Abstract
Most ethanol is broken down in the liver in two steps by alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH2) enzymes, which metabolize down ethanol into acetaldehyde and then acetate. Some individuals from the Asian population who carry a mutation in the aldehyde dehydrogenase gene (ALDH2*2) cannot metabolize acetaldehyde as efficiently, producing strong effects, including facial flushing, dizziness, hypotension, and palpitations. This results in an aversion to alcohol intake and protection against alcoholism. The large prevalence of this mutation in the human population strongly suggests that modulation of ALDH2 expression by genetic technologies could result in a similar phenotype. scAAV2 vectors encoding ALDH2 small hairpin RNA (shRNA) were utilized to validate this hypothesis by silencing ALDH2 gene expression in human cell lines. Human cell lines HEK-293 and HepG2 were transduced with scAAV2/shRNA, showing a reduction in ALDH2 RNA and protein expression with the two viral concentration assayed (1 × 104 and 1 × 105 vg/cell) at two different time points. In both cell lines, ALDH2 RNA levels were reduced by 90% and protein expression was inhibited by 90% and 52%, respectively, 5 days post infection. Transduced HepG2 VL17A cells (ADH+) exposed to ethanol resulted in a 50% increase in acetaldehyde levels. These results suggest that gene therapy could be a useful tool for the treatment of alcoholism by knocking down ALDH2 expression using shRNA technology delivered by AAV vectors.
Keywords: : gene therapy, AAV, alcoholism, ALDH2, shRNA
Introduction
Alcohol is the most commonly used addictive substance in the world, causing approximately 3.3 million deaths every year (or 5.9% of all deaths).1 The cost of excessive alcohol consumption in the United States reached almost $250 billion in 2010, mainly due to loss of productivity, healthcare, and associated societal ills.2,3 The major health problems associated with alcohol abuse are liver-related diseases such as fibrosis, cirrhosis, hepatitis, and cancer. Others problems are brain and cardiovascular damage.4–6
Ethanol is metabolized in the liver in two steps, starting with the oxidation of ethanol to produce acetaldehyde, a reaction catalyzed by the cytosolic enzyme alcohol dehydrogenase (ADH). Acetaldehyde is then converted to acetate by a different enzyme called aldehyde dehydrogenase, mainly by its mitochondrial isoform (ALDH2).7
Approximately 50% of the Asian population are heterozygous and have a normal copy of the ALDH2 gene and a mutant copy that encodes for an inactive mitochondrial isoenzyme (ALDH2*2).8,9 These individuals have a diminished capacity to metabolize acetaldehyde. Therefore, acetaldehyde accumulates in the blood after ethanol consumption,10 producing effects including facial flushing, dizziness, hypotension, and palpitations.11 This promotes a reduction in alcohol consumption and protection against alcoholism.12,13
Currently, only three types of medication have been approved in the United States to treat alcohol dependence: disulfiram, naltrexone, and acamprosate.14,15 Unfortunately, none of these medications is widely prescribed due to the limited efficacy, side effects, poor levels of patient compliance, and variable response. This has increased the efforts to identify new medications for the treatment of alcohol use disorder (AUD).16–19
Gene therapy has emerged as an alternative treatment for AUD. Several proof-of-principle studies indicate that gene therapy using antisense or small hairpin RNA (shRNA) technology can be employed to reduce ALDH2 expression and accumulate acetaldehyde in treated cells or animal models. In 2008, a study in rats by Ocaranza et al. showed that a single intravenous administration of an anti-Aldh2 antisense gene carried by an adenoviral vector reduced liver ALDH2 activity and inhibited voluntary ethanol intake by 50%, achieving a long-term reduction of alcohol intake in alcohol-dependent animals.12,20–22
Several delivery vehicles have been used for gene therapy, including adenovirus, retrovirus, vaccinia virus, lipofection and adeno-associated virus (AAV). AAV is one of the most promising vectors because of its lack of pathogenicity, long-term expression, and broad tissue tropism. Thirteen human serotypes of AAV and >100 genomic variants have been discovered with different tropism. Among these, serotype 2 (AAV2) is the most widely used and described.23
Recent advances in vector development have provided new delivery reagents to test in vitro and in vivo. Self-complementary AAV2 (scAAV2) were used for the delivery of an ALDH2 shRNA in human cell lines. Self-complementary vectors have overcome the transduction rate-limiting step of conversion of a single-stranded (ss) to a double-stranded (ds) genome prior to expression.24,25 In addition, AAV vectors have shown great efficiency for transducing liver and hepatic cells, resulting in six clinical trials to date in humans. Of the various AAV serotypes, AAV2 is very efficient at transducing HepG2 cells.26
Small interfering RNA (siRNA) technology has been used to inhibit the expression of a target gene by degrading mRNA. One disadvantage of siRNA is its short half-life, decreasing its capability to regulate gene expression efficiently. To prolong the siRNA life-span, shRNA have been developed. shRNAs are endogenously processed into siRNAs in vivo. shRNA molecules are cleaved by Dicer, a dsRNA nuclease, producing a 21 nt dsRNA that forms a ribonucleoprotein complex called the RNA induced silencing complex (RISC) to exert their function.27
In this study, an ALDH2 shRNA delivered by a scAAV2 was tested to silence the expression of the ALDH2 enzyme in HEK-293 and HepG2 human cell lines.
Materials and Methods
Production of AAV vectors
AAV virus was produced in HEK-293 cells, as previously described, by the triple transfection method.28 This method uses three plasmids: the rep/cap plasmid pXR2, the helper plasmid pXX6-80, and the plasmid containing the AAV genome pscAAV_shRNA_ALDH2. Plasmid pscAAV_shRNA_ALDH2 was constructed by swapping the fragment containing the U6 promoter and the shRNA sequence digested with MslI and BamHI, from the plasmid pLKO_ALDH2 (provided by the Lenti-shRNA Core Facility, University of North Carolina) into the pSC-TTRmvmFIX digested with endonucleases HindIII and BglII. Cells were transfected employing polyethylenimine max (PEI) as DNA transporter. Forty-eight hours later, cells were collected, and the virus was purified by cesium chloride density gradient centrifugation. Peak fractions were dialyzed against phosphate-buffered saline (PBS), titered by quantitative polymerase chain reaction (PCR), and stored at −80°C for further experiments.
Cell lines and culture conditions
HEK-293, HepG2, and HepG2-VL17A cells were maintained at 37°C in a humidified incubator at 5% CO2. HEK-293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Sigma–Aldrich) and HepG2 cells in Eagle's minimum essential medium (EMEM; ATCC). Both media were supplemented with 10% fetal bovine serum and penicillin-streptomycin (100 IU/mL). HEK-293 cells expressing human ALDH2 were developed by transfecting cells with the plasmid pLX304-Blast-V5_ALDH2 (provided by the Lenti-shRNA Core Facility, University of North Carolina). Cells were transfected with PEI and selected in the presence of 30 μg/uL of blasticidin. The cell line designated HEK-293/ALDH2 showed ALDH2 expression by Western blot analysis.
Cell transfection and AAV infection
For cell transfection, HEK-293 cells were plated into a six-well plate and were transfected as described by Longo et al.29 using PEI max (linear, MW 25,000; Polysciences, Inc.). For AAV transduction, cells were incubated with AAV at a multiplicity of infection (MOI) of 104 or 105 vg/cell for 1 h before cells were plated. Cell transfection/transduction was followed by GFP expression. After the desired time, cells were collected, washed twice with PBS, and resuspended in M-PER reagent (Thermo Scientific) for western blotting or in Qiagen buffer for RNA extraction.
Western blot
Protein concentration was determined by Bradford (Sigma–Aldrich). For Western blot, 4 × Nupage reducing buffer was added to the protein lysate. The resulting solution was boiled for 10 min, chilled, and run in a 4–12% Bis-tris pre-cast gel. The gel was run in 1 × MOPS running buffer and transferred to a nitrocellulose membrane via iBlot 2 Dry Blotting System (Invitrogen). The membrane was blocked for 1 h with 5% nonfat milk in PBST solution (0.5% Tween 20 in 1 × PBS). ALDH2 Antibody (4G6A3) (Thermo Fisher MA5-17029) at 1:2,000 dilution in PBST or beta-actin antibody (mAbcam 8226) at 1:10,000 dilution in PBST were added to the membrane, and it was incubated overnight at 4°C with gentle agitation. The membrane was washed three times with PBST, and then incubated for an additional hour with a horseradish peroxidase conjugated secondary antibody at 1:10,000 dilution in PBST-5% nonfat dry milk. Western-Bright Sirius chemiluminescence reagent (Advansta) was used according to the protocol, and blots were developed in the Amersham Imager 600 (GE Healthcare Life Sciences). Membranes were stripped for reprobing using Restore Western Blot Stripping Buffer (Pierce) according to the manufacturer's instructions. Western blot band intensity was quantified with the ImageJ 1.50i software (https://imagej.nih.gov/ij).30
Reverse transcription PCR and real-time reverse transcription PCR
To quantify the levels of shRNA-targeted ALDH2 RNA transcript, a semi-quantitative reverse transcription PCR (RT-PCR) was performed. RNA was prepared using a Qiagen RNeasy kit according to the manufacturer's protocol. RNA samples were treated with DNase solution (Promega). For first-strand cDNA synthesis, 1 μg of RNA was used for retro transcription by SuperScript III RT (Thermo Fisher), followed by a RNase H treatment (Thermo Fisher). PCR was performed with Platinum Taq High fidelity DNA polymerase (Thermo Fisher). The reaction has a first denaturation step at 94°C for 5 min, then 25 cycles of denaturation at 94°C for 30 s, gradient annealing 62–54°C for 30 s, and extension at 72°C for 2 min, with a final step at 72°C for 10 min. The sequences of primers were as follows: GAPDH forward: ACCACAGTCCATGCCATCAC; GAPDH reverse: TCCACCACCCTGTTGCTGTA (product length: 452 bp); ALDH2 forward: CGCCTCTTGTCAGCCGC and ALDH2 reverse: GTGTATGCCTGCAGCCCG (product length: 1466 bp). A minus-reverse transcriptase control was included.
Quantitative RT-PCR was performed on a Roche 480 Lightcycler instrument using SYBR green Master Mix (Roche), following the manufacturer's protocol in 10 μL of reaction volume. Each reaction included 2 μL of cDNA, 5 μL of the probe master mix (2 × ), 0.25 μL of 20 μM forward and reverse primers, and 2.5 μL of RNAase/DNAase free water. A negative control without template was included in each run. The reaction has a hot start step at 95°C for 10 min for one cycle and 45 cycles of denaturation at 95°C for 10 s and annealing at 60°C for 10 s and extension at 72°C for 10 s. The baseline and the cp (crossing points) values were already calculated by the LC software 1.5 (Roche Applied Biosystems). The sequences of primers were as follows: GAPDH forward: ACCCAGAAGACTGTGGATGG; GAPDH reverse: TTCAGCTCAGGGATGACCTT; ALDH2 forward: TGTGTGGGTCAACTGCTATGA and ALDH2 reverse: TCACTTCAGTGTATGCCTGCA.
Ethanol and acetaldehyde measurements by gas chromatography
VL17-A Hepg2 cells (kindly provided by Dr. Dahn Clemens, University of Nebraska at Omaha) were incubated in sealed wells with 10, 25, or 100 mM of ethanol in EMEM for 24 h at 37°C. After incubation, a 60 μL aliquot of cell culture supernatant was analyzed for acetaldehyde and ethanol levels using a SRI 8610c gas chromatograph (Agilent Technologies). The ethanol standard curve ranged from 0 to 100 mM, and the acetaldehyde standard curve ranged from 0 to 250 μM. Samples were distributed into tubes containing 375 μL of water and 0.5 g of NaCl. Samples were heated in a water bath at 60°C for 10 min, and a 1.5 mL sample of headspace gas was removed and injected directly into the GC. Samples were run at 140°C through a Hayesep D column and detected with a flame ionization detector.
Hydrogen gas, carrier gas, and internal air generator flow rates were 13.3, 25, and 250 mL/min, respectively. Areas under the curve were analyzed with SRI PeakSimple software and converted to mM for ethanol and μM for acetaldehyde based on the standard curves.
Statistical analysis
Statistics were performed using Student's t-test. A p-value of <0.01 was considered a significant difference and is marked in the figures with asterisks.
Results
Knocking down effect of shRNA on ALDH2 in HEK-293 cells
shRNAs were used for knocking down the expression of the human ALDH2 gene. Five different shRNA-expressing pLKO.1 plasmids were tested for their potential to silence ALDH2 gene expression (Table 1). These shRNA sequences are specific to the human ALDH2 transcript. HEK-293 cells do not express a measurable amount of ALDH2 protein. Therefore, HEK-293 cells were co-transfected with a plasmid encoding the ALDH2 cDNA and with each shRNA plasmid, including a scramble-shRNA control and a GFP plasmid (as transfection efficiency control).
Table 1.
shRNA sequences and target regions
| N° | Sequence | Target region (NM_000690.2) |
|---|---|---|
| shRNA1 | GAGGACATCTATGATGAGTTT | 985:1005 |
| shRNA2 | GCAGATCATTCCGTGGAATTT | 540:560 |
| shRNA3 | CCCAACATCATCATGTCAGAT | 871:891 |
| shRNA4 | GCAGGCATACACTGAAGTGAA | 1497:1517 |
| shRNA5 | GCTGATAAGTACCACGGGAAA | 457:477 |
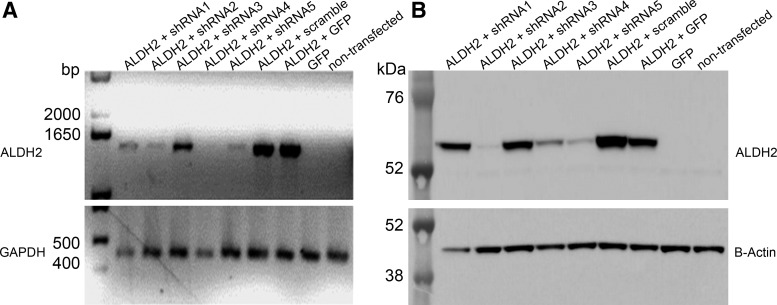
After 48 h, cells were collected, and ALDH2 gene expression was detected by RT-PCR (Fig. 1A) and Western blot (Fig. 1B). Three of the five shRNA plasmids exerted ALDH2 silencing. shRNA2 was chosen for further experiments and was cloned into the scAAV plasmid (pscAAV_shRNA), which was encapsidated into the AAV serotype 2 virion.
Figure 1.
Small hairpin RNA (shRNA) sequence selection. HEK-293 cells were collected 48 h post transfection, and RNA and protein were extracted. Aldehyde dehydrogenase (ALDH2) cDNA was co-transfected with five different shRNA plasmids (lanes 1–5); scramble and GFP plasmids co-transfection were used as ALDH2 expression control (lanes 6–7). GFP transfection (lane 8) and non-transfected cells (lane9). (A) Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR). ALDH2 expression was determined using primers designed to amplify a 1,460 bp product. Integrity of RNA was determined by amplifying a 425 bp region of the human GAPDH gene and is shown in the bottom panel. (B) Western blot of ALDH2 (56 kDa) and β-actin (42 kDa, bottom panel) in the HEK-293 cells treated as described above.
pscAAV_shRNA plasmid transfection to knock down ALDH2 in HEK-293/ALDH2 cells
A suitable cell line for testing shRNA should be a hepatic cell line because alcohol is metabolized in the liver, but HepG2 cells (human hepatocellular carcinoma) are difficult to transfect. To test the shRNA, HEK-293 cells could be used, however as they do not express enough enzyme, a stable ALDH2-expressing cell line was developed (HEK-293/ALDH2). ALDH2 expression in the selected cells was validated by RT-PCR and by Western blot (data not shown).
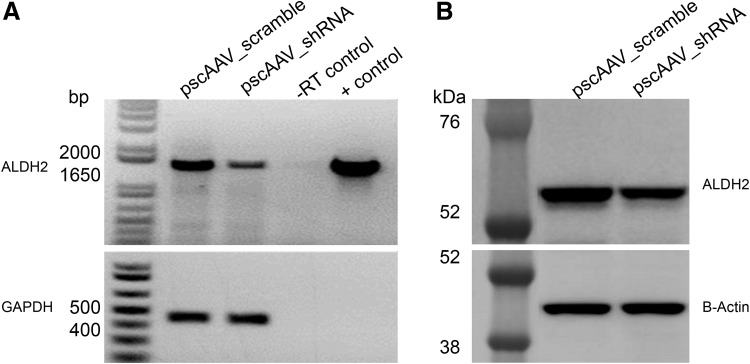
ALDH2-expressing HEK-293 cells were transfected with pscAAV_shRNA to corroborate that shRNA was still silencing ALDH2 expression. RNA and protein were extracted from the transfected cells and a semi-quantitative RT-PCR (Fig. 2A) and a Western blot were performed. Cells treated with the pscAAV_shRNA plasmid showed a reduction in ALDH2 expression compared to cells treated with the pscAAV_scramble control plasmid. A 40% reduction in ALDH2 expression was estimated by Western blot image analysis (Fig. 2B).
Figure 2.
ALDH2 silencing by adeno-associated virus (AAV) plasmid in HEK-293/ALDH2 transfected cells. ALDH2 stable-expressing HEK-293 cells were transfected with pscAAV_shRNA plasmid. After 48 h of transfection, RNA and proteins were collected for evaluation of ALDH expression. (A) ALDH2 RNA expression was evaluated by a semi-quantitative RT-PCR. pscAAV_scramble transfected cells were used as control (lane 1), pscAAV_shRNA transfected (lane 2), RT control (lane 3), and positive PCR control (lane 4). Integrity of RNA was determined by amplifying a 425 bp region of the human GAPDH gene and is shown in the bottom panel. (B) Western blot of ALDH2 (56 kDa) and β-actin (42 kDa, bottom panel) in the HEK-293/ALDH2 cells. Scramble control (lane 1) and shRNA treated (lane 2).
scAAV2/shRNA transduction to silence ALDH2 in HEK-293/ALDH2 cells
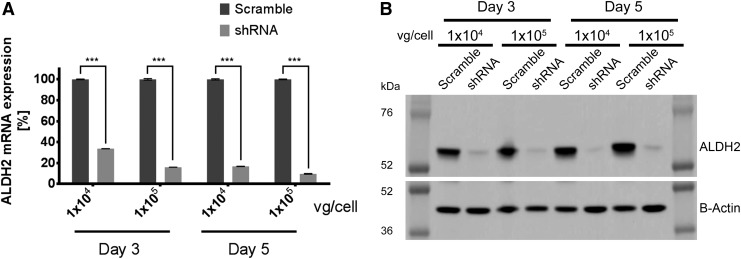
To examine whether the shRNA carried by the AAV viral vector was able to silence the ALDH2 gene, ALDH2 shRNA was packaged into double-stranded AAV serotype 2 (scAAV2/shRNA). HEK-293/ALDH2 cells were transduced at two different MOIs (1 × 104 and 1 × 105 vg/cell), and ALDH2 transcript and protein expression were evaluated at days 3 and 5 post infection (p.i.). As a control, cells were transduced with a scAAV2 carrying a scramble shRNA sequence (scAAV2/scramble). Transduction efficiency was >90%, as shown by GFP expression in HEK-293/ALDH2 cells transduced with scAAV2/GFP virus (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum). ALDH2 transcripts were quantified by reverse transcription followed by real-time PCR and compared to the scAAV2/scramble-treated cells. A reduction of 66% and 84% was observed 3 days p.i. for 1 × 104 and 1 × 105 vg/cell, respectively. For the RNA levels measured 5 days p.i., a reduction of 83% and 90% was achieved for the assayed MOIs (Fig. 3A). ALDH2 silencing was also evaluated at protein level by Western blot (Fig. 3B), and image analysis showed a reduction >90% for each of the four tested conditions.
Figure 3.
The knockdown efficiency of scAAV2/shRNA in HEK-293/ALDH2 cells. HEK-293/ALDH2 cells were infected with 1 × 104 or 1 × 105 vg/cell of scAAV2 carrying a scramble or shRNA sequence. At days 3 and 5 post infection, RNA and proteins were collected. The inhibition of scAAV2/shRNA on ALDH2 expression was analyzed by (A) real-time RT-PCR for ALDH2 transcript expression, where the ALDH2 mRNA copy number was calculated with normalization to GAPDH, and (B) Western blot of ALDH2 protein expression normalized by β-actin. Error bars indicate standard deviation. ***p < 0.001 compared to scramble-shRNA treated cells.
scAAV2/shRNA transduction to knock down ALDH2 in HepG2 cells
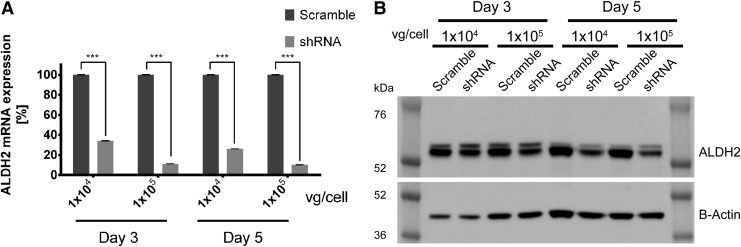
The final target of this therapy was silencing ALDH2 expression in the liver. AAV2 has shown in vitro a good transduction efficiency for the hepatic cells HepG2.26 Therefore, scAAV2/shRNA virus was used to infect HepG2 cells. Cell transduction efficiency was followed by GFP expression for 5 days. Transduced cells expressed GFP from the first day p.i., reaching the highest expression at the third day, followed by decaying GFP expression, as observed by fluorescent microscopy (Supplementary Fig. S2). RNA and protein were extracted from infected HepG2 cells (MOI 1 × 104 and 1 × 105 vg/cell) on the third and fifth day p.i. Real-time RT-PCR and Western blot were performed. Aldh2 transcript reduction of 66% and 74% was reached for 1 × 104 vg/cell on the third and fifth day p.i. respectively, and 89% and 90% reduction was observed for 1 × 105 vg/cell at both assayed time points (Fig. 4A). Protein expression was evaluated by Western blotting. For HepG2 cells, two bands were visualized for ALDH2 antibody. This might be due to the expression of different ALDH isoforms. For image analysis, only the small size band was considered. At day 3 p.i., a reduction of ALDH2 expression of 20% and 37% was achieved for the assayed MOIs (1 × 104 and 1 × 105 vg/cell). For the same assayed MOIs, protein expression was reduced by 33% and 52% on the fifth day p.i. (Fig. 4B).
Figure 4.
Inhibition of scAAV2/shRNA on ALDH2 expression in HepG2 cells. HepG2 cells were infected with 1 × 104 or 1 × 105 vg/cell of scAAV2 carrying a scramble or shRNA sequence. At days 3 and 5 post infection, RNA and proteins were collected. The inhibition of scAAV2/shRNA on ALDH2 expression was analyzed by (A) real-time RT-PCR for ALDH2 transcript expression, where the ALDH2 mRNA was normalized to GAPDH, and (B) Western blot of ALDH2 protein expression normalized by β-actin. Error bars indicate standard deviation. ***p < 0.001 compared to scramble-shRNA-treated cells.
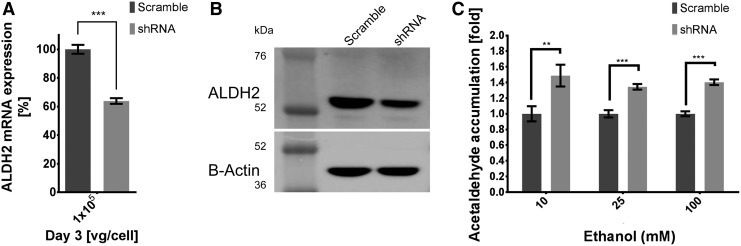
scAAV2 delivery of shRNA increases acetaldehyde levels
As a functional assay, HepG2 cells were exposed to ethanol, and acetaldehyde accumulation was measured. However, HepG2 cells do not express ADH and CYP2E1, the enzymes responsible for ethanol oxidation to acetaldehyde.31 VL-17A HepG2 cells were kindly provided by Dr. Dahn Clemens (Omaha, NE), and this cell line exhibits hepatocyte-like characteristics in response to ethanol. ALDH2 silencing was evaluated in VL-17A HepG2 cells transduced with 1 × 105 vg/cell on the third day p.i. by real time RT-PCR (Fig. 5A) and Western blot (Fig. 5B), showing an expression reduction of 40% compared to the scramble control in both analyses. Cells were incubated with ethanol (10, 25, and 100 mM) and acetaldehyde accumulation was measured by gas chromatography after 24 h of incubation. Samples treated with the scAAV2/shRNA virus showed an increase of acetaldehyde levels of 50%, 30%, and 40% for each ethanol concentration assayed (Fig. 5C).
Figure 5.
Acetaldehyde accumulation by ALDH2 knockdown on VL-17A HepG2 cells. VL-17A HepG2 cells were infected with 1 × 105 vg/cell of scAAV2/shRNA for 3 days. Inhibition on ALDH2 mRNA transcript was quantified by (A) real-time RT-PCR normalized with GAPDH expression. Silencing by scAAV2-carried shRNA of ALDH2 protein expression was compared to scramble-treated cells by (B) ALDH2 Western blot normalized by β-actin. VL-17A HepG2 hepatocyte-like ethanol response in shRNA treated cells compared to scramble treated cells was assayed by (C) acetaldehyde accumulation measured via gas chromatography at 10, 25, and 100 mM ethanol concentrations. Error bars indicate standard deviation. **p < 0.01 and ***p < 0.001 compared to scramble-shRNA treated cells.
Discussion
This study has demonstrated that a single shRNA was effective at inhibiting ALDH2 enzyme expression in vitro using scAAV in human cell lines.
ALDH2 silencing is transduced in a lower acetaldehyde metabolism, inducing its accumulation and dysphoric effects. Drinking alcohol with an impaired acetaldehyde metabolism is known as an Asian phenotype, resulting in an intolerance to alcohol consumed and thus preventing alcohol abuse.20
Several studies have shown that gene therapy could be an alternative for alcoholism treatment. These studies have used different silencing methodologies for reducing ALDH2 expression. The first approach was the use of molecules with a short half-life such as phosphorothioate nucleotides, but these were not a good alternative, since they required continuous administration.20 Another approach was the use of long antisense molecules delivered by adenovirus showing a longer half-life, but due to their long sequence and the existence of several ALDH isoforms, these molecules do not exclusively silence ALDH2 expression.12,22,32
Due to the disadvantages of the silencing molecules mentioned above, shRNA appears to be a better alternative for knocking down ALDH2 expression,21 since it is designed to target only one specific region of the mRNA. Another tool used for silencing ALDH2 are hairpin ribozymes.32 This strategy has shown a good efficacy at reducing ALDH2 activity in vitro or in vivo using a well-established rodent as model,33 showing a reduction of voluntary alcohol intake of 50%.12 It is important to highlight that the different silencing tools have never been tested for the human gene of ALDH2.
In spite of the great homology between the rat and human gene (88% identity), it is important to test the knocking down potential for the human gene, especially when the therapeutic molecule is designed to improve the specificity for targeting only one gene and not to affect the expression of relative genes or isoforms of the same protein. Some rules and guides have been established for the design of active shRNA molecules27,34,35 and many shRNA commercial libraries are available. Nevertheless, the efficiency of a shRNA sequence for knocking down the expression of a desired gene must be tested. In this work, five sequences were tested, of which three showed activity in the assayed conditions.
Studies have shown that despite the small packaging capacity of the scAAV (2.3 kb),36 multiple shRNA sequences could be cloned in tandem between the ITR viral sequences.37 This study have tested five shRNA sequences separately, showing different efficiency for knocking down ALDH2 expression at the RNA and protein level. For simplicity, only one of the tested shRNA was chosen to be cloned into the scAAV plasmid. Further experiments could use a combination of these shRNA sequences in the search for an additive effect to knock down ALDH2 expression.
AAVs have shown to be a promising choice as vectors for gene therapy. Several clinical trials have been conducted using AAV for many diseases, including hemophilia B.38–40 Different serotypes and variants have been described to target many tissues.23 Serotype 8 is the best for transducing liver in vivo, showing a reduced capacity to transduce the HepG2 cell line.41 For in vitro studies, AAV2s have shown the greatest ability to transduce different cell types.26 scAAV vectors have shown an enhanced transduction over AAV and have emerged as an important vehicle for delivering the shRNA sequence.24,42,43
scAAV2 carrying an shRNA for hALDH2 were used to transduce human cell lines in order to test their efficacy for reducing ALDH2 expression. HEK-293 cells were chosen, since they are easy to grow in culture and are known to be efficiently transduced by AAV2.
A stable ALDH2-expressing HEK-293 cell line was established and transduced with scAAV2/shRNA viral particles. Protein expression was reduced to minimal levels at 5 days p.i., even when cells were infected at low MOI (1 × 104 vg/cell). Due to the effectiveness for knocking down the ALDH2 expression in this cell line, a lower viral dose could be used, providing a safer profile for future clinical trials.44
HepG2 cells have been used as a model for ethanol metabolism and express constitutively measurable amounts of ALDH2.31,45 It is known that mitochondrial proteins are synthesized as larger precursors carrying a N-terminal extension that is cleaved off by specific peptidases.46 UNIPROT information of ALDH2 protein sequence reports that the displayed sequence is further processed into a mature form (www.uniprot.org/uniprot/P05091#sequences). ALDH2 protein sequence was analyzed by the MitoFates web server (http://mitf. cbrc.jp/MitoFates/),47 and a cleavage site was predicted. The immature isoform of ALDH2 would be the complete protein (517 aa) with a predicted molecular weight of 56.381 kDa, corresponding to the higher and less intense band of the Western blot, and the mature form (501 aa) with a predicted molecular weight of 54.558 kDa, the lower and more intense band of the Western blot.
HepG2 cells were transduced by scAAV2/shRNA at 1 × 105 vg/cell. ALDH2 expression was analyzed by Western blotting, reaching 52% protein expression inhibition on day 5 p.i. The scAAV2/shRNA was more efficient at knocking down the ALDH2 expression in the HEK-293/ALDH2 cell line compared to HepG2 cells. The difference could be explained by the efficiency of the virus to transduce and deliver the shRNA, the expression levels of the shRNA, and the half-life of the ALDH2 protein could be different in the tested cell lines, resulting in different levels of ALDH2 silencing. Previous studies have shown that a reduction >40% in ALDH2 activity resulted in a noticeable inhibition of alcohol intake in a rat model.12,20 This moderate reduction of ALDH2 activity can achieve the desirable effect of reducing alcohol consumption, minimizing the risks of complete silencing.32
As a functional assay, acetaldehyde accumulation was measured by gas chromatography in scAAV2/shRNA transduced cells. Transduced cells were cultured at three ethanol concentrations simulating light, moderate, and alcohol-dependent consumers.48,49 Acetaldehyde concentration increased by 50%, 30%, and 40%, respectively, compared to samples treated with the scramble control vector.
The present study has shown that scAAV2 vectors encoding a single ALDH2 shRNA are effective in decreasing mitochondrial ALDH2 in HEK-293/ALDH2, HepG2, and VL-17A HepG2 cell lines and increases acetaldehyde levels.
Supplementary Material
Acknowledgments
We are grateful to Dahn L. Clemens for providing the HepG2 VL17A cells, and Violeta Zaric, Darin Knapp, Melisa Soland, Matthew Hirsch, Xinghua Zeng, Matthew Smith, Ping Zhang, Alicia Lucero, and Sergio Mercado for their excellent technical assistance. A.C. Sanchez received National PhD Scholarships (#21120422) and Visiting Scholar Scholarships (2015) from the National Commission for Scientific and Technological Research (CONICYT). This work was partially supported by the Basal Programme of Conicyt (Chile) for funding of the Centre for Biotechnology and Bioengineering, CeBiB (project FB0001), by PAI project from Conicyt (Chile 2015 #7815120002), and by National Institutes of Health Grants R01DK084033 and R01AI117408 (to C.L. and R.J.S.), R01HL125749 (to C.L.), and P01HL112761 and R01AI072176 (to R.J.S.).
Author Disclosure
No competing financial interests exist.
References
- 1.World Health Organization. Global Status Report on Alcohol and Health 2014. Geneva, Switzerland: WHO Press, 2014 [Google Scholar]
- 2.Sacks JJ, Gonzales KR, Bouchery EE, et al. 2010 National and state costs of excessive alcohol consumption. Am J Prev Med 2015;49:e73–e79 [DOI] [PubMed] [Google Scholar]
- 3.Carr GD. Alcoholism: a modern look at an ancient illness. Prim Care 2011;38:9–21 [DOI] [PubMed] [Google Scholar]
- 4.Rundio A., Jr Understanding alcoholism. Nurs Clin North Am 2013;48:385–390 [DOI] [PubMed] [Google Scholar]
- 5.McKillop IH, Schrum LW. Alcohol and liver cancer. Alcohol 2005;35:195–203 [DOI] [PubMed] [Google Scholar]
- 6.Go VLW, Gukovskaya A, Pandol SJ. Alcohol and pancreatic cancer. Alcohol 2005;35:205–211 [DOI] [PubMed] [Google Scholar]
- 7.Cederbaum AI. Alcohol metabolism. Clin Liver Dis 2012;16:667–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng G-S, Yin S-J. Effect of the allelic variants of aldehyde dehydrogenase ALDH2*2 and alcohol dehydrogenase ADH1B*2 on blood acetaldehyde concentrations. Hum Genomics 2009;3:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CC, Lu RB, Chen YC, et al. Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. Am J Hum Genet 1999;65:795–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T. Pharmacogenetics of responses to alcohol and genes that influence alcohol drinking. J Stud Alcohol 2000;61:5–12 [DOI] [PubMed] [Google Scholar]
- 11.Fuller RK, Branchey L, Brightwell DR, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA 1986;256:1449–1455 [PubMed] [Google Scholar]
- 12.Ocaranza P, Quintanilla ME, Tampier L, et al. Gene therapy reduces ethanol intake in an animal model of alcohol dependence. Gene Ther 2008;32:52–57 [DOI] [PubMed] [Google Scholar]
- 13.Tu GC, Israel Y. Alcohol consumption by orientals in North America is predicted largely by a single gene. Behav Genet 1995;25:59–65 [DOI] [PubMed] [Google Scholar]
- 14.Zindel LR, Kranzler HR. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J Stud Alcohol Drugs Suppl 2014;75:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute on Alcohol Abuse and Alcoholism. Treatment for Alcohol Problems: Finding and Getting Help. NIH Publication No. 14–7974
- 16.Jones JD, Comer SD, Kranzler HR. The pharmacogenetics of alcohol use disorder. Alcohol Clin Exp Res 2015;39:391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helton SG, Lohoff FW. Pharmacogenetics of alcohol use disorders and comorbid psychiatric disorders. Psychiatry Res 2015;230:121–129 [DOI] [PubMed] [Google Scholar]
- 18.Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol 2008;75:34–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mark TL, Kranzler HR, Song X, et al. Physicians' opinions about medications to treat alcoholism. Addiction 2003;98:617–626 [DOI] [PubMed] [Google Scholar]
- 20.Garver E, Tu G, Cao Q, et al. Eliciting the low-activity aldehyde dehydrogenase Asian phenotype by an antisense mechanism results in an aversion to ethanol. J Exp Med 2001;194:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortínez G, Sapag A, Israel Y. RNA interference against aldehyde dehydrogenase-2: development of tools for alcohol research. Alcohol 2009;43:97–104 [DOI] [PubMed] [Google Scholar]
- 22.Karahanian E, Ocaranza P, Israel Y. Aldehyde dehydrogenase (ALDH2) activity in hepatoma cells is reduced by an Adenoviral vector coding for an ALDH2 antisense mRNA. Alcohol Clin Exp Res 2005;29:1384–1389 [DOI] [PubMed] [Google Scholar]
- 23.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther 2012;20:699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarty DM, Fu H, Monahan PE, et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther 2003;:2112–2118 [DOI] [PubMed] [Google Scholar]
- 25.McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther 2008;16:1648–1656 [DOI] [PubMed] [Google Scholar]
- 26.Ellis BL, Hirsch ML, Barker JC, et al. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol J 2013;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bofill-De Ros X, Gu S. Guidelines for the optimal design of miRNA-based shRNAs. Methods 2016;103:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc 2006;1:1412–1428 [DOI] [PubMed] [Google Scholar]
- 29.Longo PA, Kavran JM, Kim M, et al. Transient mammalian cell transfection wtih polyethylenimine. Methods Enzymol 2013;529:227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donohue TM, Osna NA, Clemens DL. Recombinant Hep G2 cells that express alcohol dehydrogenase and cytochrome P450 2E1 as a model of ethanol-elicited cytotoxicity. Int J Biochem Cell Biol 2006;38:92–101 [DOI] [PubMed] [Google Scholar]
- 32.Sapag A, Irrazábal T, Lobos-González L, et al. Hairpin ribozyme genes curtail alcohol drinking: from rational design to in vivo effects in the rat. Mol Ther Acids 2016;5:e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quintanilla ME, Israel Y, Sapag A, et al. The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addict Biol 2006;11:310–323 [DOI] [PubMed] [Google Scholar]
- 34.Elbashir SM, Harborth J, Weber K, et al. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 2002;26:199–213 [DOI] [PubMed] [Google Scholar]
- 35.Reynolds A, Leake D, Boese Q, et al. Rational siRNA design for RNA interference. Nat Biotechnol 2004;22:326–330 [DOI] [PubMed] [Google Scholar]
- 36.McCarty D, Monahan P, Samulski R. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 2001;8:1248–1254 [DOI] [PubMed] [Google Scholar]
- 37.Grimm D, Kay MA. Therapeutic short hairpin RNA expression in the liver: viral targets and vectors. Gene Ther 2006;13:563–575 [DOI] [PubMed] [Google Scholar]
- 38.Nathwani AC, Edward GD, Tuddenham SR, et al. Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginn SL, Alexander IE, Edelstein ML, et al. Gene therapy clinical trials worldwide to 2012—an update. J Gene Med 2013;15:65–77 [DOI] [PubMed] [Google Scholar]
- 40.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev 2011;12:341–355 [DOI] [PubMed] [Google Scholar]
- 41.Davidoff AM, Gray JT, Ng CYC, et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5 and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol Ther 2005;11:875–888 [DOI] [PubMed] [Google Scholar]
- 42.Li C, Xiao P, Gray SJ, et al. Combination therapy utilizing shRNA knockdown and an optimized resistant transgene for rescue of diseases caused by misfolded proteins. Proc Natl Acad Sci U S A 2011;108:14258–14263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Z, Sun J, Zhang T, et al. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of Hemophilia B at low vector dose. Mol Ther 2008;16:280–289 [DOI] [PubMed] [Google Scholar]
- 44.Nair N, Rincon MY, Evens H, et al. Computationally designed liver-specific transcriptional modules and hyperactive factor IX improve hepatic gene therapy. Blood 2014;123:3195–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clemens DL, Halgard CM, Miles RR, et al. Establishment of a recombinant hepatic cell line stably expressing alcohol dehydrogenase. Arch Biochem Biophys 1995;321:311–318 [DOI] [PubMed] [Google Scholar]
- 46.Gakh O, Cavadini P, Isaya G. Mitochondrial processing peptidases. Biochim Biophys Acta 2002;1592:63–77 [DOI] [PubMed] [Google Scholar]
- 47.Fukasawa Y, Tsuji J, Fu S-C, et al. MitoFates: improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol Cell Proteomics 2015;14:1113–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones A, Sternebring B. Kinetics of ethanol and methanol in alcoholics during detoxification. Alcohol Alcohol 1992;27:641–647 [PubMed] [Google Scholar]
- 49.Eckardt M, File S, Gessa G, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res 1998;22:998–1040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.