Abstract
Camptocormia is an axial postural deformity characterised by abnormal thoracolumbar spinal flexion. The symptom usually presents while standing, walking or exercising and is alleviated while sitting, lying in a recumbent position, standing against a wall or using walking support. There is no consensus on the degree of thoracolumbar flexion to define camptocormia. However, most authors usually use an arbitrary number of at least 45° flexion of the thoracolumbar spine when the individual is standing or walking. Aetiologies of camptocormia are heterogeneous, and Parkinson’s disease (PD) is one of its many causes. The prevalence of camptocormia in PD ranges from 3% to 18%. Central and peripheral mechanisms might both contribute to its pathogenesis. Although there is no established consensus for treatment of camptocormia in PD, there are non-pharmacological, pharmacological and surgical approaches that can be used.
INTRODUCTION
Camptocormia is an axial postural deformity characterised by abnormal thoracolumbar spinal flexion. The term camptocormia is composed of two Greek words: kamptos (to bend) and kormos (trunk)1 and was coined by two French neurologists, Rosanoff-Saloff and Souques, in 1915.2 However, this particular symptom was initially described in the literature by Brodie in 1837, who used the term ‘hysterical bent back’.3 Historically, most cases of camptocormia were considered conversion disorders.4–7 However, it is now clear that many organic disorders may produce camptocormia. The possible aetiologies causing camptocormia are summarised in box 1.8–49 Parkinson’s disease (PD) is a neurodegenerative disease that prominently affects dopaminergic neurons in the substantia nigra pars compacta resulting in dopamine depletion in the basal ganglia-thalamocortical circuits, causing motor and non-motor symptoms. In addition to the cardinal features of PD including classic parkinsonian tremor, rigidity, bradykinesia and postural instability, presentation of abnormal postures such as camptocormia, anterocollis, dropped head syndrome or Pisa syndrome (a coronal plane deformity defined as marked lateral flexion of the trunk, that is typically mobile) are not uncommon.50 Patients with PD are largely elderly. Another issue that might confuse the diagnosis of camptocormia in patients with PD is axial skeletal disorders especially kyphoscoliosis. The main pathology of kyphoscoliosis is abnormality of bony or articular structures of the vertebral column such as fracture, degenerative process or osteoporosis causing misalignment and fixed abnormal posture. The key feature to differentiate between camptocormia and kyphoscoliosis is the ability to correct the abnormal posture by using various manoeuvres such as lying down, using a high-frame walker (HFW) or standing against the wall. However, as noted earlier, the major population of PD is elderly; therefore both conditions can occur concomitantly and this would make it challenging to make the diagnosis. In this article, we will review camptocormia in PD, focusing on the definition, epidemiology, clinical manifestations, pathogenesis and treatment modalities including non-pharmacological, pharmacological and surgical approaches.
Box 1. Possible aetiologies of camptocormia.
SEARCH STRATEGY AND SELECTION CRITERIA
Much of the literature in this area consists of prevalence reports, case report, case series and observational studies. Relevant studies of all types were reviewed if they added new knowledge in this area. References for this review were found by searching PubMed using the terms “camptocormia” or “bent spine”, “Parkinson’s disease and camptocormia” or “camptocormia and treatment” from January 1946 to February 2015. Only reports published in English were included. The final reference list was generated on the basis of relevance to the topics covered in this review.
DEFINITION AND EPIDEMIOLOGY
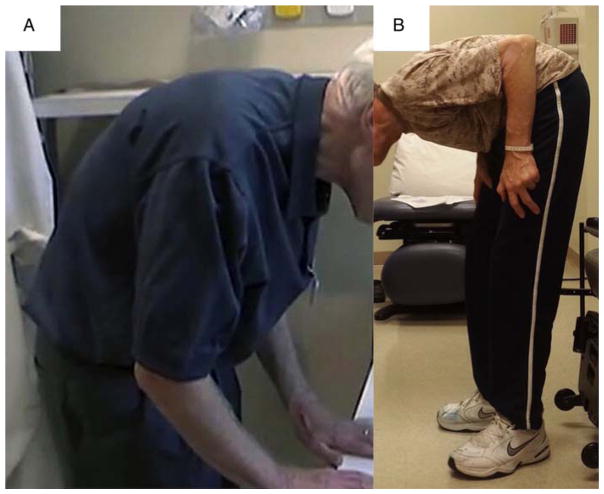
There is no consensus on the degree of thoracolumbar flexion for defining camptocormia. Most authors use an arbitrary number of at least 45° flexion of the thoracolumbar spine when the individual is standing or walking to define camptocormia.51–55 Some authors subclassified camptocormia into upper and lower camptocormia with upper camptocormia defined as abnormal truncal flexion at a point between the lower thoracic and upper lumbar spine (figure 1A) and lower camptocormia defined as flexion at the hip joint (figure 1B).56 We prefer this classification since it is useful for guiding selection of the appropriate muscles for injecting chemodenervation agents. The muscles that might play a major role in the upper subtype are bilateral abdominal external oblique together with bilateral abdominal internal oblique and rectus abdominis muscles, whereas rectus abdominis and iliopsoas muscles might be responsible for the lower subtype.
Figure 1.
Patients with Parkinson’s disease presenting with truncal flexion greater than 45° while standing. (A) Upper camptocormia defined as abnormal truncal flexion at a point between the lower thoracic and upper lumbar spine. (B) Lower camptocormia defined as flexion at the hip joint.
Since the aetiology of camptocormia is multifactorial, its prevalence in general has not been studied. However, there was a study conducted by Laroche and Cintas57 to evaluate the causes of camptocormia in 63 cases. The results showed that 40 of 63 cases were diagnosed as delayed-onset isolated paraspinal myopathy, including 4 cases concomitant with the diagnosis of PD. Twenty-three of 63 cases were diagnosed with various aetiologies including camptocormia due to PD (4 cases without evidence of paraspinal myopathy), combination of paraspinal myopathy and bilateral glutaeus medius myopathy (2 cases), limb girdle muscular dystrophy of unknown cause (8 cases), myotonic dystrophy (3 cases), facioscapulomuscular dystrophy (2 cases), inclusion body myositis (2 cases), polymyositis (1 case) and adult-onset progeria (1 case). Therefore, according to this study, only 8 of 63 cases (12.7%) were diagnosed as PD, of which 4 cases showed paraspinal myopathy. Azher and Jankovic58 investigated the aetiology of 16 patients with camptocormia and found that 11 cases (68.8%) were compatible with a diagnosis of PD. The prevalence of camptocormia in PD has also been studied. The largest study involving 1453 patients with PD was conducted by Yoritaka et al59 and reported a 9.5% rate of camptocormia. However, the prevalence of camptocormia in idiopathic PD (IPD) from all studies ranged from 3.0% to 17.7%.51–55,59,60 Most of these studies defined camptocormia using a minimum of 45° of thoracolumbar flexion; however, several studies did not mention the degree of spinal flexion.59,60 Studies of the prevalence of camptocormia in IPD are summarised in table 1.
Table 1.
Demographic data and prevalence of camptocormia in PD
| Year | Reference | Location | Number Surveyed |
Age (mean years±SD) |
Sex M/F | Disease duration of PD (mean years±SD) |
UPDRS III (mean score±SD) |
H&Y staging (mean±SD) |
MMSE (mean score±SD) |
Cognitive impairment (%) |
T-L flexion to define camptocormia (degrees) |
Prevalence (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | Ashour et al51 | USA | 164 | 71.3±n | 13/13 | 7.5±n | 61.3±n* | n | n | n | >45 | 12.2 |
| 2006 | Lepoutre et al60 | France | 700 | 68.6±7.4 | 15/8 | 10.3±5.1 | 26.7±10.0 | n | 27.1±2.3 | n | n | 3.0 |
| 2009 | Tiple et al52 | Italy | 294 | 74.6±6.8 | 13/6 | 13.0±7.2 | 42.3±14.5 | 3.5±0.8 | n | 26.3 | ≥45 | 6.9 |
| 2010 | Abe et al53 | Japan | 153 | 69.2±10.1 | 11/16 | 6.0±2.7 | 30.4±5.3 | 3.1±0.5 | 27.6±n | n | ≥45 | 17.7 |
| 2011 | Seki et al54 | Japan | 531 | 76.0±5.6 | 7/15 | 8.4±6.9 | 17.4±3.0 | 3.6±0.7 | n | 18.2 | ≥45 | 4.1 |
| 2013 | Yoritaka et al59 | Japan | 1453 | n | 650/803 | n | n | n | n | n | n | 9.5 |
| 2014 | Song et al55 | China | 705 | 65.7±10.8 | 28/18 | 9.0±5.2 | 64±13.8 | 4.1±0.7 | 21.3±5.4 | n | ≥45 | 6.5 |
Total score of all parts of UPDRS.
F, female; H&Y, Hoehn and Yahr; M, male; MMSE, Mini-Mental State Examination; n, not reported; PD, Parkinson’s disease; T-L, thoracolumbar; UPDRS III, Unified PD Rating Scale part III, motor subscore.
CLINICAL MANIFESTATIONS
Typically, camptocormia consists of gradually progressive thoracolumbar flexion without fixed kyphosis. In more severe cases, the patient might present with an anthropoid posture (severe flexion is defined as having the head and trunk parallel with the ground with arms swinging normally).61 Most patients with PD have associated low-back pain together with a history of degenerative spinal disease or back surgery.8,56,58,62,63 The symptom usually presents while standing or walking. In addition, some patients with PD reported that their symptoms were aggravated by stress, fatigue and strenuous exercise.60,62 Alleviating manoeuvres are sitting, lying in a recumbent position, standing against a wall or using walking support. Previous studies showed that camptocormia usually presented following a diagnosis of PD with the disease duration ranging from 6 to 8 years.8,58,60,62–64 Camptocormia can also present prior to a diagnosis of PD. There is one report of a patient who developed camptocormia 4 months prior to the diagnosis of PD.65 In addition, one patient in a case series that described clinical characteristics of 23 patients with PD with camptocormia developed camptocormia 3 years prior to the diagnosis of PD.60 Thus, the occurrence of camptocormia prior to PD is rare. The clinical differences between PD with and without camptocormia were reported in many studies. Patients with PD who developed camptocormia were likely women of advanced age, having a long duration of PD, high score on the Unified PD Rating Scale (UPDRS) part III, advanced Hoehn and Yahr stage, cognitive impairment, requiring a larger daily dose of levodopa, higher rate of motor fluctuation, and presenting autonomic dysfunctions such as urinary incontinence and constipation, compared with PD without camptocormia.51–55,59,60 However, it is premature to conclude that each of these factors is aetiologically related with camptocormia in PD. Camptocormia produces many negative consequences such as self-embarrassment or postural instability, whereas respiratory compromise is still controversial.66 A recent study conducted by Yamane et al67 showed that patients with PD with camptocormia frequently have deep venous thrombosis of their lower extremities when compared with PD without camptocormia (17% vs 4%, respectively).
PATHOGENESIS OF CAMPTOCORMIA IN PD
In general, pathogenesis of camptocormia can be explained by its aetiology, such as motor neuron disease, neuromuscular junction disease, muscle disease or dystonia. The pathogenesis of camptocormia in PD is not clearly understood, and central and peripheral mechanisms have both been proposed. Most explanations are derived from observations in patients with PD who responded to some type of treatment. However, the possible pathogenesis of camptocormia in PD can be subdivided into four groups (1) part of the disease progression seen in PD; (2) a form of dystonia occurring with PD; (3) a consequence of paraspinal myopathy due to the pathophysiology of PD or concomitantly occurring with PD and (4) caused by medications that were used in patients with PD.
Camptocormia is a part of the disease progression seen in PD
Camptocormia associated with PD usually emerges as the disease progresses.68 There are several lines of evidence supporting this contention. First, camptocormia is likely to develop in PD with a long duration of disease, high score on the UPDRS part III, and advanced Hoehn and Yahr stage. Second, camptocormia was reported in patients with PD who had no evidence of paraspinal myopathy proven by electromyography or histopathology.8,57 Third, there have been a few cases showing that camptocormic symptoms could be reversed following levodopa.63,69 Fourth, camptocormic symptoms were also improved by high-frequency subthalamic nucleus19,70–75 or globus pallidus interna deep brain stimulation76,77 (STN or GPi-DBS) together with improved motor symptoms in patients with PD. The argument against this idea is that most cases of camptocormia in PD did not respond to either levodopa8,58 or DBS.10,58,78 However, as is the nature of axial motor symptoms of PD, such as freezing of gait, some cases respond to either dopaminergic medications or DBS but some cases do not respond to any of these treatments. Furthermore, the roles of non-dopaminergic systems in PD with camptocormia are also unclear. Evidence has shown that anticholinergics, benzodiazepines and baclofen, failed to provide benefit for most patients with camptocormia.19,58 Such mixed evidence about neurotransmitter systems makes it difficult to draw any conclusions, but does suggest that there is not a simple relationship. Investigations of which structures in the central nervous system are involved in the pathogenesis of camptocormia in PD, such as measuring brain metabolism or functional connectivity, are limited because the camptocormic symptoms disappear while lying in the scanner. However, in a structural brain MRI study of camptocormia in patients with PD, the severity of the camptocormia was negatively correlated with the normalised sagittal surface of the pons and whole brain volume.79 There was no difference in [123I] β-CIT SPECT in PD with and without camptocormia.11 Therefore, we cannot specify which parts of the brain play a role in the pathogenesis of camptocormia in PD.
Camptocormia is a form of dystonia occurring with PD
Dystonia can occur in any part of the body in patients with PD. Many features of camptocormia are compatible with the definition of dystonia. Camptocormia presents and usually worsens while walking or exercising. This is compatible with action-induced dystonia. Dystonia can cause an abnormal posture in any body part, which is similar to camptocormia in terms of abnormal spinal flexion that might be the effect of strong abdominal muscle contraction.80 There are several manoeuvres to alleviate camptocormic symptoms that might be similar to ‘sensory tricks’ that present in dystonia such as standing against a wall or wearing a low-slung backpack. In axial dystonia such as cervical dystonia (CD), previous studies have shown that patients with CD have an internal postural perceptive distortion. When asking patients to direct their heads forward, patients with CD had a greater deviation of their head position compared with normal patients.81 Similarly, patients with early camptocormia might have a distorted concept of what an erect spine is. Finally, in isolated primary dystonic camptocormia, treating with GPi-DBS might show clinical improvement as rated by the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS), ranging from 33% to 100% after surgery.82 There were case reports of dopa-responsive dystonia presenting with camptocormia in which the symptom totally disappeared after a low dose of levodopa; no neurological disorders appeared during follow-up many years later.17,18 However, this hypothesis still lacks supportive physiological studies. Cortical inhibition such as short-intracortical inhibition, longintracortical inhibition and cortical silent period (investigated by transcranial magnetic stimulation), and blink reflex recovery cycle, that show abnormalities in dystonia,83–85 have not been explored in camptocormia.
Camptocormia is a consequence of paraspinal myopathy due to the pathophysiology of PD or concomitantly occurs with PD
The possibility of a myopathy causing camptocormia has been controversial. In this regard, camptocormia might be considered analogous to dropped head syndrome, also thought sometimes due to a myopathy. There are two hypotheses to explain the thoracolumbar paraspinal myopathy in camptocormia caused by PD itself.86 First, camptocormia might be a consequence of overusing paraspinal muscles due to rigidity in patients with PD. However, the results of muscle biopsies in camptocormia due to overuse myopathy were different compared to camptocormia in patients with PD. Muscle biopsy of the former condition revealed marked fibre necrosis, inflammation and macrophage reaction compared to the latter, which lacked an inflammatory process. The second hypothesis is proprioceptive dysregulation. Patients with PD have a poor ability to estimate the amplitude of joint motion in terms of accuracy as a result of abnormal proprioception compared to normal controls; the abnormal proprioception can also occur in axial musculature. According to the proprioceptive dysregulation hypothesis, inappropriate proprioceptive information will be sent back to supraspinal areas; at that point, supraspinal control provides inappropriate feed forward information to spinal interneuron circuits for adjusting the tone of axial muscles resulting in inappropriate muscle loading that might cause rigidity and myopathy, and, eventually, camptocormia. In addition, impaired proprioception of the axial musculature in PD correlated with the severity of the UPDRS part III.86 Additional evidence that might support the role of proprioceptive dysregulation causing paraspinal myopathy, which would be the proximate cause of camptocormia, comes from experimental Achilles tenotomy in rats.87 Tenotomy can alter the proprioceptive function of the muscle that attaches to the tendon. Histopathological findings of soleus muscle in the rat, following tenotomy, showed core-like lesions in centre and periphery of type 1 fibres with reducing activity of oxidative enzymes including succinate dehydrogenase and ATPase while increasing activity of acid phosphatase. These histopathological findings were similar to the typical biopsy of paraspinal muscles of PD with camptocormia.86 In this regard, camptocormia might develop due to secondary paraspinal myopathy that is influenced by proprioceptive dysregulation. Another possible pathophysiology of camptocormia in PD is that there is a concomitant myopathy not necessarily directly related to PD. There are many reports that bothinherited and acquired myopathies (box 1) could both be an aetiologies of camptocormia and these myopathies could occur as a concomitant condition in patients with PD. In this circumstance, we can consider that myopathy is a primary aetiology of camptocormia without any correlation to the pathophysiology of PD. However, there are strong arguments against camptocormia being due to paraspinal myopathy. First, there is no good evidence of truncal weakness in patients with camptocormia that should be present with myopathy. Second, the oedema of paraspinal muscles seen with muscle MRI is not specific and cannot confirm a myopathy.68 According to the cited evidence, the main pathogenesis of camptocormia in patients with PD does not appear to be solely explained by myopathy.
Camptocormia is caused by medications that are used in PD
To date, there is only one study suggesting that camptocormia in PD is the result of administering a dopaminergic agent.42 Galati et al reported a patient who was initially well controlled with 4 mg daily of ropinirole extended release tablets. However, she slowly developed a combination of camptocormia and Pisa syndrome. Levodopa was added and her motor symptoms improved; however, her abnormal posture worsened. The authors decided to withdraw ropinirole without modifying the levodopa dose. After withdrawing ropinirole, her posture returned to nearly normal within 3 months. Other cases of medication-induced camptocormia were reported in a patient with vascular parkinsonism who received pramipexole,41 and severe anxiety depression in a patient who received multiple antipsychotic medications including olanzapine and clozapine.39 In the former case, the patient’s camptocormia improved within a month after discontinuation of pramipexole. In the latter case, after her depression was controlled by multiple sessions of electroconvulsive therapy and antipsychotic medications were stopped, the patient’s posture was totally upright and she did not show any abnormal posture within the 6-month follow-up period. The possible explanation for antipsychotics-induced camptocormia might be related to their extrapyramidal side effects that might cause truncal flexion. However, the reason dopaminergic agents would induce camptocormia is unknown.
TREATMENT MODALITIES FOR CAMPTOCORMIA
Since there are a variety of aetiologies for camptocormia, accordingly there are different treatment modalities. Such treatment might be divided into three categories; non-pharmacological, pharmacological and surgical approaches including DBS (box 2).
Box 2. Potential treatment modalities for treating camptocormia in Parkinson’s disease.
Non-pharmacological approaches
Plaster corset
Low-slung backpack with weight
High-frame walker with forearm support
Thoraco-pelvic anterior distraction orthosis
-
Physiotherapies
Proprioceptive and tactile stimulation
Stretching
Postural re-education
Kinesiotaping on thoracolumbar paraspinal muscle
Pharmacological approaches
Levodopa
Botulinum neurotoxin injection
Lidocaine injection
Surgical approaches
Orthopaedic spinal surgical correction
Unilateral pallidotomy
-
Bilateral high-frequency deep brain stimulation
Subthalamic nucleus
Globus pallidus interna
Repetitive trans-spinal magnetic stimulation (immediate and short-lasting effect)
Non-pharmacological approaches
Historically, during World War I, Rosanoff-Saloff and Souques described a French soldier who was diagnosed with painful camptocormia with almost a 90° thoracolumbar flexion due to conversion disorder; the symptoms were improved by applying a plaster corset.88 Subsequently, there was a case report of a patient with PD who developed camptocormia resistant to dopaminergic treatment. His camptocormia totally disappeared while the patient wore a 6 kg low-slung backpack and returned after the backpack was removed.89 Another manoeuvre that was reported to alleviate camptocormia was using a HFW with forearm support.90 Three patients with IPD, using the HFW, improved their walking distances; using the HFW also reduced the degree of camptocormia and lessened these patients’ back pain. The evidence of wearing a corset, carrying a weighted backpack and using HFW with forearm support has only been reported in single cases or small case series. However, de Sèze et al91 conducted a prospective study with 15 camptocormic patients using a thoracopelvic anterior distraction orthosis and a physiotherapy programme; 2 of 15 were diagnosed with PD. The authors showed that an orthosis improved pain and quality of life (QoL), as assessed with a visual analogue scale. Average pain scores were reduced by 69% and 70% on days 30 and 90, respectively, when compared to day 0. The average improvement of QoL was 87% and 92% on days 30 and 90, respectively, when compared with day 0. Current evidence from a meta-analysis showed that physiotherapy could improve motor symptoms, especially gait and balance, in patients with PD.92 However, no strong evidence is available concerning the efficacy of physiotherapy for postural abnormalities in patients with PD. However, there has been a recent single-blind, randomised controlled trial that compared efficacy between postural rehabilitation (PR; n=7), PR plus using kinesiotaping (KT) on thoracolumbar paraspinal muscles (n=6) and no intervention (n=7) involving 20 patients with PD with anterior and/or lateral trunk bending. PR was targeted on proprioceptive and tactile stimulation, stretching and postural re-education through active movement execution. At the end of the first month, the physiotherapy groups, either PR or PR plus using KT, showed significant improvement in anterior trunk bending, gait and balance compared with pretreatment, and also showed significant improvement in anterior and lateral trunk bending, gait and balance compared with the no intervention group.93 Therefore, physiotherapy might be an option for improving the postural abnormality in patients with PD. Recently, there has been a randomised, single-blind, crossover, placebo-controlled study of 37 patients using repetitive trans-spinal magnetic stimulation (rTSMS). Eight 1 s trains of 5 Hz stimulation were given with intertrain interval of 10 s. The stimulation was delivered with a circular coil over the area of maximal thoracolumbar flexion. The primary outcome showed that rTSMS produced immediate relief of camptocormia in term of reduction of the degree of thoracolumbar flexion compared with sham stimulation (mean of 10.9° vs −0.1°, respectively). However, the authors measured the outcome only immediately after completing stimulation and did not investigate a longer lasting effect. Therefore, the value of rTSMS for treating camptocormia is not clear.94
Pharmacological approaches
The efficacy of oral levodopa for alleviating camptocormic symptoms is uncertain. Reports have indicated that oral levodopa could attenuate camptocormic symptoms in some cases of dopa-responsive dystonia,17,18 PD63,69 and multiple system atrophy.13,95 However, for PD, the effect of levodopa for reducing camptocormic symptoms was completely unpredictable. Bloch et al63 reported that approximately 20% of patients with PD with camptocormia received some benefit from oral levodopa. There was no report of other dopaminergic medications that improved camptocormic symptoms in patients with PD. Other oral antidystonic and antispasmodic medications, including trihexiphenidyl, baclofen, amantadine, biperiden, tetrabenazine, clonazepam and bromazepam were also disappointing.19
Botulinum neurotoxin (BoNT) injection and lidocaine have been used to treat camptocormia (table 2). In summary of this literature, BoNT serotype A, including abobotulinumtoxin A,96 onabotulinumtoxin A58,97 and incobotulinumtoxin A,98 was studied in patients with PD with camptocormia. Two studies used ultrasound-guided BoNT injection, one study used CT-guided BoNT injection and one study used a blind injection technique. Rectus abdominis and iliopsoas muscles were the main muscles injected. Several outcome measurements including objective58,96,97 and subjective97,98 outcome measurements were used to evaluate the efficacy of BoNT injection. Overall, the efficacy of BoNT injection is controversial. It is premature to draw the conclusion that BoNT is ineffective. There are many reasons, including small sample sizes, not injecting BoNT into other muscles (eg, abdominal external and internal oblique muscles) that might contribute to camptocormia, insufficient data for appropriate doses and types of BoNT, and lack of a standard clinical outcome measurement.
Table 2.
Studies of BoNT A and lidocaine for treating camptocormia in PD
| Study | Type of study |
Agents | Number of PD cases |
Targeted muscles | Dose (per each side) |
Procedure | Outcome measurements | Results | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|
| Azher and Jankovic58 | Case series | Onabotulinumtoxin A | 6 | IP (bilaterally in 2) RA (bilaterally in 2) IP and RA (bilaterally in 2) |
200 units for IP* and 150–400 for RA | No | Unclear methodologies of measurement | Good response in 3 patients; lasts for 8–24 weeks No response in 3 patients |
No |
| von Coelln et al96 | Case series | Abobotulinumtoxin A | 4 (3 PD, 1 MSA-P) | IP (bilaterally in 2 PD, unilaterally in 1 PD and 1 MSA-P) | 500–1500 units | US guided | Height measurement ▶ After standing for 5 min ▶ With maximal effort to stand upright without arm support at baseline and 2 weeks, 4 weeks and 4 months after the highest dose of BoNT injection |
No significant improvement of body height comparing baseline and 2 weeks, 4 weeks and 4 months after injection in all patients | Mild-to-moderate degree of weakness of hip flexion in all patients Transient painful itching sensation around the injection site in 1 patients |
| Colosimo and Salvatori97 | Case series | Onabotulinum toxinA | 2 | IP and RA (bilaterally) | 300 units for IP and 100 unit per RA | CT guided | Unclear methodologies of measurement (subjective and objective measurements) | No significant improvement of subjective and objective measurements 1 day, 1 weeks and 2 weeks after injection | No |
| Fietzek et al98 | Open label | Incobotulinumtoxin A | 10 (10 parkinsonism) | IP (bilaterally in 5) RA (bilaterally in other 5) |
220±40 units for IP and 200 ±63 units for RA |
US guided | Evaluation of goal attainment† 3 weeks after injection | No significant difference in goal attainment evaluated either by patients or physician | No |
| Furusawa et al56 | Open label | 1% lidocaine | 5 (with upper camptocormia) | RA and AEO (bilaterally in 5) AIO (bilaterally in 2) |
50 mg for RA, AEO and AIO | US guided | Reduction of average flexion angle‡ comparing before and after injection | Significant reduction of average flexion angle from 49.6±6.0 to 37.6±10° | No |
| Furusawa et al99 | Open label | 1% lidocaine | 12 (with upper camptocormia) | AEO (bilaterally) | 50 mg for AEO (first injection then repeated injection once daily for 4–5 days) | US guided | Reduction of average flexion angle‡ comparing before and after first injection, last day of repeated injection and 90 days after first injection | After first injection; 8/12 showed significant reduction of average flexion angle from 62.1±13.4 to 54.0 ±16.8° After last day of repeated injection, 9/12 showed significant reduction of average flexion angle from 62.1±13.4 to 49.0±18.5° At 90 days after first injection, 8/9 maintained the benefits |
One patient developed low-back pain after repeated injections with subsequent deterioration in posture |
200 units of onabotulinumtoxinA were injected into each side of iliopsoas muscle in two patients; however, there was no report of dosage of onabotulinumtoxinA in the other two patients.
All patients were asked to determine their subjective treatment goals including: upright gait, pain relief, grasping items that were out of reach, performing easier activities, for example, shopping or sports, and less stigmatisation. Each goal was based on the three levels of disability of the international classification of functioning, including impairment, activity limitations, or participation restrictions. Patients could choose to set 1, 2, or 3 goals. Then, they self-evaluated their goal attainment and were evaluated by a physician after 3 weeks.
The angle formed between a line perpendicular to the ground and a line linking the C7 vertebra with the inflection point of the trunk.
AEO, abdominal external oblique; AIO, abdominal internal oblique; BoNT, botulinum neurotoxin; IP, iliopsoas; MSA-P, multiple system atrophy; parkinsonian subtype; PD, Parkinson’s disease; RA, rectus abdominis; US, ultrasound.
Another injection agent investigated was lidocaine (with rehabilitation). Furusawa et al99 conducted a study using 50 mg of 1% lidocaine, which was injected bilaterally into the abdominal external oblique muscles under ultrasound guidance to treat 12 PD with upper camptocormia followed by rehabilitation, which emphasised truncal extension. Initially, a single injection was used and then repeated once daily for 4–5 days in all patients. The result showed that eight patients showed significant improvement in posture after a single injection together with rehabilitation. However, the effect diminished in several days. Repeated intervention produced long-term improvement in nine patients while eight of these patients revealed a lasting effect during the 90-day follow-up period. However, the data need to be reproduced in a randomised study involving a large population.
Surgical approaches
Surgical approaches to treat camptocormic symptoms in PD are orthopaedic surgical correction, pallidotomy, and DBS targeting the STN and GPi. For orthopaedic surgical correction, all reports showed some benefit from surgery in terms of pain reduction and postural correction compared to the preoperative stage.78,100,101 Only one report showed excellent results and the benefits were maintained at least 29 months after surgery.100 However, the surgical procedure was complicated in all patients. There was a case report of a patient with PD with 60° of camptocormia who received a unilateral right-sided pallidotomy 2 years after the onset of camptocormia that significantly improved her posture and gait.9 The patient reported that the benefit of the pallidotomy immediately occurred after surgery and lasted at least 6 months. At present, DBS is an option to treat symptoms of various types of dystonia and PD. However, there is no solid evidence that DBS is an appropriate treatment for camptocormia. The evidence for DBS to treat camptocormia either as an outcome of various types of dystonia or associated with PD came from case reports or a series of small cases. Recently, there was a case series of 16 patients (3 cases from the authors82 and 13 cases from the literature) who presented with camptocormia due to generalised dystonia, segmental dystonia or isolated camptocormia, and who received bilateral GPi-DBS.82 The results showed that GPi-DBS improved clinical outcome in terms of improved total and trunk subscores for the BFMDRS. These improved scores ranged from 33% to 100% for the total score and 50%–100% for the trunk subscore. The time of last follow-up ranged from 6 to 60 months after surgery. In PD with camptocormia, STN-DBS10,19,58,70–75,78,102 and GPi-DBS19,76–78 have both been used. The details of studies of DBS in PD with camptocormia are summarised in table 3. In summary, 56 patients with PD with camptocormia who received DBS to treat their camptocormic symptoms have been reported in the literature. Fifty-one patients received STN-DBS whereas five patients received GPi-DBS. Thirty-four of 56 patients (61%) noted that their posture had improved following DBS. However, the efficacy of STN-DBS or GPi-DBS to treat camptocormia in patients with PD should be measured cautiously because there are a variety of outcome measurement tools in every study. Therefore, the exact efficacy of STN-DBS and GPi-DBS on camptocormia in patients with PD is still inconclusive but it might be considered an option to treat levodopa non responsive camptocormia in patients with PD.
Table 3.
Studies of DBS for treating camptocormia in PD
| Study | Type of study |
Age (years)/sex |
PD duration (years) |
Target of bilateral DBS |
UPDRS III (preoperative) |
UPDRS III (postoperative with ‘on’ stimulation) |
Outcomes of postural abnormality | Efficacy of DBS on camptocormia |
Adverse effects |
|---|---|---|---|---|---|---|---|---|---|
| Schäbitz et al10 | Case report | 61/M | 30 | STN | n | n | No improvement | Not effective | n |
| 65/M | 12 | STN | n | n | No improvement | Not effective | n | ||
| Azher and Jankovic58 | Case series | n/n | n | STN | n | n | No improvement | Not effective | n |
| Micheli et al76 | Case report | 62/M | 9 | GPi | On med=24 Off med=52 |
3 months after DBS; off med=37 | Immediate effect after DBS that lasted up to 3 weeks; after that, effect wore off 6 months after DBS; obvious improvement of posture 14 months after DBS; only a slight forward flexion of the trunk |
Effective | No |
| Yamada et al70 | Case report | 71/F | 11 | STN | On med=38 Off med=47 |
3 months after DBS; on med=12 Off med=12 |
Immediate effect after DBS lasting for more than 20 months | Effective | No |
| Hellmann et al71 | Case report | 53/M | 25 | STN | On med=47* Off med=91* |
10 months after DBS; on med=29* Off med=58* |
Immediate effect after DBS, lasting at least 10 months | Effective | No |
| Sako et al72 | Case series | 60/F | 11 | STN | On med=38 Off med=47 |
46 months after DBS; on med=20 Off med=20 |
T-L angle (degrees); baseline=90 46 months=30 |
Effective | n |
| 54M | 10 | STN | On med=56 Off med=56 |
15 months after DBS; on med=15 Off med=15 |
T-L angle (degrees); baseline=90 15 months=30 |
Effective | n | ||
| 47/F | 8 | STN | On med=32 Off med=67 |
18 months after DBS; on med=32 Off med=32 |
T-L angle (degrees); baseline=90 18 months=10 |
Effective | n | ||
| 48/F | 5 | STN | On med=33 Off med=33 |
5 months after DBS; on med=5 Off med=5 |
T-L angle (degrees); baseline=50 5 months=10 |
Effective | n | ||
| 54/M | 11 | STN | On med=25 Off med=25 |
8 months after DBS; on med=7 Off med=7 |
T-L angle (degrees); baseline=60 8 months=10 |
Effective | n | ||
| 44/F | 9 | STN | On med=62 Off med=62 |
9 months after DBS; on med=11 Off med=11 |
T-L angle (degrees); baseline=60 19 months=10 |
Effective | n | ||
| Upadhyaya et al78 | Case report | 59/M | n | STN | n | n | 2 years after DBS: no improvement of camptocormia | Not effective | n |
| 59/M | n | GPi | n | n | 15 months after DBS: no improvement of camptocormia | Not effective | n | ||
| Umemura et al73 | Case series | 63/F | 19 | STN | On med=26 Off med=34 |
1 month after DBS; off med=21 12 months after DBS; off med=26 |
UPDRS item 28†; baseline=2 1 month=1 12 months=2 |
Not effective | No |
| 60/F | 20 | STN | On med=22 Off med=70 |
1 month after DBS; off med=11 12 months after DBS; off med=13 |
UPDRS item 28†; baseline=2 1 month=1 12 months=1 |
Effective | No | ||
| 59/M | 8 | STN | On med=9 Off med=29 |
1 month after DBS; off med=3 12 months after DBS; off med=4 |
UPDRS item 28†; 1 month=1 12 months=1 |
Effective | No | ||
| 63/F | 20 | STN | On med=20 Off med=40 |
1 month after DBS; off med=3 12 months after DBS; off med=10 |
UPDRS item 28†; baseline=2 1 month=1 12 months=1 |
Effective | No | ||
| 63/F | 13 | STN | On med=32 Off med=55 |
1 months after DBS; off med=22 12 months after DBS; off med=28 |
UPDRS item 28†; baseline=2 1 month=2 12 months=1 |
Effective | No | ||
| 79/M | 15 | STN | On med=42 Off med=50 |
1 months after DBS; off med=18 12 months after DBS; off med=27 |
UPDRS item 28†; baseline=2 1 month=2 12 months=2 |
Not effective | No | ||
| 66/F | 19 | STN | On med=42 Off med=79 |
1 months after DBS; off med=17 12 months after DBS; off med=21 |
UPDRS item 28†; baseline=3 1 month=3 12 months=2 |
Effective | No | ||
| 68/F | 10 | STN | On med=36 Off med=48 |
1 month after DBS; off med=35 12 months after DBS; off med=34 |
UPDRS item 28†; baseline=4 1 month=4 12 months=4 |
Not effective | No | ||
| Capelle et al19 | Case series | 73/M | 12 | STN | On med=21 Off med=43 |
16 months after DBS; off med=20 | BFMDRS-trunk‡; baseline=8 16 months=6 |
Effective | No |
| 65/M | 15 | STN | On med=15 Off med=36 |
12 months after DBS; off med=14 | BFMDRS-trunk‡; baseline=12 12 months=12 |
Not effective | No | ||
| 64/M | 10 | GPi | On med=25 Off med=47 |
36 months after DBS; off med=24 | BFMDRS-trunk‡; baseline=9 36 months=6 |
Effective | No | ||
| Asahi et al74 | Case series | 60/F | 13 | STN | On med=23 Off med=25 |
18 months after DBS; on med=7 Off med=7 |
T-L angle (degrees); baseline=50 18 months=28 |
Effective | n |
| 69/M | 12 | STN | On med=25 Off med=52 |
21 months after DBS; on med=20 Off med=26 |
T-L angle (degrees); baseline=40 21 months=21 |
Effective | n | ||
| 61/F | 12 | STN | On med=26 Off med=26 |
40 months after DBS; on med=15 Off med=25 |
T-L angle (degrees); baseline=36 40 months=23 |
Effective | n | ||
| 61/F | 9 | STN | On med=16 Off med=34 |
24 months after DBS; on med=16 Off med=22 |
T-L angle (degrees); baseline=50 24 months=51 |
Not effective | n | ||
| Thani et al77 | Case report | 57/F | 7 | GPi | On med=3 Off med=25 |
14 months after DBS; on med=14 Off med=14 |
2 months after DBS; obvious improvement of posture S-H-K angle (degrees); baseline=133 12 months=160 |
Effective | n |
| Lyons et al75 | Case report | 63/F | 1 | STN | On med=18 Off med=35 |
3 months after DBS; on med=15 Off med=20 |
T-L angle improved ~90% at 3 months after DBS | Effective | n |
| Schulz-Schaeffer Et al102 | Case series | Responders; n=13; age=65.8§; M:F=11:2 Nonresponders; n=12; age=68.6§; M:F=10:2 |
Responders=14.7§ Nonresponders=17§ |
STN=24; GPi=1 | Responders; On med=21.4§ Off med=n Non-responders On med=24.1§ Off med=n |
6–12 months after DBS; responders; on med=12.9§ Off med=n Non-responders; on med=18.2§ Off med=n |
Responder; 67–100% improvement of degree of trancal flexion within an average follow-up period of 30 months; VAS-handicap; 48% improvement; VAS-pain; 39.9% improvement Non-responders; no improvement of all outcomes (including 1 case on whom GPi stimulation was performed) |
Effective | n |
Total score of all part of UPDRS.
Postural abnormality sub-score of UPDRS (score is ranged from 0; normal to 4; severe).
Trunk sub-score of BFMDRS (score is ranged from 0–12).
Reported as a mean of age, duration of PD, and scores of UPDRS III.
BFMDRS-trunk, Burke-Fahn-Marsden Dystonia Rating Scale, trunk subscore; DBS, deep brain stimulation; F, female; GPi, globus pallidus interna; M, male; med, medication; n, not reported; PD, Parkinson’s disease; S-H-K angle, shoulder-hip-knee angle; STN, subthalamic nucleus; T-L angle, thoracolumbar angle; UPDRS III, Unified PD Rating Scale part III, motor subscore; VAS, visual analogue scales.
CONCLUSION
Although camptocormia in PD is not uncommon, there are several unresolved issues. For instance, upper and lower camptocormia might need separate consideration. This is a crucial point, because we would like to know which area, that is, thoracolumbar or hip, needs to be measured for the degree of camptocormia and which muscles need to be injected for treating with BoNT injection. Another issue is that the pathogenesis of this disorder is unknown. However, we believe that central and peripheral mechanisms can contribute to the pathogenesis, and, of course, the precise diagnosis should be helpful in designing therapy. Oral levodopa, BoNT injection or DBS might be considered treatment options for camptocormia in patients with PD. However, the efficacy of any of these treatments needs to be further explored in well-designed studies involving a large population of patients. Another critical issue is the lack of a uniform method to evaluate camptocormic symptoms at baseline and after receiving treatment. In the future, we anticipate that better understanding of the pathogenesis of camptocormia in PD will lead to more effective treatments.
Acknowledgments
The authors would like to acknowledge Devera Schoenberg, MSc for skilful editing. The Faculty of Medicine, Siriraj Hospital, Mahidol University has awarded a fellowship to Dr PS for carrying out research on Parkinson’s disease and Movement Disorders at Human Motor Control Section (HMCS), National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH), Bethesda, USA.
Funding NINDS Intramural Programme.
Footnotes
Contributors PS contributed to the manuscript preparation by writing the first draft, as well as by reviewing and critiquing it. MH contributed to the manuscript preparation by reviewing, critiquing, revising and editing it.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Competing interests MH serves as Chair of the Medical Advisory Board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6 780 413 B2 (Issued: 24 August 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7 407 478 (Issued: 5 August 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received licence fee payments from the NIH (from Brainsway) for licencing of this patent. He is on the Editorial Board of 20 journals, and has received royalties from publishing from Cambridge University Press, Oxford University Press, John Wiley & Sons, Wolters Kluwer and Elsevier. He has received honoraria for lecturing from Columbia University and the Parkinson and Aging Research Foundation. MH’s research at the NIH is largely supported by the NIH Intramural Programme. Supplemental research funds came from the Kinetics Foundation, for studies of instrumental methods to monitor Parkinson’s disease, and BCN Peptides, SA, for treatment studies of blepharospasm.
References
- 1.Pérez-Sales P. Camptocormia. Br J Psychiatry. 1990;157:765–7. doi: 10.1192/bjp.157.5.765. [DOI] [PubMed] [Google Scholar]
- 2.Wartenberg R. Camptocormia. Arch Neurol Psychiatry. 1946;56:327. doi: 10.1001/archneurpsyc.1946.02300200084005. [DOI] [PubMed] [Google Scholar]
- 3.Rosen JC, Frymoyer JW. A review of camptocormia and an unusual case in the female. Spine. 1985;10:325–7. doi: 10.1097/00007632-198505000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Sandler SA. Camptocormia: a functional condition of the back in neurotic soldiers. Arch Neurol Psychiatry. 1946;55:158–60. [PubMed] [Google Scholar]
- 5.Kosbab SP. Camptocormia-a rare case in the female. Am J Psychiatry. 1961;117:839–40. doi: 10.1176/ajp.117.9.839. [DOI] [PubMed] [Google Scholar]
- 6.Simon RC. A case of camptocormia: conversion in a schizophrenic process. Arch Gen Psychiatry. 1964;11:277–81. doi: 10.1001/archpsyc.1964.01720270049005. [DOI] [PubMed] [Google Scholar]
- 7.Lazare A, Klerman GL. Camptocormia in a female: a five-year study. Br J Med Psychol. 1970;43:265–70. doi: 10.1111/j.2044-8341.1970.tb02125.x. [DOI] [PubMed] [Google Scholar]
- 8.Djaldetti R, Mosberg-Galili R, Sroka H, et al. Camptocormia (bent spine) in patients with Parkinson’s disease-characterization and possible pathogenesis of an unusual phenomenon. Mov Disord. 1999;14:443–7. doi: 10.1002/1531-8257(199905)14:3<443::aid-mds1009>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Sławek J, Derejko M, Lass P. Camptocormia as a form of dystonia in Parkinson’s disease. Eur J Neurol. 2003;10:107–8. doi: 10.1046/j.1468-1331.2003.00503_1.x. [DOI] [PubMed] [Google Scholar]
- 10.Schäbitz WR, Glatz K, Schuhan C, et al. Severe forward flexion of the trunk in Parkinson’s disease: focal myopathy of the paraspinal muscles mimickingcamptocormia. Mov Disord. 2003;18:408–14. doi: 10.1002/mds.10385. [DOI] [PubMed] [Google Scholar]
- 11.Holler I, Dirnberger G, Pirker W, et al. Camptocormia in idiopathic Parkinson’s disease: [(123)I]beta-CIT SPECT and clinical characteristics. Eur Neurol. 2003;50:118–20. doi: 10.1159/000072514. [DOI] [PubMed] [Google Scholar]
- 12.Skidmore F, Mikolenko I, Weiss H, et al. Camptocormia in a patient with multiple system atrophy. Mov Disord. 2005;20:1063–4. doi: 10.1002/mds.20521. [DOI] [PubMed] [Google Scholar]
- 13.Sławek J, Derejko M, Lass P, et al. Camptocormia or Pisa syndrome in multiple system atrophy. Clin Neurol Neurosurg. 2006;108:699–704. doi: 10.1016/j.clineuro.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Diederich NJ, Goebel HH, Dooms G, et al. Camptocormia associated with focal myositis in multiple-system atrophy. Mov Disord. 2006;21:390–4. doi: 10.1002/mds.20686. [DOI] [PubMed] [Google Scholar]
- 15.Gavrylova N, Limousin N, Belin J, et al. Camptocormia as presenting sign in dementia with Lewy bodies. Clin Neurol Neurosurg. 2013;115:2397–8. doi: 10.1016/j.clineuro.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Brucki S, Nitrini R. Camptocormia in Alzheimer’s disease: an association? Mov Disord. 2008;23:156–7. doi: 10.1002/mds.21817. [DOI] [PubMed] [Google Scholar]
- 17.Van Gerpen JA. Dopa-responsive dystonic camptocormia. Neurology. 2006;66:1779. doi: 10.1212/01.wnl.0000218158.61678.55. [DOI] [PubMed] [Google Scholar]
- 18.Micheli F, Pardal MM. Dopa-responsive dystonic camptocormia. Neurology. 2007;68:1543. doi: 10.1212/01.wnl.0000265316.61721.63. [DOI] [PubMed] [Google Scholar]
- 19.Capelle HH, Schrader C, Blahak C, et al. Deep brain stimulation for camptocormia in dystonia and Parkinson’s disease. J Neurol. 2011;258:96–103. doi: 10.1007/s00415-010-5695-0. [DOI] [PubMed] [Google Scholar]
- 20.Van Gerpen JA. Camptocormia secondary to early amyotrophic lateral sclerosis. Mov Disord. 2001;16:358–60. doi: 10.1002/mds.1048. [DOI] [PubMed] [Google Scholar]
- 21.Castrillo Sanz A, Rodríguez Vico J, Hernández Barral M, et al. Early appearance of camptocormia in motor neuron disease: an association? J Clin Neuromuscul Dis. 2013;15:43–4. doi: 10.1097/CND.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 22.Kottlors M, Kress W, Meng G, et al. Fascioscapulohumeral muscular dystrophy presenting with isolated axial myopathy and bent spine syndrome. Muscle Nerve. 2010;42:273–5. doi: 10.1002/mus.21722. [DOI] [PubMed] [Google Scholar]
- 23.Jordan B, Eger K, Koesling S, et al. Camptocormia phenotype of FSHD: a clinical and MRI study on six patients. J Neurol. 2011;258:866–73. doi: 10.1007/s00415-010-5858-z. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos C, Papadimas GK, Spengos K, et al. Bent spine syndrome in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2011;43:615. doi: 10.1002/mus.21971. [DOI] [PubMed] [Google Scholar]
- 25.Dupeyron A, Stober N, Gelis A, et al. Painful camptocormia: the relevance of shaking your patient’s hand. Eur Spine J. 2010;19(Suppl 2):S87–90. doi: 10.1007/s00586-009-1086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Findlay AR, Lewis S, Sahenk Z, et al. Camptocormia as a late presentation in a manifesting carrier of duchenne muscular dystrophy. Muscle Nerve. 2013;47:124–7. doi: 10.1002/mus.23497. [DOI] [PubMed] [Google Scholar]
- 27.Kemta Lekpa F, Chevalier X, Dubourg O, et al. Isolated camptocormia revealing sporadic late onset nemaline myopathy. Presse Med. 2013;42:1142–4. doi: 10.1016/j.lpm.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Renard D, Castelnovo G, Fernandez C, et al. Camptocormia as presenting sign in myofibrillar myopathy. Neuromuscul Disord. 2012;22:987–9. doi: 10.1016/j.nmd.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Gómez-Puerta JA, Peris P, Grau JM, et al. Camptocormia as a clinical manifestation of mitochondrial myopathy. Clin Rheumatol. 2007;26:1017–19. doi: 10.1007/s10067-006-0259-5. [DOI] [PubMed] [Google Scholar]
- 30.Sakiyama Y, Okamoto Y, Higuchi I, et al. A new phenotype of mitochondrial disease characterized by familial late-onset predominant axial myopathy and encephalopathy. Acta Neuropathol. 2011;121:775–83. doi: 10.1007/s00401-011-0818-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zenone T, Streichenberger N, Puget M. Camptocormia as a clinical manifestation of polymyositis/systemic sclerosis overlap myositis associated with anti-Ku. Rheumatol Int. 2013;33:2411–15. doi: 10.1007/s00296-012-2412-6. [DOI] [PubMed] [Google Scholar]
- 32.Kim JM, Song EJ, Seo JS, et al. Polymyositis-like syndrome caused by hypothyroidism, presenting as camptocormia. Rheumatol Int. 2009;29:339–42. doi: 10.1007/s00296-008-0679-4. [DOI] [PubMed] [Google Scholar]
- 33.Goodman BP, Liewluck T, Crum BA, et al. Camptocormia due to inclusion body myositis. J Clin Neuromuscul Dis. 2012;14:78–81. doi: 10.1097/CND.0b013e3182650718. [DOI] [PubMed] [Google Scholar]
- 34.Ma H, McEvoy KM, Milone M. Sporadic inclusion body myositis presenting with severe camptocormia. J Clin Neurosci. 2013;20:1628–9. doi: 10.1016/j.jocn.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Kataoka H, Kiriyama T, Ueno S. Myasthenia gravis can cause camptocormia. J Neurol Neurosurg Psychiatry. 2012;83:469–70. doi: 10.1136/jnnp-2011-300311. [DOI] [PubMed] [Google Scholar]
- 36.Devic P, Choumert A, Vukusic S, et al. Myopathic camptocormia associated with myasthenia gravis. Clin Neurol Neurosurg. 2013;115:1488–9. doi: 10.1016/j.clineuro.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Terashima M, Kataoka H, Sugie K, et al. Coexistence of chronic inflammatory demyelinating polyneuropathy and camptocormia. J Neurol Neurosurg Psychiatry. 2009;80:1296–7. doi: 10.1136/jnnp.2008.155770. [DOI] [PubMed] [Google Scholar]
- 38.Kiuru S, Iivanainen M. Camptocormia, a new side effect of sodium valproate. Epilepsy Res. 1987;1:254–7. doi: 10.1016/0920-1211(87)90033-7. [DOI] [PubMed] [Google Scholar]
- 39.Vela L, Jiménez Morón D, Sánchez C, et al. Camptocormia induced by atypical antipsychotics and resolved by electroconvulsive therapy. Mov Disord. 2006;21:1977–80. doi: 10.1002/mds.21101. [DOI] [PubMed] [Google Scholar]
- 40.Robert F, Koenig M, Robert A, et al. Acute camptocormia induced by olanzapine: a case report. J Med Case Rep. 2010;4:192. doi: 10.1186/1752-1947-4-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakayama Y, Miwa H. Drug-induced camptocormia: a lesson regarding vascular Parkinsonism. Intern Med. 2012;51:2843–4. doi: 10.2169/internalmedicine.51.8469. [DOI] [PubMed] [Google Scholar]
- 42.Galati S, Möller JC, Städler C. Ropinirole-induced pisa syndrome in Parkinson disease. Clin Neuropharmacol. 2014;37:58–9. doi: 10.1097/WNF.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 43.Duman I, Baklaci K, Tan AK, et al. Unusual case of camptocormia triggered by lumbar-disc herniation. Clin Rheumatol. 2008;27:525–7. doi: 10.1007/s10067-007-0763-2. [DOI] [PubMed] [Google Scholar]
- 44.Nieves AV, Miyasaki JM, Lang AE. Acute onset dystonic camptocormia caused by lentricular lesions. Mov Disord. 2001;16:177–80. doi: 10.1002/1531-8257(200101)16:1<177::aid-mds1035>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 45.Jang W, Kwon HS, Kim JS, et al. A Parkinson’s disease patient with camptocormia caused by an esophageal hiatal hernia. Mov Disord. 2012;27:922–3. doi: 10.1002/mds.24989. [DOI] [PubMed] [Google Scholar]
- 46.Psimaras D, Maisonobe T, Delanian S, et al. Late onset radiation-induced camptocormia. J Neurol. 2011;258:1723–5. doi: 10.1007/s00415-011-5997-x. [DOI] [PubMed] [Google Scholar]
- 47.Kelly L, Perju-Dumbrava LD, Thyagarajan D, et al. Delayed postirradiation camptocormia. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-200083. pii: bcr2013200083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zwecker M, Iancu I, Zeilig G, et al. Camptocormia: a case of possible paraneoplastic aetiology. Clin Rehabil. 1998;12:157–60. doi: 10.1191/026921598670972368. [DOI] [PubMed] [Google Scholar]
- 49.Turkmen S, Hoffmann K, Demirhan O, et al. Cerebellar hypoplasia, with quadrupedal locomotion, caused by mutations in the very low-density lipoprotein receptor gene. Eur J Hum Genet. 2008;16:1070–4. doi: 10.1038/ejhg.2008.73. [DOI] [PubMed] [Google Scholar]
- 50.Doherty KM, van de Warrenburg BP, Peralta MC, et al. Postural deformities in Parkinson’s disease. Lancet Neurol. 2011;10:538–49. doi: 10.1016/S1474-4422(11)70067-9. [DOI] [PubMed] [Google Scholar]
- 51.Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Mov Disord. 2006;21:1856–63. doi: 10.1002/mds.21058. [DOI] [PubMed] [Google Scholar]
- 52.Tiple D, Fabbrini G, Colosimo C, et al. Camptocormia in Parkinson disease: an epidemiological and clinical study. J Neurol Neurosurg Psychiatry. 2009;80:145–8. doi: 10.1136/jnnp.2008.150011. [DOI] [PubMed] [Google Scholar]
- 53.Abe K, Uchida Y, Notani M. Camptocormia in Parkinson’s disease. Parkinsons Dis. 2010;2010 doi: 10.4061/2010/267640. pii: 267640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seki M, Takahashi K, Koto A, et al. Camptocormia in Japanese patients with Parkinson’s disease: a multicenter study. Mov Disord. 2011;26:2567–71. doi: 10.1002/mds.23955. [DOI] [PubMed] [Google Scholar]
- 55.Song W, Guo X, Chen K, et al. Camptocormia in Chinese patients with Parkinson’s disease. J Neurol Sci. 2014;337:173–5. doi: 10.1016/j.jns.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Furusawa Y, Mukai Y, Kobayashi Y, et al. Role of the external oblique muscle in upper camptocormia for patients with Parkinson’s disease. Mov Disord. 2012;27:802–3. doi: 10.1002/mds.24930. [DOI] [PubMed] [Google Scholar]
- 57.Laroche M, Cintas P. Bent spine syndrome (camptocormia): a retrospective study of 63 patients. Joint Bone Spine. 2010;77:593–6. doi: 10.1016/j.jbspin.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Azher SN, Jankovic J. Camptocormia: pathogenesis, classification, and response to therapy. Neurology. 2005;65:355–9. doi: 10.1212/01.wnl.0000171857.09079.9f. [DOI] [PubMed] [Google Scholar]
- 59.Yoritaka A, Shimo Y, Takanashi M, et al. Motor and non-motor symptoms of 1453 patients with Parkinson’s disease: prevalence and risks. Parkinsonism Relat Disord. 2013;19:725–31. doi: 10.1016/j.parkreldis.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Lepoutre AC, Devos D, Blanchard-Dauphin A, et al. A specific clinical pattern of camptocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:1229–34. doi: 10.1136/jnnp.2005.083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandler SA. Camptocormia, or the functional bent back. Psychosom Med. 1947;9:197–204. doi: 10.1097/00006842-194705000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Margraf NG, Wrede A, Rohr A, et al. Camptocormia in idiopathic Parkinson’s disease: a focal myopathy of the paravertebral muscles. Mov Disord. 2010;25:542–51. doi: 10.1002/mds.22780. [DOI] [PubMed] [Google Scholar]
- 63.Bloch F, Houeto JL, Tezenas du Montcel S, et al. Parkinson’s disease with camptocormia. J Neurol Neurosurg Psychiatry. 2006;77:1223–8. doi: 10.1136/jnnp.2006.087908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spuler S, Krug H, Klein C, et al. Myopathy causing camptocormia in idiopathic Parkinson’s disease: a multidisciplinary approach. Mov Disord. 2010;25:552–9. doi: 10.1002/mds.22913. [DOI] [PubMed] [Google Scholar]
- 65.Oh YS, Kim JS, Chung SW, et al. Camptocormia: as the first sign of Parkinson’s disease. Can J Neurol Sci. 2011;38:370–2. doi: 10.1017/s0317167100011665. [DOI] [PubMed] [Google Scholar]
- 66.Marinelli P, Colosimo C, Ferrazza AM, et al. Effect of camptocormia on lung volumes in Parkinson’s disease. Respir Physiol Neurobiol. 2013;187:164–6. doi: 10.1016/j.resp.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 67.Yamane K, Kimura F, Unoda K, et al. Postural abnormality as a risk marker for leg deep venous thrombosis in Parkinson’s disease. PLoS ONE. 2013;8:e66984. doi: 10.1371/journal.pone.0066984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jankovic J. Camptocormia, head drop and other bent spine syndromes: heterogeneous etiology and pathogenesis of Parkinsonian deformities. Mov Disord. 2010;25:527–8. doi: 10.1002/mds.23139. [DOI] [PubMed] [Google Scholar]
- 69.Ho B, Prakash R, Morgan JC, et al. A case of levodopa-responsive camptocormia associated with advanced Parkinson’s disease. Nat Clin Pract Neurol. 2007;3:526–30. doi: 10.1038/ncpneuro0584. [DOI] [PubMed] [Google Scholar]
- 70.Yamada K, Goto S, Matsuzaki K, et al. Alleviation of camptocormia by bilateral subthalamic nucleus stimulation in a patient with Parkinson’s disease. Parkinsonism Relat Disord. 2006;12:372–5. doi: 10.1016/j.parkreldis.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 71.Hellmann MA, Djaldetti R, Israel Z, et al. Effect of deep brain subthalamic stimulation on camptocormia and postural abnormalities in idiopathic Parkinson’s disease. Mov Disord. 2006;21:2008–10. doi: 10.1002/mds.21090. [DOI] [PubMed] [Google Scholar]
- 72.Sako W, Nishio M, Maruo T, et al. Subthalamic nucleus deep brain stimulation for camptocormia associated with Parkinson’s disease. Mov Disord. 2009;24:1076–9. doi: 10.1002/mds.22529. [DOI] [PubMed] [Google Scholar]
- 73.Umemura A, Oka Y, Ohkita K, et al. Effect of subthalamic deep brain stimulation on postural abnormality in Parkinson disease. J Neurosurg. 2010;112:1283–8. doi: 10.3171/2009.10.JNS09917. [DOI] [PubMed] [Google Scholar]
- 74.Asahi T, Taguchi Y, Hayashi N, et al. Bilateral subthalamic deep brain stimulation for camptocormia associated with Parkinson’s disease. Stereotact Funct Neurosurg. 2011;89:173–7. doi: 10.1159/000324907. [DOI] [PubMed] [Google Scholar]
- 75.Lyons M, Boucher O, Patel N, et al. Long-term benefit of bilateral subthalamic deep brain stimulation on camptocormia in Parkinson’s disease. Turk Neurosurg. 2012;22:489–92. doi: 10.5137/1019-5149.JTN.3857-10.0. [DOI] [PubMed] [Google Scholar]
- 76.Micheli F, Cersósimo MG, Piedimonte F. Camptocormia in a patient with Parkinson disease: beneficial effects of pallidal deep brain stimulation. Case report. J Neurosurg. 2005;103:1081–3. doi: 10.3171/jns.2005.103.6.1081. [DOI] [PubMed] [Google Scholar]
- 77.Thani NB, Bala A, Kimber TE, et al. High-frequency pallidal stimulation for camptocormia in Parkinson disease: case report. Neurosurgery. 2011;68:E1501–5. doi: 10.1227/NEU.0b013e318210c859. [DOI] [PubMed] [Google Scholar]
- 78.Upadhyaya CD, Starr PA, Mummaneni PV. Spinal deformity and Parkinson disease: a treatment algorithm. Neurosurg Focus. 2010;28:E5. doi: 10.3171/2010.1.FOCUS09288. [DOI] [PubMed] [Google Scholar]
- 79.Bonneville F, Bloch F, Kurys E, et al. Camptocormia and Parkinson’s disease: MR imaging. Eur Radiol. 2008;18:1710–19. doi: 10.1007/s00330-008-0927-8. [DOI] [PubMed] [Google Scholar]
- 80.Kataoka H, Tonomura Y, Eura N, et al. Painful abdominal contractions in patients with Parkinson disease. J Clin Neurosci. 2012;19:624–7. doi: 10.1016/j.jocn.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 81.Müller SV, Gläser P, Tröger M. Disturbed ergocentric space representation in cervical dystonia. Mov Disord. 2005;20:58–63. doi: 10.1002/mds.20293. [DOI] [PubMed] [Google Scholar]
- 82.Reese R, Knudsen K, Falk D, et al. Motor outcome of dystonic camptocormia treated with pallidal neurostimulation. Parkinsonism Relat Disord. 2014;20:176–9. doi: 10.1016/j.parkreldis.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 83.Edwards MJ, Talelli P, Rothwell JC. Clinical applications of transcranial magnetic stimulation in patients with movement disorders. Lancet Neurol. 2008;7:827–40. doi: 10.1016/S1474-4422(08)70190-X. [DOI] [PubMed] [Google Scholar]
- 84.Cohen LG, Ludlow CL, Warden M, et al. Blink reflex excitability recovery curves in patients with spasmodic dysphonia. Neurology. 1989;39:572–7. doi: 10.1212/wnl.39.4.572. [DOI] [PubMed] [Google Scholar]
- 85.Eekhof JL, Aramideh M, Bour LJ, et al. Blink reflex recovery curves in blepharospasm, torticollis spasmodica, and hemifacial spasm. Muscle Nerve. 1996;19:10–15. doi: 10.1002/(SICI)1097-4598(199601)19:1<10::AID-MUS2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 86.Wrede A, Margraf NG, Goebel HH, et al. Myofibrillar disorganization characterizes myopathy of camptocormia in Parkinson’s disease. Acta Neuropathol. 2012;123:419–32. doi: 10.1007/s00401-011-0927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karpati G, Carpenter S, Eisen AA. Experimental core-like lesions and nemaline rods: a correlative morphological and physiological study. Arch Neurol. 1972;27:237–51. doi: 10.1001/archneur.1972.00490150045008. [DOI] [PubMed] [Google Scholar]
- 88.Finsterer J, Strobl W. Presentation, etiology, diagnosis, and management of camptocormia. Eur Neurol. 2010;64:1–8. doi: 10.1159/000314897. [DOI] [PubMed] [Google Scholar]
- 89.Gerton BK, Theeler B, Samii A. Backpack treatment for camptocormia. Mov Disord. 2010;25:247–8. doi: 10.1002/mds.22909. [DOI] [PubMed] [Google Scholar]
- 90.Schroeteler FE, Fietzek UM, Ziegler K, et al. Upright posture in parkinsonian camptocormia using a high-frame walker with forearm support. Mov Disord. 2011;26:1560–1. doi: 10.1002/mds.23585. [DOI] [PubMed] [Google Scholar]
- 91.de Sèze MP, Creuzé A, de Sèze M, et al. An orthosis and physiotherapy programme for camptocormia: a prospective case study. J Rehabil Med. 2008;40:761–5. doi: 10.2340/16501977-0252. [DOI] [PubMed] [Google Scholar]
- 92.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2013;9:CD002817. doi: 10.1002/14651858.CD002817.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Capecci M, Serpicelli C, Fiorentini L, et al. Postural rehabilitation and Kinesio taping for axial postural disorders in Parkinson’s disease. Arch Phys Med Rehabil. 2014;95:1067–75. doi: 10.1016/j.apmr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 94.Arii Y, Sawada Y, Kawamura K, et al. Immediate effect of spinal magnetic stimulation on camptocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1221–6. doi: 10.1136/jnnp-2014-307651. [DOI] [PubMed] [Google Scholar]
- 95.Song IU, Kim JS, Lee KS. Dopa-responsive camptocormia in a patient with multiple system atrophy. Parkinsonism Relat Disord. 2008;14:161–3. doi: 10.1016/j.parkreldis.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 96.von Coelln R, Raible A, Gasser T, et al. Ultrasound-guided injection of the iliopsoas muscle with botulinum toxin in camptocormia. Mov Disord. 2008;23:889–92. doi: 10.1002/mds.21967. [DOI] [PubMed] [Google Scholar]
- 97.Colosimo C, Salvatori FM. Injection of the iliopsoas muscle with botulinum toxin in camptocormia. Mov Disord. 2009;24:316–17. doi: 10.1002/mds.22249. [DOI] [PubMed] [Google Scholar]
- 98.Fietzek UM, Schroeteler FE, Ceballos-Baumann AO. Goal attainment after treatment of parkinsonian camptocormia with botulinum toxin. Mov Disord. 2009;24:2027–8. doi: 10.1002/mds.22676. [DOI] [PubMed] [Google Scholar]
- 99.Furusawa Y, Mukai Y, Kawazoe T, et al. Long-term effect of repeated lidocaine injections into the external oblique for upper camptocormia in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:350–4. doi: 10.1016/j.parkreldis.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 100.Peek AC, Quinn N, Casey AT, et al. Thoracolumbar spinal fixation for camptocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80:1275–8. doi: 10.1136/jnnp.2008.152736. [DOI] [PubMed] [Google Scholar]
- 101.Wadia PM, Tan G, Munhoz RP, et al. Surgical correction of kyphosis in patients with camptocormia due to Parkinson’s disease: a retrospective evaluation. J Neurol Neurosurg Psychiatry. 2011;82:364–8. doi: 10.1136/jnnp.2009.176198. [DOI] [PubMed] [Google Scholar]
- 102.Schulz-Schaeffer WJ, Margraf NG, Munser S, et al. Effect of neurostimulation on camptocormia in Parkinson’s disease depends on symptom duration. Mov Disord. 2015;30:368–72. doi: 10.1002/mds.26081. [DOI] [PMC free article] [PubMed] [Google Scholar]