Abstract
Osteoarthritis (OA) is a degenerative joint disease affecting a large population of people. Although the elevated expression of PKR (double stranded RNA-dependent protein kinase) and MMP-13 (collagenase-3) have been indicated to play pivotal roles in the pathogenesis of OA, the exact mechanism underlying the regulation of MMP-13 by PKR following inflammatory stimulation was relatively unknown. The purpose of this study was to determine the signaling pathway involved in the PKR-mediated induction of MMP-13 after TNF-α-stimulation. In this study, cartilages of knee joint were obtained from OA subjects who underwent arthroplastic knee surgery. Cartilages were used for tissue analysis or for chondrocytes isolation. In results, the upregulated expression of PKR was observed in damaged OA cartilages as well as in TNF-α-stimulated chondrocytes. Phosphorylation of PKC (protein kinase C) was found after TNF-α administration or PKR activation using poly(I:C), indicating PKC was regulated by PKR. The subsequent increased activity of NADPH oxidase led to oxidative stress accumulation and antioxidant capacity downregulation followed by an exaggerated inflammatory response with elevated levels of COX-2 and IL-8 via ERK/NF-κB pathway. Activated ERK pathway also impeded the inhibition of MMP-13 by PPAR-γ. These findings demonstrated that TNF-α-induced PKR activation triggered oxidative stress-mediated inflammation and MMP-13 in human chondrocytes. Unraveling these deregulated signaling cascades will deepen our knowledge of OA pathophysiology and provide aid in the development of novel therapies.
Keywords: Osteoarthritis, Human chondrocyte, PKR, Oxidative stress, MMP-13
Graphical abstract
1. Introduction
Osteoarthritis (OA) is one of the critical degenerative orthopedic diseases that affect a significant proportion of the population. Clinical symptoms included joint pain, stiffness and decrease of mobility, leading to physical disability and reduced quality of life [1]. To date, a number of factors are believed to trigger OA, such as abnormal mechanical stress, compressive forces, failure of nutrient intake or genetic issues [2]. However, current interventions are still restricted to pain control and total knee arthroplasty is often suggested for late-stage OA cases [3].
Chondrocytes, the only cell type present in the articular cartilage, express various genes to maintain homeostasis. And numerous genes involved in extracellular matrix (ECM) formation and oxidative damage defense have been found to be altered in late-stage OA cartilage [4]. In addition, it is well established that pathogenesis of OA is closely related with pro-inflammatory cytokines produced by chondrocytes, such as interleukin (IL)-1 and tumor necrosis factor (TNF)-α, which eventually results in activation of matrix metalloproteinases (MMPs) and deterioration of OA [5]. In particular, MMP-13 has been shown to be upregulated [6] and identified as a critical target during the progression of OA [7]. Also, it has been previously suggested structural cartilage damage in animal OA model is dependent on MMP-13 activity [8]. As such, elucidation of the detailed mechanism underlying the increased expression of MMP-13 may be beneficial to prevent the development of OA.
The double stranded (ds) RNA-dependent protein kinase, PKR, is a ubiquitously expressed serine/threonine kinase. Once PKR is activated by dimerization and autophosphorylation, it subsequently phosphorylates the α-subunit of the eukaryotic translation initiation factor 2 (eIF2α) [9]. Various studies have implicated PKR signaling pathways in the cartilage degradation that occurs in arthritic diseases [10], [11], [12]. It has been proven that PKR inhibitor could antagonize the IL-1α-activated eIF2α phosphorylation, thereby suppressing proteoglycan degradation and cyclooxygenase (COX)−2 accumulation [11]. PKR also regulates the TNF-α-induced proteoglycan degradation and chondrocyte cell death [10]. Moreover, PKR has been indicated to mediate the TNF-α-induced activation of MMP-2 and −9 [10]. Hence, it is tempting to speculate that PKR regulates MMP-13 expression in OA cartilages, which leads to ECM degradation.
The aim of the present study was to decipher the mechanism of PKR-mediated MMP-13 upregulation in chondrocytes following TNF-α stimulation. To this end, we employed the chondrocytes obtained from OA patients and examined the associated signaling pathways in order to understand the role of PKR in the onset of OA. Our data also demonstrated the relationship between accumulated oxidative stress and increased inflammation and MMP-13.
2. Materials and methods
2.1. Reagents
Trypsin-EDTA and Dulbecco's modified Eagle's medium (DMEM) were bought from Gibco (Grand Island, NY, USA). PD98059, dihydroethidium (DHE), Apocynin, Diphenyleneiodonium (DPI), Polyinosinic-polycytidylic acid (poly(I:C)), collagenase B, Pyrrolidine dithiocarbamate (PDTC), penicillin and streptomycin were all purchased from Sigma (St. Louis, MO, USA). Anti-PKR, anti-p-PKR,anti-PKC, anti-p-PKC, anti-NOX-1, anti-p47, anti-Rac-1, were all obtained from Abcam (Cambridge, UK). Anti-β-actin, anti-ERK, anti-p-ERK, anti-COX-2, anti-PPAR-γ were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HRP-conjugated anti-rabbit secondary antibodies were purchased from Transduction Laboratories (CA, USA). Antioxidant enzymes kits were obtained from EMD Millipore (Calbiochem, Gibbstown, NJ). IL-8 ELISA kit was purchased from R&D Systems (Minneapolis, MN, USA).
2.2. Human chondrocytes isolation
The study group included 30 patients diagnosed with knee OA over 5 years. The study protocol was approved by the Ethics Committee of E-Da Hospital (EMRP-105-077), and each participant provided written informed consent. Cartilages of knee joint were obtained from OA subjects who underwent arthroplastic knee surgery. Articular cartilage tissues were gathered from the resected bone and cartilages. The damaged cartilage tissues were gathered for tissue analysis. The non-damaged cartilage tissues were gathered for tissue analysis and digested for in vitro investigations. Cartilage samples were cut into small pieces and washed with PBS for three times. Cartilage fragments were digested with collagenase B in DMEM at 37 °C overnight on a shaker. The isolated chondrocytes were centrifuged and washed two times with PBS. Chondrocytes were cultured in MDEM with 10% FBS, 2 mM L-glutamine, 25 mM HEPES, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37 °C in a humidified atmosphere of 95% air and 5% CO2 [13].
2.3. Western blot analysis
RIPA, which was purchased from Millipore, was used to extract lysate. The proteins were transferred on to a PVDF membrane after the proteins were separated by SDS/PAGE. The membranes were blocked by the buffer for 1 h at 37 °C. Then, the membranes were incubated with primary antibodies overnight at 4 °C, followed by hybridization with HRP (horseradish peroxidase)-conjugated secondary antibody for 1 h. The intensities were quantified by densitometric analysis.
2.4. PKR activity assay
PKR activity was tested using commercial CycLex® PKR/EIF2AK2 Kinase Assay Kit according to the manufacturer's instructions. In brief, 100 μL of reaction mixtures were added to the non-coated wells. After 1 h, 10 μL of EDTA Solution was added to stop kinase reaction. Next, 100 μL of reaction mixtures were loaded to the antibody-coated well, followed by hybridization with HRP conjugated anti-GST antibody. PKR activity was measured by O.D 450 nm.
2.5. NAPDH oxidase activity assay
The lucigenin method was used to determine NAPDH oxidase activity in chondrocytes. Total protein concentration was adjusted to 1 mg/ml. An aliquot of 200 μL of protein (100 μg) was incubated in the presence of 5 μL of lucigenin and 100 μM NADPH. Luminescence was assessed after 10 min using a plate reader (VICTOR3; PerkinElmer) to determine the relative changes in NADPH oxidase activity.
2.6. Measurement of ROS production
ROS levels were investigated by DHE. Confluent cells (104 cells/well) in 96-well plates were preincubated with various concentrations of TNF-α for 24 hrs. The cells were incubated with 10 μM DHE for 1 h. Fluorescence intensity was assessed with a fluorescent microplate reader (Labsystem, California) that had been calibrated at 540 nm excitation and 590 nm emission.
2.7. Transfection with small-interfering RNA
ON-TARGET plus SMART pool small-interfering RNAs (siRNAs) for si-Controls were obtained from Dharmacon Research (Lafayette, Colorado). si-PKC and si-PKR were purchased from Santa Cruz. Transient transfection was performed using INTERFERin siRNA transfection reagent (Polyplus Transfection, Huntingdon, UK) according to the manufacturer's guide.
2.8. NF-κB activity assay
NF-κB activity was measured by an NF-κB p65 ActiveELISA kit (Imgenex Corp, San Diego, CA) according to the manufacturer's instructions. The absorbance at 405 nm was determined using a microplate reader (spectraMAX 340).
2.9. Assay for IL-8 and MMP-13 secretion
Cells were seeded in 24-well plates at 0.5 × 105 cells. After 2 days, cells were treated TNF-α for 24 h. Cell supernatants were removed and assayed for IL-8 and MMP-13 concentrations using an ELISA kit obtained from R&D Systems (Minneapolis, MN).
2.10. Isolation of mRNA and quantitative real-time PCR
Total RNA was isolated from chondrocytes using the RNeasy kit (Qiagen, Valencia, CA, USA). Oligonucleotides for MMP-13 and β-actin were designed using the computer software package Primer Express 2.0 (Applied Biosystems, Foster City, CA, USA). All of the oligonucleotides were synthesized by Invitrogen (Breda, The Netherlands). Oligonucleotide specificity was determined by a homology search within the human genome (BLAST, National Center for Biotechnology Information, Bethesda, MD, USA) and confirmed by dissociation curve analysis. The oligonucleotide sequences were as follows: MMP-13 sense primer, 5′-TCCCAGGAATTGGTGATAAAGTAGA-3′; anti-sense primer, 5′-CTGGCATGACGCGAACAATA-3′; β-actin sense primer, 5′-AGGTCATCACTATTGGCAACGA-3′; anti-sense primer, 5′-CACTTCATGATGGAATTGAATGTAGTT-3′. PCR was performed with SYBR Green in an ABI 7000 sequence detection system (Applied Biosystems) according to the manufacturer's guidelines.
2.11. Statistical analyses
The results are expressed as mean ± SD. Statistical analyses were performed using one-way or two-way ANOVA, following by Tukey's test as appropriate. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Activation of PKR following inflammation of cartilage at knee joint
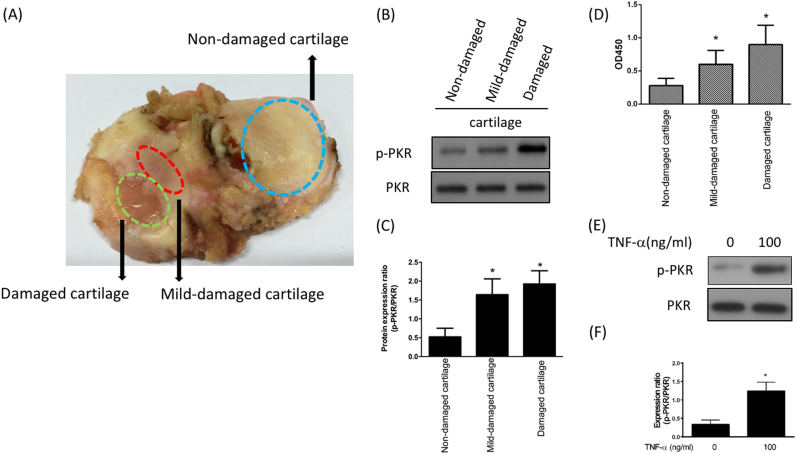
In order to investigate the influence of gradual wear and tear to the cartilage, we examined the expression of phosphorylated and total PKR in these three regions of damaged knee joint (Fig. 1A) from a patient with total knee replacement. Western blotting analysis revealed that phospho-PKR was up-regulated in the mid-damaged and damaged cartilages (Fig. 1B and C). In agreement with this finding, the activity of PKR was elevated in the mid- and damaged regions (Fig. 1D). We observed the similar result of increased expression of phospho-PKR after adding TNF-α to mimic inflammatory condition in chondrocytes which were isolated from non-damaged cartilage (Fig. 1E and F). Our results showed that the expression and activity of phospho-PKR were up-regulated at the onset of OA and by treatment of TNF-α in chondrocytes.
Fig. 1.
Upregulation of PKR following cartilage inflammation. A. Representative image of cartilage from patient with total knee replacement showing non-damaged, mid-damaged and damaged regions; Protein expression (B) and ratio (C) of p-PKR to total PKR; (D) Kinase activity of p-PKR from three different regions; protein expression (E) and ratio (F) of p-PKR to total PKR after exogenous administration of TNF-α for 12 h in human chondrocytes. (n = 3; * p < .05 compared to non-damaged cartilage or control group).
3.2. Increased PKC expression after inflammation is mediated by PKR
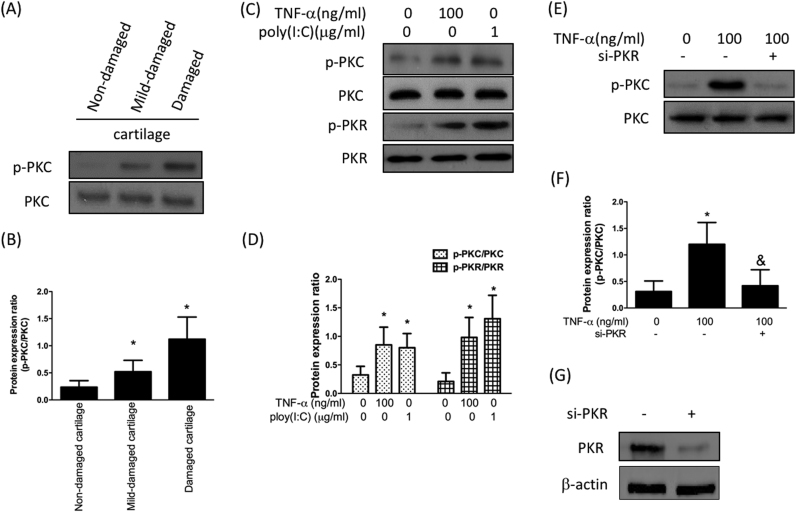
Previously, elevated expression of protein kinase C (PKC) was found in human OA articular cartilages and was required for TNF-α or IL-1-induced NF-κB activation in chondrocytes [14]. Therefore, we sought to examine the relationship between PKR and PKC. As shown in Fig. 2A and B, protein expression of phospho-PKC was up-regulated in the mid-damaged and damaged cartilages. And the increased expression levels of phospho-PKC and phospho-PKR were observed after TNF-α treatment in human chondrocytes which were isolated from non-damaged cartilage (Fig. 2C and D). Next, we assessed the effect of a synthetic analog of dsRNA polyinosinic-polycytidylic acid, poly(I:C), on the expression of PKC and PKR in chondrocytes. As expected, poly(I:C) enhanced the expression of phospho-PKR (Fig. 2C and D). It was noteworthy that the expression of phospho-PKC was up-regulated as well, indicating that activation of PKR possibly led to phosphorylation of PKC. As such, we utilized si-PKR to hinder the expression of PKR and found that the TNF-α-induced activation of PKC was abrogated by si-PKR (Fig. 2E and F). These results demonstrated that increased expression of PKC after inflammation was via up-regulation of phospho-PKR.
Fig. 2.
Increased expression of PKC after cartilage inflammation is due to PKR upregulation Protein expression (A) and the ratio (B) of p-PKC to total PKC from three different regions; Protein expression (C) and quantification (D) of PKR as well as PKC activation by addition of TNF-α and poly(I:C), which is known to activate PKR. Protein expression (E) and the ratio (F) of p-PKC to total PKC after treatment of TNF-α with or without the addition of si-PKR. (G)Western blotting confirming PKR knockdown efficiency. (n = 3; * p < .05 compared to non-damaged cartilage or no treatment control group; & p < .05 compared to TNF-α-treated group).
3.3. Upregulation of NADPH oxidase (NOX) activity under the inflammatory condition is regulated by PKR
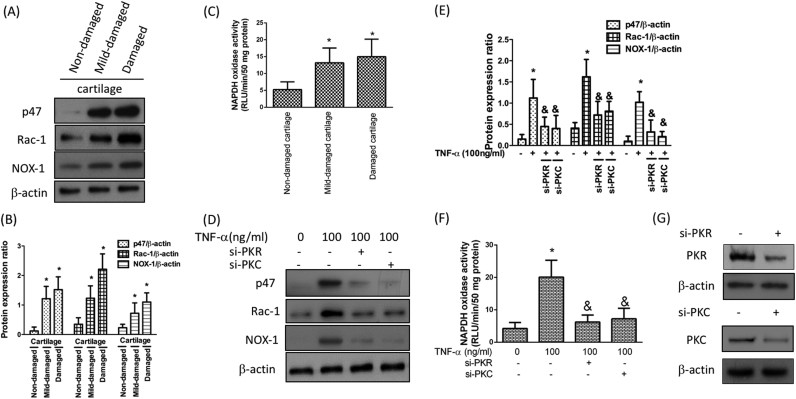
Reactive oxygen species (ROS) could be generated by chondrocytes following activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [15] and oxidative stress has been shown to induce the expression of OA markers [16]. Moreover, IL-1β-mediated MMP secretion in chondrocytes has been proven by up-regulation of NADPH oxidase (NOX) [17]. In the current study, we examined whether the effect of inflammatory stimulation on NOX activity was via PKR signaling pathway. First, we demonstrated that the subunits (p47 and Rac-1) as well as the isoform (NOX-1) of NADPH oxidase were elevated in the mid-damaged and damaged cartilages (Fig. 3A and B). Likewise, the activity of NOX was also increased in these damaged cartilages (Fig. 3C). Next, we showed the TNF-α-induced up-regulation of subunits and isoform (Fig. 3D and E) as well as NOX activity (Fig. 3F) in chondrocytes using si-PKR or si-PKC. Together, these findings suggested that the up-regulation of NOX following inflammation was mediated by PKR.
Fig. 3.
Activation of NADPH oxidase (NOX) under the inflammatory condition is mediated by increased level of PKR or PKC. Protein expression (A) and quantification (B) of NADPH oxidase cytosolic subunits, including p47 and Rac-1, as well as NOX1; (C) Activity of NOX from three different regions; The protein expression levels (D) and quantification of NOX subunits and isoform (E) in TNF-α-stimulated chondrocytes in the presence of si-PKR or si-PKC. The activity of NOX was tested by NADPH oxidase activity assay (F). (G) Western blotting confirming PKR and PKC knockdown efficiency. (n = 3; * p < .05 compared to non-damaged cartilage or no treatment control group; & p < .05 compared to TNF-α only group).
3.4. Reduced antioxidant activity by inflammation in cartilage is repressed by inhibition of PKR/PKC/NOX pathway
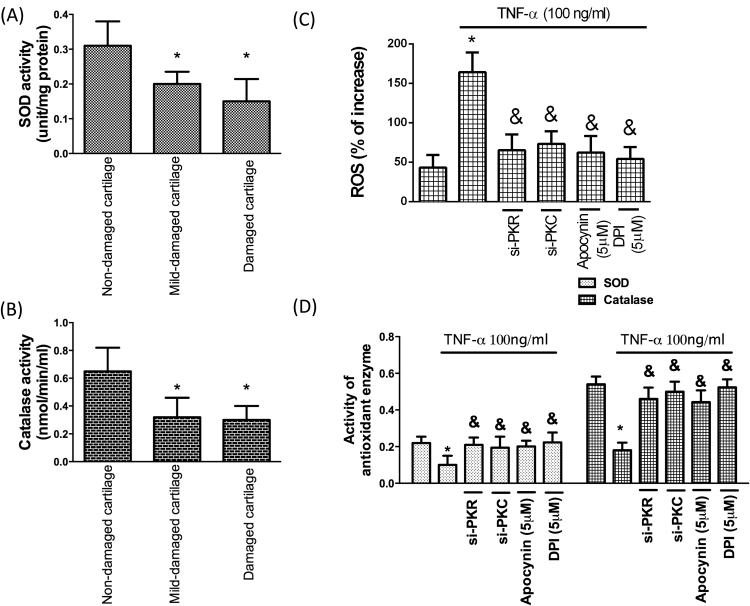
In associated with the previous study [15], the activity of antioxidant enzymes, superoxide (SOD; Fig. 4A) and catalase (Fig. 4B), was downregulated in OA cartilages. But ROS induced by TNF-α was suppressed by administration of si-PKR or si-PKC. NADPH oxidases have several sub-units, therefore, two different ROS inhibitors were used in this study. We also confirmed that ROS activated by TNF-α was inhibited by administration of ROS inhibitors (apocynin and DPI) (Fig. 4C). Most importantly, we demonstrated that the reduced antioxidant activity in chondrocytes by TNF-α was reversed using si-PKR, si-PKC or ROS inhibitors. (Fig. 4D). These data indicated that the impaired antioxidant capacity in inflammatory chondrocytes may be enhanced by modulation of PKR/PKC/NOX pathway.
Fig. 4.
Accumulated oxidative stress by inflammation in cartilage is reversed by inhibition of PKR/PKC/NOX pathway Activities of the antioxidant enzymes, (A) SOD and (B) catalase, in three different regions of cartilage; (C) TNF-α-induced ROS in chondrocytes was interfered by si-PKR or si-PKC; (D) Activities of antioxidant enzymes (SOD and catalase) following TNF-α exposure with si-PKR, si-PKC or two NOX inhibitors (apocynin and DPI). (n = 3; * p < .05 compared to non-damaged cartilage or no treatment control group; & p < .05 compared to TNF-α only group).
3.5. Inflammation-induced phosphorylation of ERK is modulated by PKR/PKC/NOX pathway
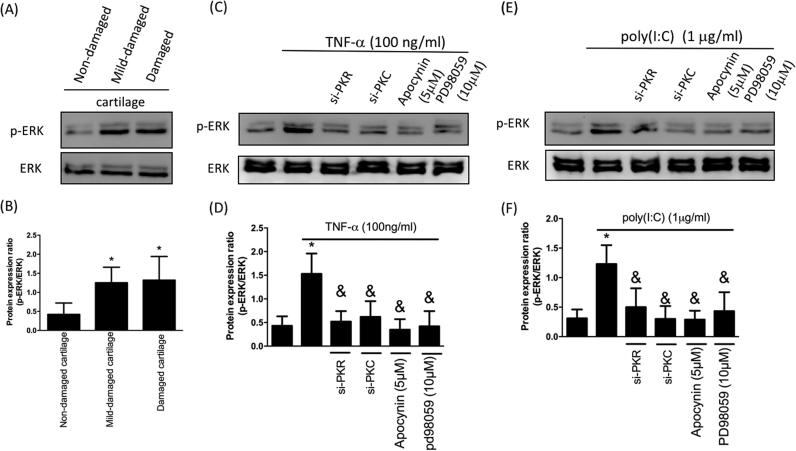
MAPK signaling pathways are involved in the regulation of various biological activities, including inflammation [18]. Previous studies have demonstrated that ex vivo cartilage compression stimulates the phosphorylation of ERK1/2 [19] and TNF-α-induced MMP-13 expression was associated with ERK and NF-κB [20]. Here, we observed upregulated phosphorylation of ERK in OA cartilages (Fig. 5A and B) and TNF-α-stimulated ERK phosphorylation was quenched by si-PKR, si-PKC or NOX inhibitor apocynin (Fig. 5C and D). Furthermore, we showed that ERK phosphorylation was increased following enhanced expression of phospho-PKR using poly(I:C) (Fig. 5E and F). And this upregulation was inhibited by si-PKR, si-PKC or apocynin (Fig. 5E and F), indicating phosphorylation of ERK by the inflammatory mediator in chondrocytes was regulated through PKR/PKC/NOX pathway.
Fig. 5.
Inflammation-induced p-ERK is downregulated by modulation of PKR/PKC/NOX pathway. Protein expression (A) and ratio (B) of p-ERK to total ERK in three different regions; Protein expression (C) and ratio (D) of p-ERK to total ERK after TNF-α treatment with si-PKR, si-PKC or NOX inhibitor apocynin; Protein expression (E) and ratio (F) of p-ERK to total ERK following poly(I:C) treatment with si-PKR, si-PKC or NOX inhibitor apocynin. (n = 3; * p < .05 compared to non-damaged cartilage or no treatment control group; & p < .05 compared to TNF-α only or poly(I:C) only group).
3.6. Mitigation of PPAR-γ is via activation of PKR/PKC/NOX pathway, leading to MMP-13 upregulation
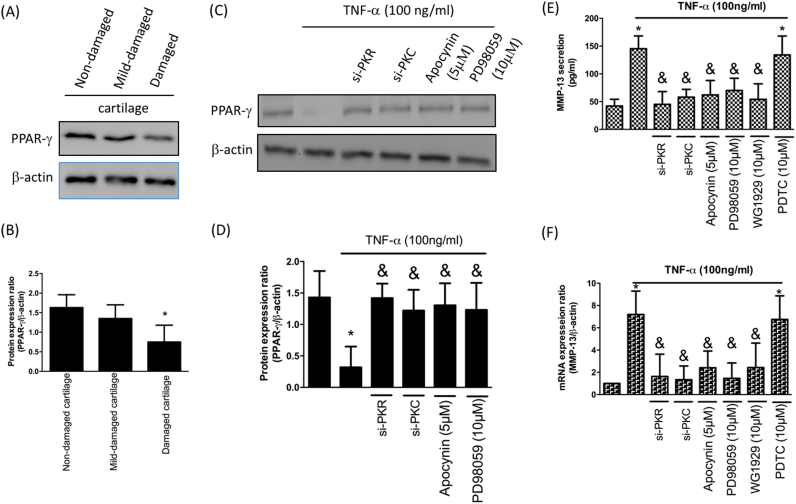
Peroxisome proliferator-activated receptor-γ (PPAR-γ) is known to exhibit chondroprotective property. It has been shown that IL-1-induced downregulation of PPAR-γ was via activation of the MAPKs and NF-κB signaling pathways [21]. Our result was in accordance with the previous study showing reduced expression of PPAR-γ in OA cartilages (Fig. 6A and B). We not only demonstrated that the diminished expression of PPAR-γ after TNF-α treatment was via PKR/PKC/NOX pathway (Fig. 6C and D), but also revealed ERK was required for this phenomenon using ERK inhibitor PD98059 since PD98059 has frequently been utilized to conduct in vitro investigations (Fig. 6C and D). Besides, our data proved that activation of MMP-13 by TNF-α in chondrocytes was also through PKR/PKC/NOX pathway (Fig. 6E). To confirm downregulation of PPAR-γ affected MMP-13 production in human chondrocytes, we added PPAR-γ agonist GW1929 and found that MMP-13 expression was reduced. However, PDTC treatment (NF-κB inhibitor) did not mitigate TNF-α-induced MMP-13 secretion, indicating that TNF-α-induced MMP-13 production is NF-κB independent (Fig. 6E). This finding was further confirmed by real-time PCR (Fig. 6F).
Fig. 6.
Mitigation of PPAR-γ is via activation of PKR/PKC/NOX/ERK pathway, leading to reduction of MMP-13. Protein expression (A) and quantification (B) of PPAR-γ in three different regions; Protein expression (C) and quantification (D) of PPAR-γ by TNF-α stimulation in chondrocytes along with si-PKR, si-PKC, NOX inhibitor apocynin or ERK inhibitor PD98059; The up-regulation of MMP-13 secretion (E) and MMP-13 mRNA expression (F) by TNF-α in chondrocytes was hindered by si-PKR, si-PKC, apocynin, PD98059 or PPAR-γ agonist GW1929. (n = 3; * p < .05 compared to non-damaged cartilage or no treatment control group; & p < .05 compared to TNF-α only group).
3.7. TNF-α-activated NF-κB expression in chondrocytes is via PKR/PKC/NOX/ERK signaling, resulting in increased expression level of COX-2 and IL-8
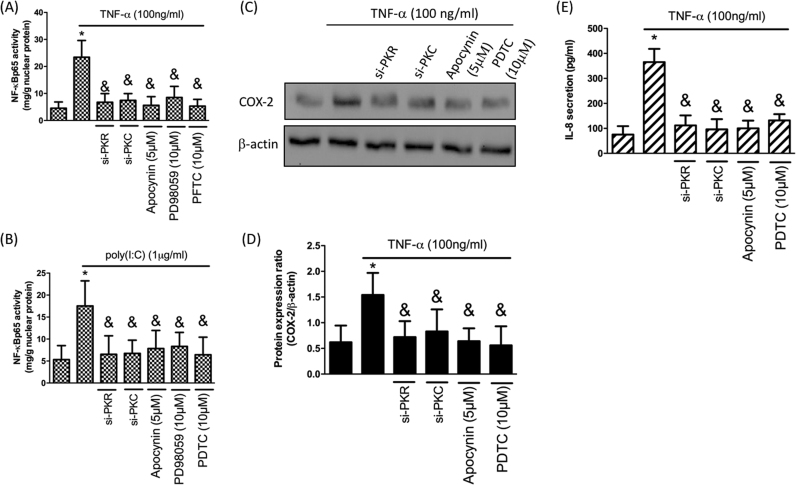
NF-κB is crucial for the inflammatory response in chondrocytes [22], hence, we addressed the impact of PKR/PKC/NOX/ERK pathway following TNF-α treatment. First, we showed that elevated expression of NF-κB p65 by TNF-α (Fig. 7A) or poly(I:C) (Fig. 7B) was reduced when adding si-PKR, si-PKC, apocynin (NOX inhibitor) or PD98059 (ERK inhibitor). To further investigate the proinflammatory response after NF-κB activation, we examined the expression of COX-2 and secretion IL-8 since they both are important mediators in the pathophysiology of OA [23], [24]. By utilizing si-PKR, si-PKC, apocynin or pyrrolidine dithiocarbamate (PDTC; a potent NF-κB inhibitor), we confirmed that upregulation of COX-2 and IL-8 after TNF-α treatment were mediated by PKR/PKC/NOX/NF-κB signaling (Fig. 7C-E).
Fig. 7.
PKR-mediated elevation of COX-2 and IL-8 expression. N-FκB p65-activity in response to TNF-α (A) or poly(I:C)-induction (B) with si-PKR, si-PKC, apocynin or PD98059; Protein expression (C) and quantification (D) of COX-2 by TNF-α stimulation in chondrocytes with si-PKR, si-PKC, apocynin or NF-κB inhibitor PDTC; (E) IL-8 secretion after TNF-α treatment in chondrocytes with si-PKR, si-PKC, apocynin or PDTC. (n = 3; * p < .05 compared with no treatment control group; & p < .05 compared to TNF-α only or poly(I:C) only group).
4. Discussion
Over the past decades, the role of PKR in the pathogenesis of OA has been investigated in numerous studies. It has been shown that PKR is involved in COX-2 accumulation [11] as well as MMP-9 up-regulation [12] in IL-1α-activated cartilage. The pro-inflammatory molecule TNF-α is involved in regulation of osteoblast differentiation [25] and contributes to arthritis by increasing of bone resorption and activation of pro-inflammatory responses [26]. The TNF-α-increased release of proteoglycan and activation of MMP-2 and -9 in cartilage explants are both PKR dependent [10]. Moreover, TNF-α-induced chondrocyte cell death is associated with PKR pathway [10]. TNF-α has also been found in diseased synovial fluid, hence it was selected to facilitate OA-like changes in vitro [27]. In this present study, TNF-α was used to induce OA-like degenerative responses in chondrocytes. A previous study has shown that mitigation of PKR inhibited the TNF-α-caused activation of NF-κB and MAPK in RAW264.7 cells. This finding also indicated that PKR is required for TNF-α-induced osteoclast differentiation [28]. In consistent with these findings, we found that the upregulated expression of PKR in human chondrocytes by TNF-α stimulation led to increase in COX-2, IL-8 and MMP-13 production (Fig. 6, Fig. 7). Several studies have indicated IL-8 promote the pathophysiology of OA, including the release of MMP-13 [29], neutrophil accumulation [30] and chondrocyte hypertrophic differentiation [29], [31]. And COX-2 contributes to cartilage proteoglycan degradation [32] and is associated with differentiation status [33] and cell death [34], [35] in OA chondrocytes.
MMP-13 (collagenase-3) has been suggested to play a role in physiological turnover of cartilage via cleavage of various ECM molecules, such as type II collagen [36], [37], [38]. MMP-13 is constitutively produced and rapidly endocytosed by chondrocytes under steady condition [39]. During the development of OA, chondrocyte hypertrophy was observed accompanied by induction of MMP-13 and collagenase activity [40]. Apart from matrix degradation and hypertrophic changes, MMP-13 was also associated with chondrocyte differentiation [41], [42] and denaturation of type II collagen [36], [43] in OA cartilages. And it has been proved to be modulated by pro-inflammatory cytokines, IL-1 and TNF-α, in chondrocytes [38], [44]. Recently, it has been revealed that the increase in MMP-13 transcription in human chondrocytes after inflammation was regulated by activating transcription factor 3 (ATF3) [45], NF-κB [20] or via MAPK/c-Fos/AP-1 and JAK/STAT pathways [46]. In addition, Singh's group has reported that MMP-13 deficiency plays an important role in mitigating local pro-inflammatory events, suggesting MMP-13 is critical in the pathogenesis of arthritis [47]. Data from the current study further demonstrated the signaling pathway in the regulation of MMP-13 expression following TNF-α stimulation was via PKR/PKC/NOX/ERK/PPAR-γ pathway in chondrocytes.
Oxidative stress has been defined as the imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses. The implication of oxidative stress in the progression of OA has been extensively examined recently, and numerous studies have concluded that OA progression is significantly related to oxidative stress accumulation [48], [49]. In addition to mitochondria, NOX is the major source of ROS in cells [50]. ROS serve as integral factors contributing to the maintenance of cartilage homeostasis as they modulate chondrocyte apoptosis, inflammatory cytokine production and ECM breakdown and synthesis [51], [52], [53], leading to cartilage degradation and joint inflammation. In consistent with these findings, we showed that the activity of antioxidant enzymes was reduced in OA cartilages or in response to TNF-α treatment concurrent with an increase in ROS (Fig. 4). And we also found the elevated inflammatory response following the accumulated oxidative stress in human chondrocytes (Fig. 7), which may contribute to synovium inflammation in OA patients.
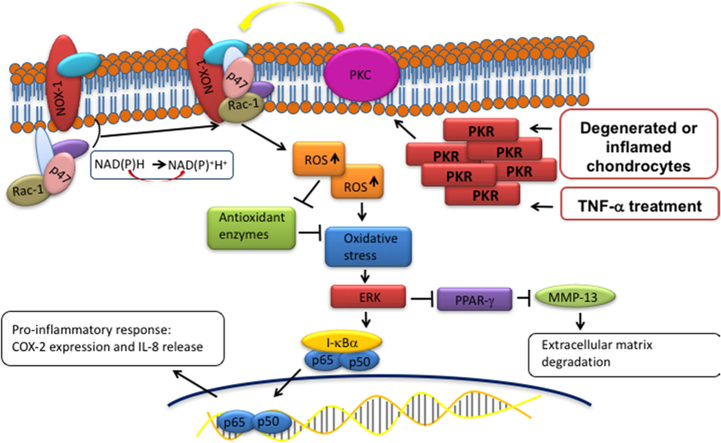
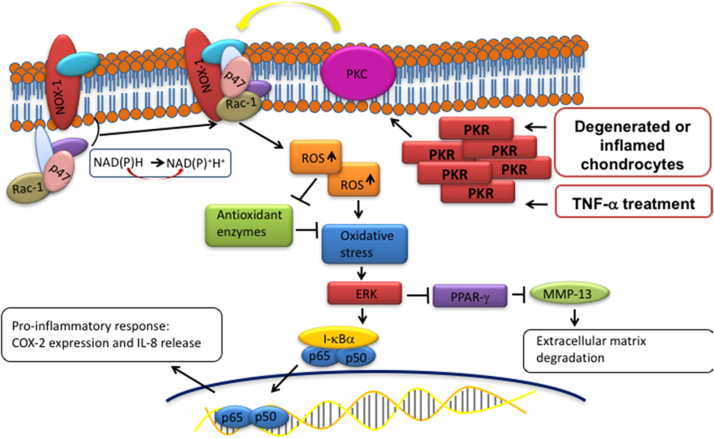
In conclusion, the present study demonstrated substantial evidence that PKR contributes to OA pathogenesis through regulation of molecular events involved in oxidative stress, inflammation, and matrix degradation. PKR causes phosphorylation of PKC following exposure to TNF-α in human chondrocytes, leading to the elevated expression of NOX and the subsequent increased ROS. The accumulated oxidative stress eventually exaggerates inflammatory response, such as an increase in COX-2 and IL-8, via ERK/NF-κB pathway. The activated ERK signaling also suppresses the inhibition of PPAR-γ to MMP-13, thereby resulting in cartilage degradation (Fig. 8). These findings revealed PKR signaling pathway in human chondrocytes following inflammatory stimulation and suggested PKR may be able to serve as a pharmacological target for OA treatment.
Fig. 8.
Schematic diagram of PKR signaling pathways that are activated by inflammatory stimulation. Phosphorylation of PKC by PKR activation induces the cytosolic complex (such as p47 and Rac1) to translocate to the membrane and associate with the integral membrane components, resulting in activation of NOX1 followed by accumulation of reactive oxidase species (superoxide) and down-regulation of antioxidant enzymes activity (SOD and catalase). Subsequently, the up-regulated oxidative stress triggers the ERK pathway which leads to enhanced inflammatory responses (increased COX-2 and IL-8) via NF-κB. In addition, the activated ERK hinder the inhibition of PPAR-γ to MMP-13, allowing MMP-13-mediated ECM degradation/remodeling.
Conflict of interest
None.
Acknowledgments
This study was supported by grants from National Cheng Kung University and E-Da Hospital (NCKUEDA10613). E-Da Hospital (EDCHP106002). This study was also supported by grants from the Ministry of Science and Technology (MOST 105-2311-B-006-008 and 106-2314-B-006-023).
Contributor Information
Pei-Ling Hsieh, Email: akinosha@hotmail.com.
Kun-Ling Tsai, Email: kunlingtsai@mail.ncku.edu.tw.
References
- 1.Goldring S.R., Goldring M.B. Clinical aspects, pathology and pathophysiology of osteoarthritis. J. Musculoskelet. Neuron. Interact. 2006;6(4):376–378. [PubMed] [Google Scholar]
- 2.Goldring M.B., Goldring S.R. Osteoarthritis. J. Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 3.Malemud C.J., Islam N., Haqqi T.M. Pathophysiological mechanisms in osteoarthritis lead to novel therapeutic strategies. Cells Tissues Organs. 2003;174(1–2):34–48. doi: 10.1159/000070573. [DOI] [PubMed] [Google Scholar]
- 4.Aigner T., Fundel K., Saas J., Gebhard P.M., Haag J., Weiss T., Zien A., Obermayr F., Zimmer R., Bartnik E. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54(11):3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 5.Goldring M.B. Osteoarthritis and cartilage: the role of cytokines. Curr. Rheumatol. Rep. 2000;2(6):459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 6.Roach H.I., Yamada N., Cheung K.S., Tilley S., Clarke N.M., Oreffo R.O., Kokubun S., Bronner F. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheumatol. 2005;52:3110–3124. doi: 10.1002/art.21300. [DOI] [PubMed] [Google Scholar]
- 7.Wang M., Sampson E.R., Jin H., Li J., Ke Q.H., Im H.J., Chen D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 2013;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little C.B., Barai A., Burkhardt D., Smith S.M., Fosang A.J., Werb Z., Shah M., Thompson E.W. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60(12):3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fremont M., Vaeyens F., Herst C.V., De Meirleir K.L., Englebienne P. Double-stranded RNA-dependent protein kinase (PKR) is a stress-responsive kinase that induces NFkappaB-mediated resistance against mercury cytotoxicity. Life Sci. 2006;78(16):1845–1856. doi: 10.1016/j.lfs.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert S.J., Duance V.C., Mason D.J. Does protein kinase R mediate TNF-alpha- and ceramide-induced increases in expression and activation of matrix metalloproteinases in articular cartilage by a novel mechanism? Arthritis Res. Ther. 2004;6:R46–R55. doi: 10.1186/ar1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tam C.L., Hofbauer M., Towle C.A. Requirement for protein kinase R in interleukin-1alpha-stimulated effects in cartilage. Biochem. Pharmacol. 2007;74:1636–1641. doi: 10.1016/j.bcp.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert S.J., Blain E.J., Al-Sabah A., Zhang Y., Duance V.C., Mason D.J. Protein kinase R plays a pivotal role in oncostatin M and interleukin-1 signalling in bovine articular cartilage chondrocytes. Eur. Cells Mater. 2012;23:41–57. doi: 10.22203/ecm.v023a04. [DOI] [PubMed] [Google Scholar]
- 13.Yui N., Yoshioka H., Fujiya H., Musha H., Beppu M., Karasawa R., Yudoh K. The DNA repair enzyme apurinic/apyrimidinic endonuclease (Apex nuclease) 2 has the potential to protect against down-regulation of chondrocyte activity in osteoarthritis. Int. J. Mol. Sci. 2014;15(9):14921–14934. doi: 10.3390/ijms150914921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaVallie E.R., Chockalingam P.S., Collins-Racie L.A., Freeman B.A., Keohan C.C., Leitges M., Dorner A.J., Morris E.A., Majumdar M.K., Arai M. Protein kinase Czeta is up-regulated in osteoarthritic cartilage and is required for activation of NF-kappaB by tumor necrosis factor and interleukin-1 in articular chondrocytes. J. Biol. Chem. 2006;281:24124–24137. doi: 10.1074/jbc.M601905200. [DOI] [PubMed] [Google Scholar]
- 15.Hiran T.S., Moulton P.J., Hancock J.T. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic. Biol. Med. 1997;23:736–743. doi: 10.1016/s0891-5849(97)00054-3. [DOI] [PubMed] [Google Scholar]
- 16.Khan I.M., Gilbert S.J., Caterson B., Sandell L.J., Archer C.W. Oxidative stress induces expression of osteoarthritis markers procollagen IIA and 3B3(-) in adult bovine articular cartilage. Osteoarthr. Cartil. 2008;16:698–707. doi: 10.1016/j.joca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Rousset F., Hazane-Puch F., Pinosa C., Nguyen M.V., Grange L., Soldini A., Rubens-Duval B., Dupuy C., Morel F., Lardy B. IL-1beta mediates MMP secretion and IL-1beta neosynthesis via upregulation ofp22(phox) and NOX4 activity in human articular chondrocytes. Osteoarthr. Cartil. 2015;23:1972–1980. doi: 10.1016/j.joca.2015.02.167. [DOI] [PubMed] [Google Scholar]
- 18.Kyriakis J.M., Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol. Rev. 2012;92 doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 19.Fanning P.J., Emkey G., Smith R.J., Grodzinsky A.J., Szasz N., Trippel S.B. Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. J. Biol. Chem. 2003;278:50940–50948. doi: 10.1074/jbc.M305107200. [DOI] [PubMed] [Google Scholar]
- 20.Liacini A., Sylvester J., Li W.Q., Huang W., Dehnade F., Ahmad M., Zafarullah M. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp. Cell Res. 2003;288:208–217. doi: 10.1016/s0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 21.Afif H., Benderdour M., Mfuna-Endam L., Martel-Pelletier J., Pelletier J.P., Duval N., Fahmi H. Peroxisome proliferator-activated receptor gamma1 expression is diminished in human osteoarthritic cartilage and is downregulated by interleukin-1beta in articular chondrocytes. Arthritis Res. Ther. 2007;9:R31. doi: 10.1186/ar2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saklatvala J. Inflammatory signaling in cartilage: mapk and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr. Drug Targets. 2007;8:305–313. doi: 10.2174/138945007779940115. [DOI] [PubMed] [Google Scholar]
- 23.Chauffier K., Laiguillon M.C., Bougault C., Gosset M., Priam S., Salvat C., Mladenovic Z., Nourissat G., Jacques C., Houard X., Berenbaum F., Sellam J. Induction of the chemokine IL-8/Kc by the articular cartilage: possible influence on osteoarthritis. Jt. Bone Spine. 2012;79:604–609. doi: 10.1016/j.jbspin.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Hogue J.H., Mersfelder T.L. Pathophysiology and first-line treatment of osteoarthritis. Ann. Pharmacother. 2002;36:679–686. doi: 10.1345/aph.1A132. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert L., He X., Farmer P., Rubin J., Drissi H., van Wijnen A.J., Lian J.B., Stein G.S., Nanes M.S. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J. Biol. Chem. 2002;277(4):2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 26.Choy E.H., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 27.Horiuchi T., Yoshida T., Koshihara Y., Sakamoto H., Kanai H., Yamamoto S., Ito H. The increase of parathyroid hormone-related peptide and cytokine levels in synovial fluid of elderly rheumatoid arthritis and osteoarthritis. Endocr. J. 1999;46(5):643–649. doi: 10.1507/endocrj.46.643. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara H., Teramachi J., Okamura H., Yang D., Nagata T., Haneji T. Double stranded RNA-Dependent protein kinase is necessary for TNF-alpha-induced osteoclast formation in vitro and in vivo. J. Cell Biochem. 2015;116(9):1957–1967. doi: 10.1002/jcb.25151. [DOI] [PubMed] [Google Scholar]
- 29.Merz D., Liu R., Johnson K., Terkeltaub R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J. Immunol. 2003;171:4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 30.Lotz M., Terkeltaub R., Villiger P.M. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J. Immunol. 1992;148:466–473. [PubMed] [Google Scholar]
- 31.Cecil D.L., Rose D.M., Terkeltaub R., Liu-Bryan R. Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum. 2005;52:144–154. doi: 10.1002/art.20748. [DOI] [PubMed] [Google Scholar]
- 32.Hardy M.M., Seibert K., Manning P.T., Currie M.G., Woerner B.M., Edwards D., Koki A., Tripp C.S. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheumatol. 2002;46:1789–1803. doi: 10.1002/art.10356. [DOI] [PubMed] [Google Scholar]
- 33.Huh Y.H., Kim S.H., Kim S.J., Chun J.S. Differentiation status-dependent regulation of cyclooxygenase-2 expression and prostaglandin E2 production by epidermal growth factor via mitogen-activated protein kinase in articular chondrocytes. J. Biol. Chem. 2003;278:9691–9697. doi: 10.1074/jbc.M211360200. [DOI] [PubMed] [Google Scholar]
- 34.Notoya K., Jovanovic D.V., Reboul P., Martel-Pelletier J., Mineau F., Pelletier J.P. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E2 via the induction of cyclooxygenase-2. J. Immunol. 2000;165:3402–3410. doi: 10.4049/jimmunol.165.6.3402. [DOI] [PubMed] [Google Scholar]
- 35.Pelletier J.P., Fernandes J.C., Jovanovic D.V., Reboul P., Martel-Pelletier J. Chondrocyte death in experimental osteoarthritis is mediated by MEK 1/2 and p38 pathways: role of cyclooxygenase-2 and inducible nitric oxide synthase. J. Rheumatol. 2001;28:2509–2519. [PubMed] [Google Scholar]
- 36.Billinghurst R.C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C., Mitchell P., Hambor J., Diekmann O., Tschesche H., Chen J., Van Wart H., Poole A.R. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Investig. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell P.G., Magna H.A., Reeves L.M., Lopresti-Morrow L.L., Yocum S.A., Rosner P.J., Geoghegan K.F., Hambor J.E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Investig. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reboul P., Pelletier J.P., Tardif G., Cloutier J.M., Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J. Clin. Investig. 1996;97:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto K., Okano H., Miyagawa W., Visse R., Shitomi Y., Santamaria S., Dudhia J., Troeberg L., Strickland D.K., Hirohata S., Nagase H. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016;56:57–73. doi: 10.1016/j.matbio.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tchetina E.V., Kobayashi M., Yasuda T., Meijers T., Pidoux I., Poole A.R. Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: implications for development and arthritis. Matrix Biol. 2007;26:247–258. doi: 10.1016/j.matbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Borzí R.M., Olivotto E., Pagani S., Vitellozzi R., Neri S., Battistelli M., Falcieri E., Facchini A., Flamigni F., Penzo M., Platano D., Santi S., Facchini A., Marcu K.B. Matrix metalloproteinase 13 loss associated with impaired extracellular matrix remodeling disrupts chondrocyte differentiation by concerted effects on multiple regulatory factors. Arthritis Rheumatol. 2010;62:2370–2381. doi: 10.1002/art.27512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C.W., Tchetina E.V., Mwale F., Hasty K., Pidoux I., Reiner A., Chen J., Van Wart H.E., Poole A.R. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J. Bone Mineral. Res. 2002;17:639–651. doi: 10.1359/jbmr.2002.17.4.639. [DOI] [PubMed] [Google Scholar]
- 43.Wu W., Billinghurst R.C., Pidoux I., Antoniou J., Zukor D., Tanzer M., Poole A.R. Sites of collagenase cleavage and denaturation of type II collagen in aging and osteoarthritic articular cartilage and their relationship to the distribution of matrix metalloproteinase 1 and matrix metalloproteinase 13. Arthritis Rheumatol. 2002;46:2087–2094. doi: 10.1002/art.10428. [DOI] [PubMed] [Google Scholar]
- 44.Tardif G., Pelletier J.P., Dupuis M., Geng C., Cloutier J.M., Martel-Pelletier J. Collagenase 3 production by human osteoarthritic chondrocytes in response to growth factors and cytokines is a function of the physiologic state of the cells. Arthritis Rheumatol. 1999;42:1147–1158. doi: 10.1002/1529-0131(199906)42:6<1147::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 45.Chan C.M., Macdonald C.D., Litherland G.J., Wilkinson D.J., Skelton A., Europe-Finner G.N., Rowan A.D. Cytokine-induced MMP13 expression in human chondrocytes is dependent on activating transcription Factor 3 (ATF3) regulation. J. Biol. Chem. 2017;292:1625–1636. doi: 10.1074/jbc.M116.756601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim H., Kim H.P. Matrix metalloproteinase-13 expression in IL-1β-treated chondrocytes by activation of the p38 MAPK/c-Fos/AP-1 and JAK/STAT pathways. Arch. Pharmacal Res. 2011;34:109–117. doi: 10.1007/s12272-011-0113-4. [DOI] [PubMed] [Google Scholar]
- 47.Singh A., Rajasekaran N., Hartenstein B., Szabowski S., Gajda M., Angel P., Brauer R., Illges H. Collagenase-3 (MMP-13) deficiency protects C57BL/6 mice from antibody-induced arthritis. Arthritis Res. Ther. 2013;15(6):R222. doi: 10.1186/ar4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henrotin Y., Kurz B., Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthr. Cartil. 2005;13:643–654. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Lepetsos P., Papavassiliou A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta. 2016;1862:576–591. doi: 10.1016/j.bbadis.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 51.Rathakrishnan C., Tiku K., Raghavan A., Tiku M.L. Release of oxygen radicals by articular chondrocytes: a study of luminol-dependent chemiluminescence and hydrogen peroxide secretion. J. Bone Mineral. Res. 1992;7:1139–1148. doi: 10.1002/jbmr.5650071005. [DOI] [PubMed] [Google Scholar]
- 52.Tiku M.L., Liesch J.B., Robertson F.M. Production of hydrogen peroxide by rabbit articular chondrocytes. Enhancement by cytokines. J. Immunol. 1990;145:690–696. [PubMed] [Google Scholar]
- 53.Henrotin Y.E., Bruckner P., Pujol J.P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]