ptilophylla. Int J Food Sci Technol 2012;47:1063–1071 [Google Scholar]
- 6.Li Y-F, et al. A comparative analysis of chemical compositions in Camellia sinensis var. puanensis Kurihara, a novel Chinese tea, by HPLC and UFLC-Q-TOF-MS/MS. Food Chem 2017;216:282–288 [DOI] [PubMed] [Google Scholar]
- 7.Li WX, Li YF, Zhai YJ, Chen WM, Kurihara H, He RR. Theacrine, a purine alkaloid obtained from Camellia assamica var. kucha, attenuates restraint stress-provoked liver damage in mice. J Agric Food Chem 2013;61:6328–6335 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Yang X, Zheng X, Li J, Ye C, Song X. Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities. Fitoterapia 2010;81:627–631 [DOI] [PubMed] [Google Scholar]
- 9.Xie G, Wu M, Huang Y, Cao Y, Li L, Zhou H, Zhu R, Liao Y, Kurihara H. Experimental study of theacrine on antidepressant effects. Chin Pharmacol Bull 2009;9:13 [Google Scholar]
- 10.Feduccia AA, et al. Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: Involvement of adenosine and dopamine receptors. Pharmacol Biochem Behav 2012;102:241–248 [DOI] [PubMed] [Google Scholar]
- 11.Xu JK, Kurihara H, Zhao L, Yao XS. Theacrine, a special purine alkaloid with sedative and hypnotic properties from Cammelia assamica var. kucha in mice. J Asian Nat Prod Res 2007;9:665–672 [DOI] [PubMed] [Google Scholar]
- 12.Lê AD, et al. Neurobiological processes in alcohol addiction. Alcohol Clin Exp Res 2001;25:144S–151S [DOI] [PubMed] [Google Scholar]
- 13.Siegel S. Tolerance: Role of conditioning processes. NIDA Res Monogr 1991;213–229 [PubMed] [Google Scholar]
- 14.Svenningsson P, Nomikos GG, Fredholm BB. The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J Neurosci 1999;19:4011–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cauli O, Pinna A, Valentini V, Morelli M. Subchronic caffeine exposure induces sensitization to caffeine and cross-sensitization to amphetamine ipsilateral turning behavior independent from dopamine release. Neuropsychopharmacology 2003;28:1752–1759 [DOI] [PubMed] [Google Scholar]
- 16.Li S-B, et al. Differing chemical compositions of three teas may explain their different effects on acute blood pressure in spontaneously hypertensive rats. J Sci Food Agric 2015;95:1236–1242 [DOI] [PubMed] [Google Scholar]
- 17.Clewell A, et al. A 90-day oral toxicological evaluation of the methylurate purine alkaloid theacrine. J Toxicol 2016;2016:6206859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang WK, et al. [Determination of theacrine in rat plasma by RP-HPLC]. Zhongguo Zhong Yao Za Zhi 2013;38:753–756 [PubMed] [Google Scholar]
- 19.Taylor L, et al. Safety of TeaCrine®, a non-habituating, naturally-occurring purine alkaloid over eight weeks of continuous use. J Int Soc Sports Nutr 2016;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi EJ, et al. Simultaneous quantification of caffeine and its three primary metabolites in rat plasma by liquid chromatography-tandem mass spectrometry. Food Chem 2013;141:2735–2742 [DOI] [PubMed] [Google Scholar]
- 21.Food and Drug Administration. Guidance for Industry: Drug Interaction Studies—Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. US Department of Health and Human Services; (2012). Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm292362.pdf [Google Scholar]
- 22.Haller CA, Jacob P, 3rd, Benowitz NL. Enhanced stimulant and metabolic effects of combined ephedrine and caffeine. Clin Pharmacol Ther 2004;75:259–273 [DOI] [PubMed] [Google Scholar]
- 23.Habowski S, Sandrock J, Kedia A, Ziegenfuss TN. The effects of Teacrine™, a nature-identical purine alkaloid, on subjective measures of cognitive function, psychometric and hemodynamic indices in healthy humans: A randomized, double-blinded crossover pilot trial. J Int Soc Sports Nutr 2014;11:P49 [Google Scholar]
- 24.Ziegenfuss TN, Habowski SM, Sandrock JE, Kedia AW, Kerksick CM, Lopez HL. A two-part approach to examine the effects of theacrine (TeaCrine®) supplementation on oxygen consumption, hemodynamic responses, and subjective measures of cognitive and psychometric parameters. J Diet Suppl 2016;1–15 [DOI] [PubMed] [Google Scholar]
- 25.Cysneiros RM, Farkas D, Harmatz JS, von Moltke LL, Greenblatt DJ. Pharmacokinetic and pharmacodynamic interactions between zolpidem and caffeine. Clin Pharmacol Ther 2007;82:54–62 [DOI] [PubMed] [Google Scholar]
- 26.Fukasawa T, et al. Effects of caffeine on the kinetics of fluvoxamine and its major metabolite in plasma after a single oral dose of the drug. Ther Drug Monit 2006;28:308–311 [DOI] [PubMed] [Google Scholar]
- 27.Kot M, Daniel WA. Caffeine as a marker substrate for testing cytochrome P450 activity in human and rat. Pharmacol Rep 2008;60:789–797 [PubMed] [Google Scholar]
- 28.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A 1989;86:7696–7700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanchard J, Sawers SJ. Comparative pharmacokinetics of caffeine in young and elderly men. J Pharmacokinet Biopharm 1983;11:109–126 [DOI] [PubMed] [Google Scholar]
- 30.Matthaei J, et al. Heritability of caffeine metabolism: Environmental effects masking genetic effects on CYP1A2 activity but not on NAT2. Clin Pharmacol Therap 2016;100:606–616 [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen BB, Brix TH, Kyvik KO, Brosen K. The interindividual differences in the 3-demthylation of caffeine alias CYP1A2 is determined by both genetic and environmental factors. Pharmacogenetics 2002;12:473–478 [DOI] [PubMed] [Google Scholar]
- 32.Quarta D, et al. Adenosine receptor-mediated modulation of dopamine release in the nucleus accumbens depends on glutamate neurotransmission and N-methyl-D-aspartate receptor stimulation. J Neurochem 2004;91:873–880 [DOI] [PubMed] [Google Scholar]
- 33.Karcz-Kubicha M, et al. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology 2003;28:1281–1291 [DOI] [PubMed] [Google Scholar]
- 34.Solinas M, Ferre S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci 2002;22:6321–6324 [DOI] [PMC free article] [PubMed] [Google Scholar]