Abstract
Background:
An estimated 4% of hospital admissions acquired healthcare-associated infections (HAIs) and accounted for $9.8 (USD) billion in direct cost during 2011. In 2010, nearly 140 000 of the 3.5 million potentially preventable hospitalizations (PPHs) may have acquired an HAI. There is a knowledge gap regarding the co-occurrence of these events.
Aims:
To estimate the period occurrences and likelihood of acquiring an HAI for the PPH population.
Methods:
Retrospective, cross-sectional study using logistic regression analysis of 2011 Texas Inpatient Discharge Public Use Data File including 2.6 million admissions from 576 acute care hospitals. Agency for Healthcare Research and Quality Prevention Quality Indicator software identified PPH, and existing administrative data identification methodologies were refined for Clostridium difficile infection, central line–associated bloodstream infection, catheter-associated urinary tract infection, and ventilator-associated pneumonia. Odds of acquiring HAIs when admitted with PPH were adjusted for demographic, health status, hospital, and community characteristics.
Findings:
We identified 272 923 PPH, 14 219 HAI, and 986 admissions with PPH and HAI. Odds of acquiring an HAI for diabetic patients admitted for lower extremity amputation demonstrated significantly increased odds ratio of 2.9 (95% confidence interval: 2.16-3.91) for Clostridium difficile infection. Other PPH patients had lower odds of acquiring HAI compared to non-PPH patients, and results were frequently significant.
Conclusions:
Clinical implications include increased risk of HAI among diabetic patients admitted for lower extremity amputation. Methodological implications include identification of rare events for inpatient subpopulations and the need for improved codification of HAIs to improve cost and policy analyses regarding allocation of resources toward clinical improvements.
Keywords: healthcare-associated infection, preventable hospitalizations, diabetes, administrative data, comorbidity
Introduction
More than 3.5 million hospital admissions were identified as potentially preventable during 2010.1 In addition to potentially misallocated resources, potentially preventable hospitalization (PPH) or any hospital admission carries the risk of acquiring a healthcare-associated infection (HAI). An estimated 1 in 25 US hospital patients acquired an HAI during 2011, translating to $9.8 billion (USD) of additional annual direct medical costs nationwide and an increased risk of death.2-5
In our review of the literature, we found little research that examined the patient population with co-occurring PPH and HAI. Since HAIs are known to be both physically and financially costly, reducing exposure to HAI risk by decreasing hospitalizations that are potentially preventable may contribute to improved population health. However, we must first understand the composition and prevalence of individuals with a PPH who acquire an HAI during the same hospitalization. This study begins to address the gap in our knowledge about the PPH population that acquires an HAI.
The primary objectives of the study were to: (1) identify and quantify the prevalence and patient characteristics of individuals who experience co-occurring PPH and HAI and (2) estimate the odds of a PPH patient acquiring an HAI during their hospital admission.
Methods
Data
The 2011 Texas Hospital Discharge Public Use Data File (PUDF) contained over 2.9 million summary abstracts of patient-level information from 1 of 576 Texas hospitals.6 Institutional review board exempt status approvals were obtained from governing research institutions.
Identification of Patients and Conditions
Identification of PPHs and comorbid conditions
Potentially preventable hospitalizations were identified from the PUDF using SAS 9.3 and program PQSAS1 from the Agency for Healthcare Research and Quality (AHRQ) Prevention Quality Indicator (PQI), version 4.5.7 The algorithms identify hospitalizations associated with ambulatory care–sensitive conditions for 1 of 14 adult or 5 pediatric conditions considered potentially preventable through appropriate use of quality preventive care.2 Thirty comorbid conditions were identified using the comorbidity software from the Health Care Utilization Project.8,9
Identification of HAIs
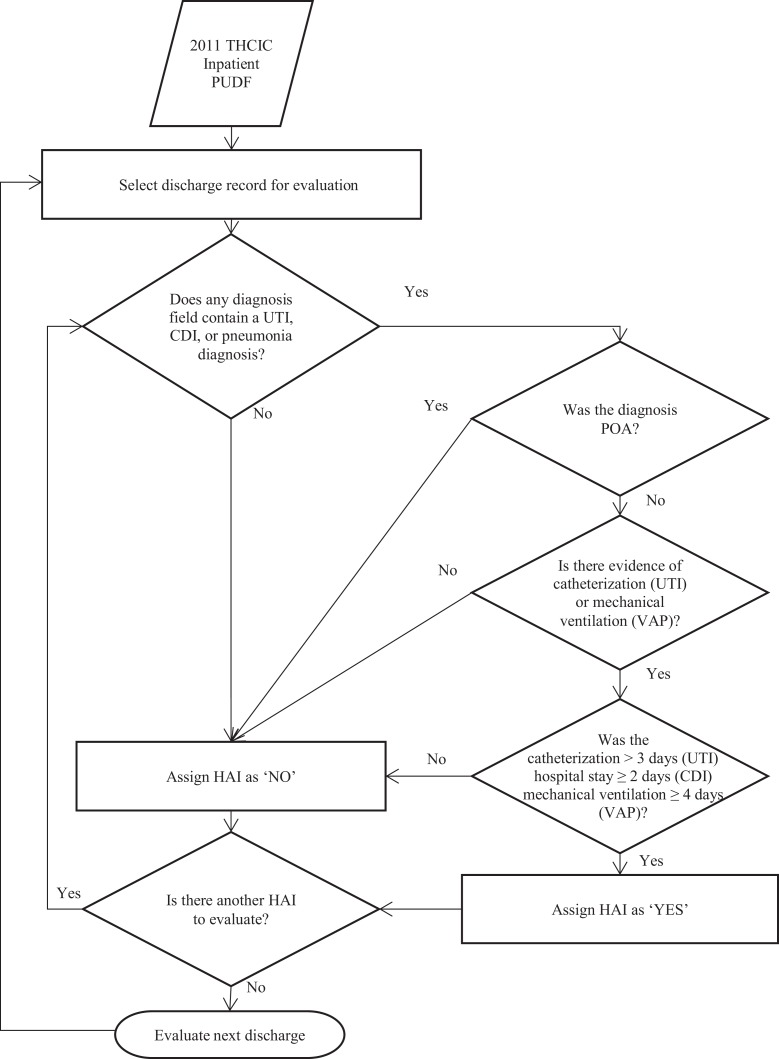
Definitions and methods of identifying HAI from inpatient discharge data were reviewed, combined, and supplemented as described below for use in this study.10-12 The process used for identifying HAI in the PUDF is represented in Figure 1.
Figure 1.
Identification of CAUTI, CDI, and VAP from administrative inpatient data. CAUTI indicates catheter-associated urinary tract infection; CDI, Clostridium difficile infection; HAI, healthcare-associated infection; POA, present on admission; PUDF, public use data file; THCIC, Texas Health Care Information Collection; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
Catheter-associated urinary tract infection
For catheter-associated urinary tract infection (CAUTI), we used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 996.64—complications related to infection from an indwelling catheter in any diagnosis field. Since code 996.64 is associated with underreporting of CAUTI, we used 17 ICD-9-CM codes (Supplemental Materials) to identify urinary tract infection not present on admission.11,13,14 We identified catheterization using ICD-9-CM procedure codes 57.94, 97.62, and 97.64 along with procedure dates to estimate the duration of catheterization. We combined urinary tract infection not present on admission with evidence of catheterization lasting more than 2 days to assign CAUTI.
Ventilator-associated pneumonia
To assign ventilator-associated pneumonia (VAP), 4 things were evaluated. First, diagnoses fields were examined for the diagnosis code 997.31—VAP. Since 997.31 historically underreports VAP, we looked for mechanical ventilation or intubation codes and 1 of 29 pneumonia infection codes (Supplemental Materials) not present on admission.11,12,15 Finally, an admission was assigned as VAP when (1) a diagnosis code of 997.31 was present in the record or (2) evidence of mechanical ventilation greater than 4 days with a pneumonia infection code not present on admission.16
Central line–associated bloodstream infections
In the AHRQ Quality Indicator modules, Patient Safety Indicator 7, Pediatric Quality Indicator 12, and Neonate Quality Indicator 3 identify central line–associated bloodstream infection (CLABSI) rates for the adult, pediatric, and neonate hospitalizations, respectively, and prior to availability of electronic health record data, all were endorsed measures of CLABSI by the National Quality Forum.17,18
Clostridium difficile infection
Clostridium difficile infection (CDI) was identified by using an ICD-9-CM diagnosis of 008.45. Since CDI can also be acquired in a community setting and requires 2 days of incubation before symptoms manifest, a CDI diagnosis was considered an HAI if not present on admission and hospital length of stay was greater than 2 days.
Exclusion Criteria
Discharge records were excluded when evaluation variables were missing or invalid. Patients with a length of stay greater than 180 days were excluded as extreme outliers. Hospitalizations identified by the PQI for perforated appendix were excluded as there is no ambulatory care–sensitive condition that precedes appendicitis, and hospitalization is required for treatment. Evaluation of the PQI for low birth weight babies was also excluded as the preventive care associated with it is prenatal care for the mother, and there was no means to appropriately link a low birth weight infant with its mother.
Finally, when regression models were applied to examine each PPH, non-PPH patient records were excluded if patient characteristics did not match the epidemiologic denominator population, or patient at-risk population specifications, of the PQI. For example, patients under 18 would not be included when evaluating the adult asthma PPH. Additionally, each PPH associated with less than 10 HAIs were excluded from PPH-specific analyses due to the inability to make meaningful inferences from statistical analyses. This eliminated all pediatric PPH and adult PPH for hypertension, angina without procedure, uncontrolled diabetes, and asthma in younger adults. However, these subpopulations were included when all PPH were evaluated collectively for an HAI.
Analyses
Period occurrences and odds ratios of PPH with HAI
A correlation matrix that included variables for PPH, HAI, and other independent characteristics was examined for confounding relationships. Period occurrences were tabulated and reported by evaluation variables. Odds ratios were calculated using 45 logistic regression models. The logistic regression equations modeled the probability of an HAI. The primary independent variable was PPH hospital admission. For example, the presence of CAUTI, VAP CLABSI, CDI, or any HAI was set as the dependent variable in the regression equations. The primary independent variable was 1 of the 8 PPH admission types or all PPH. Hospital admission records were excluded from the denominator population for 3 reasons: (1) if the patient age was less than 18 years, (2) the admission record was identified with an HAI not being evaluated, or (3) the admission record was identified with a PPH not being evaluated.
Other independent variables used to adjust the logistic regression models included age, gender, race, hospital characteristics, community characteristics, and health status as measured by the presence of comorbid conditions. For the 30 comorbid conditions, conditions were excluded from the regression models when the PPH under evaluation was associated with or similar to the comorbid condition. For example, the variable reflecting comorbid diabetes was excluded from regression models when evaluating the PPHs for short-term complications due to diabetes, long-term complications due to diabetes, and diabetes-related lower extremity amputation.
Results
Demographic and Independent Variables
Of the 2 937 134 discharges in the 2011 Texas inpatient data, 294 453 (10.0%) were excluded due to missing or invalid data. Nearly 6.5% of total discharges were excluded for missing gender and were attributed to the suppression of gender to protect the identification of individuals with a diagnosis of substance abuse or HIV. Among the remaining 2 642 681 discharges, 272 923 (10.3%) were identified as PPH, 14 219 (0.5%) included evidence of a potential HAI, and 986 (0.36% of PPH discharges) demonstrated evidence of co-occurring PPH and HAI. Compared to the general inpatient population, individuals with a PPH were older and more likely to have Medicare identified as their primary insurer (Table 1).
Table 1.
Distributions of Inpatients Across Demographic and Select Evaluation Variables by Type of Admission, 2011.a
| Variable categories | PPHb | HAIc | With Bothd | Total Discharges | ||||
|---|---|---|---|---|---|---|---|---|
| N = 272 923 | N = 14 219 | N = 986 | N = 2 642 681 | |||||
| n | % of PPH discharges | n | % of HAI discharges | n | % of both discharges | n | % of total discharges | |
| Gender | ||||||||
| Male | 116 172 | 43% | 6898 | 49% | 421 | 43% | 1 030 128 | 39% |
| Female | 156 751 | 57% | 7321 | 51% | 565 | 57% | 1 612 553 | 61% |
| Age group | ||||||||
| Under 1 year | 20 178 | 7% | 435 | 3% | 39 | 4% | 401 863 | 15% |
| 1-17 years | 14 700 | 5% | 363 | 3% | 9 | 1% | 151 462 | 6% |
| 18-24 years | 5992 | 2% | 238 | 2% | 6 | 1% | 190 732 | 7% |
| 25-44 years | 27 135 | 10% | 1165 | 8% | 69 | 7% | 513 224 | 19% |
| 45-64 years | 72 441 | 27% | 4082 | 29% | 299 | 30% | 576 503 | 22% |
| 65-74 years | 47 211 | 17% | 3315 | 23% | 214 | 22% | 334 456 | 13% |
| 75-84 years | 50 633 | 19% | 3097 | 22% | 214 | 22% | 302 540 | 11% |
| 85+ years | 34 633 | 13% | 1524 | 11% | 136 | 14% | 171 901 | 6% |
| Race | ||||||||
| White | 143 653 | 53% | 7813 | 55% | 550 | 56% | 1 323 466 | 50% |
| Black | 43 745 | 16% | 1880 | 13% | 139 | 14% | 339 738 | 13% |
| Hispanic | 69 764 | 26% | 3080 | 22% | 243 | 25% | 773 549 | 29% |
| Asian/Pacific Islander | 2720 | 1% | 197 | 1% | 10 | 1% | 45 762 | 2% |
| American Indian./Eskimo/Aleut | 2052 | 1% | 56 | 0% | 5 | 1% | 18 334 | 1% |
| Other | 10 989 | 4% | 1193 | 8% | 39 | 4% | 141 832 | 5% |
| Primary payer | ||||||||
| Private payer | 55 996 | 21% | 2819 | 20% | 146 | 15% | 842 482 | 32% |
| Medicare | 146 944 | 54% | 8842 | 62% | 646 | 66% | 909 285 | 34% |
| Medicaid | 36 971 | 13% | 1419 | 10% | 111 | 11% | 583 356 | 22% |
| Other government | 5867 | 2% | 326 | 2% | 16 | 2% | 77 403 | 3% |
| Self-pay or charity | 27 145 | 10% | 813 | 6% | 67 | 7% | 230 155 | 9% |
| Comorbid conditions | ||||||||
| Congestive heart failure | 31 194 | 11% | 1 938 | 14% | 177 | 18% | 151 997 | 6% |
| Pulmonary circulation disorders | 3455 | 1% | 485 | 3% | 36 | 4% | 28 883 | 1% |
| Hypertension | 137 217 | 50% | 5498 | 39% | 502 | 51% | 888 641 | 34% |
| Paralysis | 2809 | 1% | 510 | 4% | 21 | 2% | 27 770 | 1% |
| Diabetes without chronic complications | 56 673 | 21% | 2195 | 15% | 202 | 20% | 366 177 | 14% |
| Renal failure | 43 243 | 16% | 2401 | 17% | 265 | 27% | 216 262 | 8% |
| Obesity | 31 030 | 11% | 1411 | 10% | 155 | 16% | 205 673 | 8% |
| Weight loss | 9880 | 4% | 1853 | 13% | 116 | 12% | 82 433 | 3% |
Abbreviations: HAI, healthcare-associated infection; PPH, potentially preventable hospitalizations.
aTexas Health Care Information Collection Inpatient Public Use Data File, 2011.
bAll variable distributions were significantly different than the general inpatient population at p < .0001, except for the comorbid condition of paralysis that was not significantly different from the general inpatient population.
cAll variable distributions were significantly different than the general inpatient population at p < .0001, except for the comorbid condition of depression and measures of rurality that were not significantly different from the general inpatient population.
dAll variable distributions were significantly different than the general inpatient population at p < .05, except for hospital ownership, measures of rurality, public health benefits and the comorbid conditions lymphoma, blood loss anemia, and psychoses that were not significantly different from the general inpatient population.
Odds Ratios
When examined in aggregate, odds of acquiring an HAI in the PPH population were significantly lower than the remaining inpatient population, with odds ratios ranging from 0.335 (95% confidence interval [CI]: 0.295-0.381) for VAP to 0.729 (95% CI: 0.609-0.874) for CLABSI (Table 2). Of the significant differences, men, white individuals, and individuals with congestive heart failure, paralysis, weight loss, and renal failure had higher odds of acquiring an HAI, except for the renal failure with CLABSI group. Conversely, individuals with hypertension had significantly lower odds of acquiring any form of HAI.
Table 2.
Odds of Acquiring HAIa for All PPHs,b 2011.c,d
| Variable Categories | All HAI | CDI | CLABSI | CAUTI | VAP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 14 219 | n = 6617 | n = 1532 | n = 1139 | n = 5012 | |||||||||||
| Odds Ratio | LCL | UCL | Odds Ratio | LCL | UCL | Odds Ratio | LCL | UCL | Odds Ratio | LCL | UCL | Odds Ratio | LCL | UCL | |
| PPH admission | 0.478 | 0.447 | 0.510 | 0.541 | 0.494 | 0.592 | 0.729 | 0.609 | 0.874 | 0.561 | 0.455 | 0.693 | 0.335 | 0.295 | 0.381 |
| Gender | |||||||||||||||
| Male | 1.261 | 1.219 | 1.306 | 1.071 | 1.018 | 1.127 | 1.315 | 1.184 | 1.461 | 0.989 | 0.875 | 1.117 | 1.582 | 1.492 | 1.677 |
| Age group | |||||||||||||||
| 85+ years | Referent | Referent | Referent | Referent | Referent | ||||||||||
| Under 1 year | 0.112 | 0.099 | 0.127 | 0.026 | 0.020 | 0.035 | 0.970 | 0.690 | 1.363 | 0.006 | 0.002 | 0.016 | 0.274 | 0.223 | 0.336 |
| 1-17 years | 0.266 | 0.234 | 0.303 | 0.231 | 0.190 | 0.281 | 2.130 | 1.524 | 2.979 | 0.034 | 0.016 | 0.072 | 0.203 | 0.153 | 0.269 |
| 18-24 years | 0.148 | 0.128 | 0.172 | 0.107 | 0.084 | 0.137 | 0.638 | 0.427 | 0.954 | 0.055 | 0.031 | 0.095 | 0.256 | 0.200 | 0.328 |
| 25-44 years | 0.287 | 0.262 | 0.315 | 0.232 | 0.203 | 0.265 | 0.941 | 0.689 | 1.285 | 0.130 | 0.095 | 0.177 | 0.477 | 0.405 | 0.563 |
| 45-64 years | 0.972 | 0.907 | 1.043 | 0.791 | 0.718 | 0.870 | 2.059 | 1.569 | 2.703 | 0.434 | 0.345 | 0.545 | 1.624 | 1.422 | 1.856 |
| 65-74 years | 1.180 | 1.108 | 1.256 | 0.963 | 0.885 | 1.047 | 1.992 | 1.536 | 2.584 | 0.641 | 0.528 | 0.777 | 1.926 | 1.703 | 2.180 |
| 75-84 years | 1.185 | 1.114 | 1.261 | 1.082 | 0.997 | 1.174 | 1.461 | 1.116 | 1.911 | 0.877 | 0.732 | 1.051 | 1.608 | 1.419 | 1.823 |
| Race | |||||||||||||||
| White | Referent | Referent | Referent | Referent | Referent | ||||||||||
| Black | 0.567 | 0.533 | 0.604 | 0.478 | 0.439 | 0.520 | 0.583 | 0.483 | 0.704 | 0.700 | 0.555 | 0.883 | 0.695 | 0.619 | 0.780 |
| Hispanic | 0.618 | 0.574 | 0.666 | 0.512 | 0.460 | 0.569 | 0.727 | 0.585 | 0.902 | 0.862 | 0.654 | 1.135 | 0.755 | 0.661 | 0.863 |
| Asian/Pacific Islander | 0.570 | 0.531 | 0.612 | 0.496 | 0.449 | 0.548 | 0.519 | 0.421 | 0.639 | 0.632 | 0.485 | 0.823 | 0.734 | 0.647 | 0.833 |
| American Indian./Eskimo/Aleut | 0.651 | 0.559 | 0.758 | 0.540 | 0.430 | 0.678 | 0.638 | 0.412 | 0.989 | 0.583 | 0.302 | 1.128 | 0.925 | 0.727 | 1.177 |
| Other | 0.317 | 0.242 | 0.416 | 0.196 | 0.124 | 0.310 | 0.283 | 0.116 | 0.694 | 0.488 | 0.197 | 1.208 | 0.470 | 0.310 | 0.713 |
| Primary payer | |||||||||||||||
| Medicare | Referent | Referent | Referent | Referent | Referent | ||||||||||
| Self-pay or charity | 0.698 | 0.643 | 0.758 | 0.463 | 0.403 | 0.533 | 0.742 | 0.583 | 0.945 | 0.807 | 0.597 | 1.091 | 0.996 | 0.881 | 1.127 |
| Medicaid | 0.850 | 0.787 | 0.917 | 0.582 | 0.513 | 0.661 | 1.163 | 0.953 | 1.418 | 0.892 | 0.663 | 1.200 | 1.111 | 0.986 | 1.252 |
| Other government | 0.766 | 0.682 | 0.861 | 0.526 | 0.430 | 0.643 | 0.892 | 0.643 | 1.238 | 0.721 | 0.458 | 1.135 | 1.062 | 0.894 | 1.262 |
| Private insurance | 0.664 | 0.628 | 0.702 | 0.561 | 0.516 | 0.609 | 0.734 | 0.618 | 0.872 | 0.671 | 0.547 | 0.824 | 0.766 | 0.699 | 0.839 |
| Comorbid conditionse | |||||||||||||||
| Congestive heart failure | 1.432 | 1.357 | 1.510 | 1.369 | 1.266 | 1.480 | 1.258 | 1.041 | 1.521 | 1.223 | 1.009 | 1.482 | 1.781 | 1.636 | 1.938 |
| Pulmonary circulation disorders | 1.686 | 1.531 | 1.857 | 1.461 | 1.255 | 1.701 | 1.983 | 1.466 | 2.682 | 1.409 | 0.971 | 2.045 | 1.944 | 1.687 | 2.239 |
| Hypertension | 0.538 | 0.517 | 0.559 | 0.480 | 0.454 | 0.508 | 0.602 | 0.529 | 0.684 | 0.529 | 0.464 | 0.603 | 0.577 | 0.541 | 0.616 |
| Paralysis | 2.598 | 2.370 | 2.847 | 2.123 | 1.834 | 2.457 | 3.551 | 2.833 | 4.450 | 3.223 | 2.371 | 4.380 | 2.899 | 2.514 | 3.342 |
| Diabetes without chronic complications | 0.726 | 0.691 | 0.762 | 0.725 | 0.674 | 0.780 | 0.906 | 0.777 | 1.057 | 0.603 | 0.503 | 0.723 | 0.699 | 0.645 | 0.758 |
| Renal failure | 1.517 | 1.442 | 1.596 | 1.481 | 1.376 | 1.595 | 0.808 | 0.664 | 0.983 | 1.218 | 1.011 | 1.466 | 1.904 | 1.755 | 2.065 |
| Obesity | 1.304 | 1.231 | 1.382 | 0.978 | 0.888 | 1.076 | 1.556 | 1.316 | 1.840 | 1.624 | 1.337 | 1.971 | 1.431 | 1.306 | 1.567 |
| Weight loss | 2.627 | 2.492 | 2.770 | 2.635 | 2.446 | 2.839 | 2.696 | 2.264 | 3.211 | 1.586 | 1.279 | 1.968 | 2.892 | 2.652 | 3.153 |
Abbreviations: CAUTI, catheter-associated urinary tract infection; CDI, Clostridium difficile infection; CLABSI, central line–associated bloodstream infection; HAI, healthcare-associated infections; LCL, lower confidence limit; PPH, potentially preventable hospitalization; UCL, upper confidence limit; VAP, ventilator-associated pneumonia.
aThe denominator or at-risk population, n = 2 642 681.
bThe PPH population, n = 272 923.
cTexas Health Care Information Collection Inpatient Public Use Data File, 2011.
dOdds ratios in bold are significant at p < .0001.
eReferent group for comorbid conditions consist of individuals without the comorbid condition.
When we estimated the odds ratios for each HAI for the different types of PPH, we found the reduced odds of acquiring an HAI did not hold for patients admitted with a diabetes-related lower extremity amputation (Table 3). For the diabetes-related lower extremity amputation group, significantly higher odds of acquiring an HAI were reported for CDI (OR: 2.9; 95% CI: 2.16-3.91). However, despite increased odds of acquiring VAP (OR: 1.4; 95% CI: 0.95-2.18), CLABSI (OR: 1.7; 95% CI: 0.68-4.03), or CAUTI (OR: 2.2; 95% CI: 0.90-5.32) among this same group, the results were not significant, despite the substantial effect sizes.
Table 3.
Adjusted Odds of Acquiring an HAI by HAI and AHRQ Prevention Quality Indicator, 2011.a,b
| Type of PPH | PPH Denominator Populationc | PPH Population | HAI Population | PPH with HAI | Odds Ratio | LCL | UCL |
|---|---|---|---|---|---|---|---|
| Any HAI | |||||||
| All PPH | 2 642 681 | 272 923 | 14 219 | 986 | 0.335 | 0.295 | 0.381 |
| PQI01 diabetes short-term complications | 1 862 070 | 10 759 | 12 514 | 31 | 0.660 | 0.463 | 0.941 |
| PQI03 diabetes long-term complications | 1 872 221 | 20 910 | 12 590 | 112 | 0.629 | 0.519 | 0.762 |
| PQI05 COPD or asthma in older adults | 1 891 645 | 40 334 | 12 617 | 139 | 0.372 | 0.313 | 0.442 |
| PQI08 heart failure | 1 899 828 | 48 517 | 12 740 | 270 | 0.592 | 0.522 | 0.672 |
| PQI10 dehydration | 1 870 759 | 19 448 | 12 542 | 60 | 0.419 | 0.324 | 0.542 |
| PQI11 bacterial pneumonia | 1 893 646 | 42 335 | 12 626 | 144 | 0.382 | 0.324 | 0.452 |
| PQI12 urinary tract infection | 1 885 483 | 34 172 | 12 598 | 135 | 0.377 | 0.314 | 0.454 |
| PQI16 LEA among diabetes patients | 1 854 910 | 3599 | 12 560 | 77 | 2.067 | 1.640 | 2.606 |
| CDI | |||||||
| All PPH | 2 642 681 | 272 551 | 6617 | 530 | 0.541 | 0.494 | 0.592 |
| PQI01 diabetes short-term complications | 1 862 070 | 10 749 | 5919 | 21 | 1.123 | 0.729 | 1.728 |
| PQI03 diabetes long-term complications | 1 872 221 | 20 875 | 5970 | 76 | 0.966 | 0.763 | 1.222 |
| PQI05 COPD or asthma in older adults | 1 891 645 | 40 241 | 5939 | 42 | 0.242 | 0.178 | 0.330 |
| PQI08 heart failure | 1 899 828 | 48 358 | 5997 | 104 | 0.500 | 0.409 | 0.611 |
| PQI10 dehydration | 1 870 759 | 19 423 | 5933 | 36 | 0.501 | 0.359 | 0.699 |
| PQI11 bacterial pneumonia | 1 893 646 | 42 288 | 5994 | 97 | 0.567 | 0.463 | 0.695 |
| PQI12 urinary tract infection | 1 885 483 | 34 151 | 5992 | 96 | 0.605 | 0.492 | 0.743 |
| PQI16 LEA among diabetes patients | 1 854 910 | 3568 | 5945 | 46 | 2.904 | 2.159 | 3.906 |
| CLABSI | |||||||
| All PPH | 2 642 681 | 272 068 | 1532 | 133 | 0.729 | 0.609 | 0.874 |
| PQI01 diabetes short-term complications | 1 862 070 | 10 732 | 1120 | 4 | 0.683 | 0.255 | 1.830 |
| PQI03 diabetes long-term complications | 1 872 221 | 20 812 | 1125 | 10 | 0.618 | 0.320 | 1.196 |
| PQI05 COPD or asthma in older adults | 1 891 645 | 40 210 | 1127 | 10 | 0.387 | 0.213 | 0.703 |
| PQI08 heart failure | 1 899 828 | 48 284 | 1141 | 24 | 0.847 | 0.566 | 1.267 |
| PQI10 dehydration | 1 870 759 | 19 393 | 1120 | 4 | 0.366 | 0.137 | 0.977 |
| PQI11 bacterial pneumonia | 1 893 646 | 42 218 | 1142 | 26 | 0.889 | 0.600 | 1.317 |
| PQI12 urinary tract infection | 1 885 483 | 34 070 | 1129 | 14 | 0.514 | 0.296 | 0.892 |
| PQI16 LEA among diabetes patients | 1 854 910 | 3526 | 1121 | 4 | 1.654 | 0.679 | 4.031 |
| CAUTI | |||||||
| All PPH | 2 642 681 | 272 029 | 1139 | 94 | 0.677 | 0.556 | 0.826 |
| PQI01 diabetes short-term complications | 1 862 070 | 10 730 | 1032 | 2 | 0.638 | 0.159 | 2.565 |
| PQI03 diabetes long-term complications | 1 872 221 | 20 807 | 1035 | 4 | 0.420 | 0.174 | 1.015 |
| PQI05 COPD or asthma in older adults | 1 891 645 | 40 211 | 1042 | 11 | 0.387 | 0.219 | 0.686 |
| PQI08 heart failure | 1 899 828 | 48 303 | 1075 | 45 | 1.257 | 0.925 | 1.708 |
| PQI10 dehydration | 1 870 759 | 19 401 | 1042 | 12 | 0.922 | 0.521 | 1.632 |
| PQI11 bacterial pneumonia | 1 893 646 | 42 205 | 1043 | 13 | 0.415 | 0.239 | 0.719 |
| PQI16 LEA among diabetes patients | 1 854 910 | 3526 | 1035 | 4 | 2.185 | 0.897 | 5.322 |
| VAP | |||||||
| All PPH | 2 642 681 | 272 860 | 5012 | 258 | 0.335 | 0.295 | 0.381 |
| PQI01 diabetes short-term complications | 1 862 070 | 10 732 | 4492 | 4 | 0.197 | 0.074 | 0.525 |
| PQI03 diabetes long-term complications | 1 872 221 | 20 824 | 4509 | 21 | 0.310 | 0.201 | 0.476 |
| PQI05 COPD or asthma in older adults | 1 891 645 | 40 272 | 4560 | 76 | 0.564 | 0.446 | 0.714 |
| PQI08 heart failure | 1 899 828 | 48 352 | 4580 | 98 | 0.563 | 0.457 | 0.695 |
| PQI10 dehydration | 1 870 759 | 19 398 | 4497 | 9 | 0.193 | 0.100 | 0.371 |
| PQI11 bacterial pneumonia | 1 893 646 | 42 200 | 4496 | 8 | 0.056 | 0.028 | 0.113 |
| PQI12 urinary tract infection | 1 885 483 | 34 065 | 4496 | 8 | 0.083 | 0.041 | 0.166 |
| PQI16 LEA among diabetes patients | 1 854 910 | 3545 | 4511 | 23 | 1.440 | 0.949 | 2.184 |
Abbreviations: COPD, Chronic obstructive pulmonary disease; HAI, healthcare-associated infections; LCL, lower confidence limit; LEA, lower extremity amputation; PPH, potentially preventable hospitalization; PQI, Prevention Quality Indicator; UCL, upper confidence limit.
aTexas Health Care Information Collection Inpatient Public Use Data File, 2011.
bOdds ratios in bold are significant at p < .0001. Odds ratios are adjusted for age, gender, race, hospital characteristics, community characteristics, and comorbid conditions.
cThe denominator population for the logistic regression included all inpatient records that were not identified as potentially preventable and those records identified as the PQI identified with the PPH being evaluated.
Discussion
The reduced odds uncovered through our quantitative evaluation are consistent with PPH individuals potentially requiring less intensive acute care that translates into a decreased risk of acquiring an HAI. When considered from this perspective, comorbid conditions including congestive heart failure, valvular disease, renal failure, pulmonary circulation disorders, weight loss, and paralysis may be important HAI risk factors for PPH individuals. Regarding the reduced odds of acquiring an HAI for diabetes-related comorbidities, in addition to not requiring the invasive and antibiotic therapies, it is possible that the consciousness of providers regarding the heightened risks associated with infections and corresponding best practice treatment protocols for diabetic patients may also play a role in the reduced odds of HAIs.
Aside from lower extremity amputation among diabetic patients, individuals admitted with a PPH had odds approximately half those of the general inpatient population for acquiring an HAI. Thus, while population-based healthcare initiatives may encourage patients to use quality preventive care and chronic disease management to reduce preventable hospitalizations, it seems unlikely the reduced hospitalizations will translate to reduced HAI events.
For individuals with diabetes-related lower extremity amputation, their increased odds of acquiring any HAI are concerning. With significant odds of acquiring CDI at 2.9 times the adjusted non-PPH inpatient population, individuals admitted for diabetes-related lower extremity amputation may benefit from additional specialized care directed toward reducing contact with pathogens or the invasive procedures that increase the risk of acquiring the HAIs identified in this study.
Although administrative discharge data are not preferred or recommended for surveillance of HAI, it remains a valuable resource for policy and cost assessment. One limitation of using administrative data was the potential underidentification of HAI. Even with the enhanced methods for the identification of CAUTI and other forms of HAI, only 0.5% of discharges were identified with a potential HAI. Although our study did not attempt to identify all forms of HAI such as surgical site infection, administrative data continue to underidentify HAI according to the Centers for Disease Control and Prevention’s (CDC) estimate of 4% of discharges. This may be attributed to the inability during secondary data analysis to link infection codes to the cause using the current coding system. Also, since there are ICD-9-CM codes for CAUTI, and the CDC’s estimate of CAUTI in the hospitalized population is much higher than identified, it is probable that the data abstraction and coding processes are systemically misaligned with reporting HAI due to the disconnected billing and payment process.
However, we were able to identify a sufficient sample to evaluate the population affected. Although bias may exist toward individuals with more severe disease, we anticipate with the transition to ICD-10 more accurate reporting of CAUTI as there are at least 4 codes that specify the source of urinary tract infection as secondary to the indwelling catheter. Additionally, we were able to identify a sufficient sample size of HAI to identify significant relationships adjusting for numerous demographic and environmental factors. Therefore, this method should translate to other hospital subpopulations for examining the odds of acquiring an HAI or other rare event.
Conclusions
The increased odds of acquiring all forms of HAI by the diabetes population with lower extremity amputation are of particular interest. Although studies of amputee care during hospitalization should inform best practices related to hospital care, patient education and comparison of preventive care utilization between diabetic amputees and diabetic nonamputees could inform policy makers about key services that may reduce the occurrence of amputations and, by extension, eliminate the risk of HAI.
Although the number of individuals identified with co-occurring PPH and HAI was consistent with broad probability calculations, the potential for substantial underidentification of HAIs, especially CAUTI, suggests there is more to learn about identifying this population through administrative data. Additionally, the potential underidentification limits our ability to accurately estimate direct medical costs attributable to co-occurring PPH and HAI. However, identification may not be an issue for other rare events, making this method one to consider when exploring the period occurrence of rare events particularly in hospital subpopulations.
Supplementary Material
Author Biographies
Andrea L. Lorden, PhD, is an assistant professor in the Department of Health Administration and Policy at the University of Oklahoma Health Sciences Center. During her doctoral program in Health Services Research at Texas A&M University, Dr. Lorden received the Excellence in Research Award for the outstanding quality of her research efforts. At OUHSC, she teaches courses in health economics, cost-effectiveness, and health services research design, and participates in funded research activities that examine the economic burden of disease in vulnerable populations.
Luohua Jiang is currently an assistant professor in the department of Epidemiology at University of California Irvine. She obtained her MD degree from Peking University and PhD in Biostatistics from UCLA. Dr. Jiang has participated in multiple federally funded studies evaluating evidenced-based community chronic disease prevention interventions designed for minority populations. Her current research interests include multilevel and longitudinal data analysis, latent variable modeling, chronic disease prevention and management, and health disparities research.
Tiffany Radcliff, PhD, is an associate professor and Associate Department head for the Department of Health Policy and Management at the Texas A&M School of Public Health. She teaches courses in health economics and health services research methodology. Her areas of research expertise are in health services research, health economics, secondary data analysis, and research methods.
Kathleen Kelly, PhD, MPH, MS, FNP, is a tenured associate professor and director of the Doctor of Nursing Science Program in Education and Leadership at The Sage Colleges. Her research funded by HRSA and RWJF focuses upon quality healthcare and effective translational of new research into practice and policy. Dr. Kelly is active in inter-professional education and research leading an inter-professional asthma program and a mixed methods study to evaluate a new medical respite program for the homeless and assessment of the social determinants of health. Dr. Kelly teaches Global Health Policy and was a Distinguished Scholar Lecturer at both Mahidol University and the Thai Red Cross School of Nursing in Bangkok, Thailand.
Robert Ohsfeldt is a regents professor in the Department of Health Policy and Management in the School of Public Health at Texas A&M University. Previously, he has been a professor at the University of Iowa and the University of Alabama at Birmingham, an assistant professor at Arizona State University, and was employed as a manager of health outcomes research for Eli Lilly and Company, where he received the President’s Award from Lilly Research Laboratories. Dr. Ohsfeldt completed his Ph.D. in economics at the University of Houston, and was a Robert Wood Johnson Foundation Fellow in Healthcare Finance at Johns Hopkins.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Battelle. Quality indicators software instructions, SAS©, version 4.5a. 2013 http://www.qualityindicators.ahrq.gov/Downloads/Software/SAS/V45/Software_Instructions_SAS_V4.5.pdf. Accessed January 13, 2017.
- 2. Centers for Disease Control and Prevention. Healthcare-associated infections: HAI data and statistics, 2016. http://www.cdc.gov/HAI/surveillance/index.html. Accessed January 15, 2017.
- 3. Klevens RM, Edwards JR, Richards CL, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007;122(2):160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scott RDII. The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention, 2009. (CDC report CS200891_A). Atlanta, GA: The Centers for Disease Control and Prevention. [Google Scholar]
- 5. Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi:10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Texas Health Care Information Collection. Texas inpatient public use data file (PUDF), 2013. http://www.dshs.state.tx.us/thcic/hospitals/Inpatientpudf.shtm. Updated 2013. Accessed September 17, 2013.
- 7. Agency for Healthcare Research and Quality. QI SAS©, version 4.5, 2013. http://www.qualityindicators.ahrq.gov/software/SAS.aspx. Updated 2013. Accessed September 5, 2013. [DOI] [PubMed]
- 8. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 9. Comorbidity Software, Version 3.7, 2012. HCUP-US tools & software page www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed January 4, 2016.
- 10. Jhung MA, Banerjee SN. Administrative coding data and health care-associated infections. Clin Infect Dis. 2009;49(6):949–955. [DOI] [PubMed] [Google Scholar]
- 11. Sherman ER, Heydon KH, John KHS, et al. Administrative data fail to accurately identify cases of healthcare-associated infection. Infect Control Hospital Epidemiol. 2006;27(4):332–337. [DOI] [PubMed] [Google Scholar]
- 12. Stevenson KB, Khan Y, Dickman J, et al. Administrative coding data, compared with CDC/NHSN criteria, are poor indicators of health care–associated infections. Am J Infect Control. 2008;36(3):155–164. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN): Surveillance for urinary tract infections event protocol, 2017. http://www.cdc.gov/nhsn/acute-care-hospital/CAUTI/index.html. Updated 2017. Accessed January 10, 2017.
- 14. Agency for Healthcare Research and Quality. Prevention quality indicators technical specifications—version 4.5, 2013. http://www.qualityindicators.ahrq.gov/Modules/PQI_TechSpec.aspx. Updated 2013. Accessed July 24, 2015.
- 15. Restrepo MI, Anzueto A, Arroliga AC, et al. Economic burden of ventilator-associated pneumonia based on total resource utilization. Infect Control Hospital Epidemiol. 2010;31(5):509–515. [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN): Surveillance for ventilator-associated events protocol, 2017. http://www.cdc.gov/nhsn/acute-care-hospital/vae/index.html. Updated 2017. Accessed January 10, 2017.
- 17. Agency for Healthcare Research and Quality. U.S. Department of Health and Human Services Measures Inventory: Legacy HHS measures, 2016. https://www.qualitymeasures.ahrq.gov/hhs/legacyHHSMeasures.aspx. Updated 2016. Accessed June 15, 2017.
- 18. National Quality Forum. Measures, reports & tools, 2017. http://www.qualityforum.org/Measures_Reports_Tools.aspx. Updated 2017. Accessed June 15, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.