Abstract
Objectives:
Many that survive an aneurysmal subarachnoid hemorrhage experience lasting physical disability, which might be improved by medications with effects on the dopaminergic, serotonergic, and brain-derived neurotrophic factor neurotransmitter systems. But it is not clear which patients are most likely to benefit from these therapies. The purpose of this pilot study was to explore the relationship of genetic polymorphisms in these pathways with 12-month functional outcomes after aneurysmal subarachnoid hemorrhage.
Methods:
Subjects were recruited at the time of admission as a part of a larger parent study. Genotypes were generated using the Affymetrix genome-wide human single-nucleotide polymorphism array 6.0. Those within dopaminergic, serotonergic, and brain-derived neurotrophic factor pathways were analyzed for associations with functional outcomes at 12 months post aneurysmal subarachnoid hemorrhage using the Glasgow Outcome Scale and the Modified Rankin Scale.
Results:
The 154 subjects were 55.8 ± 11.3 years old and 74% female; they had Fisher scores of 2.95 ± 0.67, Hunt/Hess scores of 2.66 ± 1.13, and admission Glasgow Coma Scale scores of 12.52 ± 3.79. Single-nucleotide polymorphisms in the serotonin receptor genes 1B and 1E and dopamine receptor D2 were associated with greater disability (odds ratio: 3.88–3.25, confidence interval: 1.01–14.77), while single-nucleotide polymorphisms in the serotonin receptor genes 2A and 2C and dopamine receptor D5 conferred a risk of poor recovery (odds ratio: 3.31–2.32, confidence interval: 1.00–10.80). Single-nucleotide polymorphisms within the same serotonin genes, and within the dopamine receptor gene D2, were associated with greater recovery after aneurysmal subarachnoid hemorrhage (odds ratio: 0.17–0.34, confidence interval: 0.05–0.89).
Conclusions:
These data demonstrate that there may be an association between genetic factors and functional outcomes post stroke.
Keywords: Subarachnoid hemorrhage, dopamine, serotonin, brain-derived neurotrophic factor, disability
Introduction
Aneurysmal subarachnoid hemorrhages (aSAH) are a particularly devastating form of stroke; mortality rates approach 40%.1 For the survivors, many will still suffer long-lasting functional deficits after the acute recovery is complete.2 Lasting deficits greatly impact quality of life and can result in a loss of many productive years.
Genetic changes in neurotransmitter pathway genes have been associated with measures of physical function post stroke.3–5 In particular, interest has focused on the candidate dopaminergic (DA), serotonergic (5-HT), and brain-derived neurotrophic factor (BDNF) pathways as these are often targeted with pharmacologic therapy to improve functional status post stroke. For example, Parkinson’s disease medication levodopa was first shown to improve motor recovery in animal models post stroke,6 but has also more recently been shown to do the same in human stroke patients.7 Furthermore, improvements have been seen in motor outcomes in the first 3 months post stroke for patients given the selective serotonin reuptake inhibitor (SSRI) fluoxetine.8 We also know that neurotrophins like BDNF can increase neurogenesis and reduce apoptosis in the brain9—an effect of high consequence in a post-stroke environment.
However, what research that has been done has primarily been in ischemic stroke. It is unknown whether the same associations hold true in subarachnoid hemorrhage and how robust these associations are in that population. This is a ripe area for investigation, as a subarachnoid hemorrhage is different from ischemic stroke in many ways. In particular, it carries a high risk of post-stroke complication of vasospasm. The development of vasospasm can lead to delayed cerebral ischemia (DCI; and secondary infarction), which is in turn associated with poor functional outcomes.
Taken together, these pieces of evidence make it likely that variations in dopamine, serotonin, and BDNF play a role for functional outcomes after aSAH. If verified to be true, this would allow health professionals to precisely target only those with a high risk of poor functional outcomes with pharmacologic therapy. This is advantageous by first eliminating the financial burden associated with treating everyone with these therapies and second by reducing the potential side effects for those who would not benefit from this treatment. Thus, the purpose of this pilot study was to explore the relationship of genetic polymorphisms in the DA, serotonergic, and BDNF pathways with 12-month functional outcomes for aSAH subjects. This work represents a pilot inquiry into this area of research in order to justify feasibility and expansion into a larger sample.
Methods
Subjects
Subjects were recruited as a part of a larger observational study (referred to as the “parent study”) from a Level 1 trauma center at the time of admission for aSAH. Written informed consent was obtained from the subject or their legally authorized representative, and demographic and clinical information was obtained from clinical records. Inclusion criteria for the parent study were as follows: (1) new (within 5 days) diagnosis of aSAH verified via cerebral angiogram; (2) Fisher grade ⩾2; (3) ⩾18 years of age; and (4) able to read/speak English. Exclusion criteria for the parent study were as follows: (1) history of previous degenerative neurological disorder and (2) blood transfusion prior to blood sample collection. Subjects donated blood at the time of enrollment and underwent interviews at 12 months to obtain measures of functional outcomes. For inclusion in this substudy, subjects must also have genotype information that met quality control procedures (as described below) and 12-month Glasgow Outcome Scale (GOS) and Modified Rankin Scale (mRS) scores. This protocol was approved by the Institutional Review Board of the University of Pittsburgh (REN15110020/IRB980950). Data may be accessed upon request to the authors.
The severity of aSAH was measured using the subject’s Fisher score and by Hunt/Hess (HH) scores. The Fisher scale is typically used in clinical practice and is based on the size of the hemorrhage seen on computed tomography (CT) scan at the time of admission. The scale ranges from 1 to 4 such that 1 = no hemorrhage evident; 2 = subarachnoid hemorrhage less than 1 mm thick; 3 = subarachnoid hemorrhage greater than 1 mm thick; and 4 = subarachnoid hemorrhage of any thickness with intra-ventricular hemorrhage or parenchymal extension.
HH scores are also typically used in clinical practice and are based upon the symptoms of the hemorrhage, rather than appearance on CT scan. As would be expected, these two measures are highly correlated, but they do measure slightly different aspects of the hemorrhage.10 For a HH score of grade 1, the patients are typically asymptomatic or present with mild headache. A grade 2 score patient would have a moderate to severe headache but no neurological deficit outside of a cranial nerve palsy. HH grade 3 patients are drowsy, grade 4 patients are stuporous, and grade 5 patients are in a deep coma.
Outcome measures
Disability and recovery outcomes were measured by the GOS and by the mRS. The first, GOS, is a structured interview commonly used in clinical practice to measure level of disability and has established validity and reliability within the stroke population.11 Scores are hierarchical and range from 1–5 such that 1 = death, 2 = persistent vegetative state, 3 = severe disability, 4 = moderate disability, and 5 = low disability (i.e. good recovery). For the purposes of this exploratory and hypothesis-generating analysis, the outcome was dichotomized into either poor (1, 2, or 3) or good (4 or 5) outcomes.12 Cronbach’s alpha for this test ranges between 0.96 and 0.98.13
The mRS is a similar instrument, also used in clinical practice, with high inter-rater reliability,14 but captures a slightly different aspect of recovery. Scores range from 0 to 6, with lower scores indicating a greater variance in physical function. Again, this variable was dichotomized for analysis, and those with poor outcomes (3–6) were evaluated as one group and compared to those with good outcomes (0–2).15 Cronbach’s alpha for this test is α = 0.91.14
Genotype data collection
Whole blood was collected from subjects at the time of admission for aSAH but prior to any blood transfusion, as this might confound genotyping results. DNA was extracted from buffy coat, and genotyping was performed using 500 ng of DNA for the Affymetrix genome-wide human single-nucleotide polymorphism (SNP) array 6.0 (Affymetrix, Santa Clara, CA, USA). This method has been described in detail previously.16
Genome-wide microarray data were then queried for SNPs only in the DA, serotonergic, and BDNF pathways using Affymetrix NetAffx software (http://www.affymetrix.com/analysis/index.affx). This search used genome build GRCh37 (February 2009, NC_012920) with any 5’ flanking regions included. This returned 432 SNPs in these pathways, and these were then subjected to genotype quality control procedures below.
Genotype data quality control
Quality control was performed using R, PLINK17 and Eigenstrat.18 Standard quality control measures for genome-wide association studies were applied, but in brief: Reported sex of all samples was compared with observed sex by calculating the X chromosome inbreeding coefficient. Individuals whose X chromosome inbreeding coefficient estimate was greater than 0.8 were called male and less than 0.2 were called female. Individuals whose values fell in between, and those individuals whose calculated sex does not match their reported sex were flagged and reviewed manually. This scenario eliminated 11 subjects in our dataset, and was probably attributable in most cases to the age of the subjects. For adults, especially older adults, XX/XO and XY/XO mosaicism may be observed and is not a cause for concern, but may produce values outside of the expected range. Alternately, X chromosome inbreeding coefficients outside of the expected could be the result of blood transfusions that were unreported in the research record. A transfusion from male to female or vice versa could create this problem.
Missingness levels by SNP and by sample were calculated. Variants with a call rate below 95% or individuals with a call rate below 95% were excluded. The remaining variants were tested for violation of the assumption of Hardy–Weinberg equilibrium, and SNPs with a p value < 1 × 10−4 were removed from the dataset. Once all poorly performing SNPs had been excluded, samples were tested for cryptic relatedness and decisions made on how to deal with excess kinship. Heterozygosity rates were calculated genome wide, to test for excessive rates of heterozygosity which could indicate sample contamination. Finally, principal component analysis (PCA) was performed using Eigenstrat18 with HapMap anchors to test whether samples self-reported ancestry clustered with the expected HapMap ancestry group, and any significant outliers were then excluded. The PCA was then repeated without HapMap anchors for the purposes of including the top eigenvalues as covariates in the association analyses to adjust for population stratification.
Statistical analysis
All statistics were calculated using R statistical software19 (version 3.3.1; Vienna, Austria) and PLINK.17 Descriptive statistics were measured by means, standard deviations, and ranges. Chi squares, t-tests, and correlations were used as appropriate to evaluate demographic and clinical characteristics between subgroups.
Genetic association analyses were performed in PLINK using a logistic model and adjusted for age, sex, and the first three principal components from the PCA. Odds ratios (ORs) and 95% confidence intervals were calculated for each SNP. Adjusting the statistics for multiple testing using the standard Bonferroni correction approach, which is based on the number of SNPs, makes little sense in a small dataset with significant blocks of linkage disequilibrium (LD). Instead we used a modified Bonferroni approach by calculating the effective number of tests in R, using the method of Ramos et al.20 This method calculates intermarker LD as part of the correction procedure and returns the effective degrees of freedom which are used to adjust the target significance level (α). In SNPs with an OR of greater than one, the variant allele confers risk. Thus, each extra copy of the minor allele increases the risk of having a poor outcome. Similarly, SNPs with an OR of less than one means that that allele is protective, that is, that each extra copy of the minor allele decreases the risk of having a poor outcome. As this was an exploratory pilot study, based upon the number of subjects available and meeting criteria from a larger dataset, retrospective power calculations were done for the final sample size of 154 subjects. We found that for the lowest minor allele frequency (MAF) of 0.15, our sample size has have >90% power to detect variants with an OR > 2.5.
Results
Demographic characteristics
A total of 154 subjects with GOS and mRS outcomes at 12 months were included in the analysis. These subjects were pulled for meeting substudy inclusion criteria from a larger parent observational study. The process of selection is shown in Figure 1. While this is a smaller sample size than usually used for this type of work, these individuals represent a very rare phenotype in that they were collected prospectively and survived a subarachnoid hemorrhage for longer than 1 year.
Figure 1.
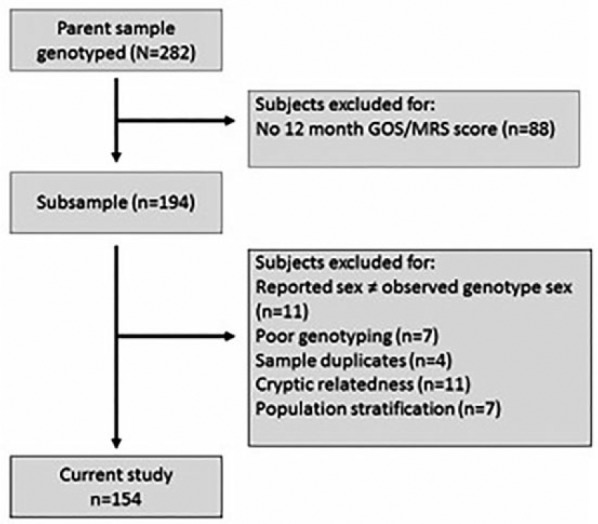
Flow diagram of subject selection procedures.
Included subjects were 55.8 ± 11.3 years old and 74% female (Table 1). While our recruitment was not limited by race, all subjects included in this analysis self-identified as being Caucasian. The majority of aneurysms were treated by endovascular coiling, rather than neurosurgical clipping. The mean Fisher score for this sample was 3.0 ± 0.7, reflecting moderate- to large-sized hemorrhages in this sample. Demographic and clinical characteristics for the total sample are listed in Table 1.
Table 1.
Demographic and clinical characteristics of the sample (N = 154).
| Characteristics | N | % | |
|---|---|---|---|
| Sex | |||
| Male | 40 | 26 | |
| Female | 114 | 74 | |
| Marital status | |||
| Married | 106 | 69 | |
| Single | 20 | 13 | |
| Divorced/separated | 10 | 7 | |
| Widowed | 13 | 8 | |
| Did not answer | 5 | 3% | |
| Aneurysm side | |||
| Right | 54 | 35 | |
| Left | 31 | 20 | |
| Central | 69 | 45 | |
| Treatment method | |||
| Clip | 65 | 42 | |
| Coil | 89 | 57 | |
| No intervention | 1 | 1 | |
| Outcome measures | |||
| Poor GOS | 30 | 20 | |
| Poor mRS | 38 | 25 | |
| Mean (SD) | Range | Mode | |
| Age (years) | 55.81 (11.29) | 25–75 | 50 |
| Glasgow Coma Scale score | 12.52 (3.79) | 3–15 | 15 |
| Hunt/Hess | 2.66 (1.13) | 1–5 | 3 |
| Fisher | 2.95 (0.67) | 2–4 | 3 |
SD: standard deviation; GOS: Glasgow Outcome Scale; mRS: Modified Rankin Scale.
All percentages are rounded.
Before any quality control measures were applied, the mean call rate was 0.98 and the number of SNPs was 909,622. After sex checking, 11 samples were excluded for sex discrepancies, 7 were excluded for poor genotyping rates, and 90,201 SNPs removed for poor array performance. An additional 5029 SNPs were excluded after violating the assumption of Hardy–Weinberg equilibrium. Four individuals were identified as sample duplicates by kinship testing and 11 subjects removed due to excess kinship with other individuals in the cohort. Heterozygosity rates for all remaining samples were within the normal range, indicating no sample contamination. PCA with HapMap anchors excluded 7 individuals for showing significant population stratification compared to the rest of the dataset.
After standard normalization procedures and quality control, we limited the genotype data to include only those SNPs that were located in DA, serotonergic, or BDNF pathways. This left us with a total of 432 SNPs (86 probes in 7 DA genes, 301 probes in 14 serotonergic genes, and 45 probes in 2 BDNF genes) that were considered for associations with functional outcomes. A final list of genes, and number of probes per gene, is given in Table 2. Analysis of local LD around each gene gave a final effective degrees of freedom of 39.12 and a target Bonferroni corrected p value of 1.28 × 10−3.
Table 2.
List of genes considered, by pathway.
| Pathway | Gene symbol | Gene name | No. of probes |
|---|---|---|---|
| Dopamine | SLC6A3 | Solute carrier family 6, member 3 (dopamine transporter) | 10 |
| Dopamine | ANKK1 | Ankyrin repeat and kinase domain containing 1 | 1 |
| Dopamine | DRD1 | Dopamine receptor D1 | 10 |
| Dopamine | DRD2 | Dopamine receptor D2 | 43 |
| Dopamine | DRD3 | Dopamine receptor D3 | 12 |
| Dopamine | DRD5 | Dopamine receptor D5 | 4 |
| Dopamine | DBH | Dopamine beta-hydroxylase | 6 |
| Serotonin | HTR1A | 5-hydroxytryptamine (serotonin) receptor 1A | 16 |
| Serotonin | HTR1B | 5-hydroxytryptamine (serotonin) receptor 1B | 31 |
| Serotonin | HTR1D | 5-hydroxytryptamine (serotonin) receptor 1D | 3 |
| Serotonin | HTR1E | 5-hydroxytryptamine (serotonin) receptor 1E | 8 |
| Serotonin | HTR1F | 5-hydroxytryptamine (serotonin) receptor 1F | 5 |
| Serotonin | HTR2A | 5-hydroxytryptamine (serotonin) receptor 2A | 107 |
| Serotonin | HTR2C | 5-hydroxytryptamine (serotonin) receptor 2C | 69 |
| Serotonin | HTR3B | 5-hydroxytryptamine (serotonin) receptor 3B | 1 |
| Serotonin | HTR3C | 5-hydroxytryptamine (serotonin) receptor 3C | 3 |
| Serotonin | HTR3D | 5-hydroxytryptamine (serotonin) receptor 3D | 1 |
| Serotonin | HTR3E | 5-hydroxytryptamine (serotonin) receptor 3E | 3 |
| Serotonin | HTR4 | 5-hydroxytryptamine (serotonin) receptor 4 | 45 |
| Serotonin | HTR6 | 5-hydroxytryptamine (serotonin) receptor 6 | 1 |
| Serotonin | HTR7 | 5-hydroxytryptamine (serotonin) receptor 7 | 8 |
| BDNF | BDNF-AS | BDNF antisense RNA | 22 |
| BDNF | BDNF | Brain-derived neurotrophic factor | 23 |
Each outcome (GOS, mRS) was assessed for associations with demographic variables using point biserial correlations, and chi-square tests as appropriate (Table 3). Age and Fisher score were found to have a significant association with GOS, but not with mRS. Sex, treatment method (clip or coil), and side of aneurysm were not significantly associated with functional outcomes, while admission Glasgow Coma Scale (GCS) and HH scores were significantly associated.
Table 3.
Associations of dichotomized GOS and mRS scores with demographic and clinical characteristics.
| Characteristics | GOS (p) | mRS (p) |
|---|---|---|
| Age (point biserial) | r = −0.2, two tailed = 0.01 | r = 0.13, two tailed = 0.12 |
| Sex (chi-square) | 0.75 | 0.74 |
| Treatment (chi-square) | 0.95 | 0.94 |
| Glasgow Coma Scale score (point biserial) | r = 0.42, two tailed < 0.0001 | r = −0.37, two tailed < 0.0001 |
| Hunt/Hess (chi-square) | 0.0005 | 0.004 |
| Fisher (chi-square) | 0.03 | 0.19 |
| Side (chi-square) | 0.94 | 0.93 |
GOS: Glasgow Outcome Scale; mRS: Modified Rankin Scale.
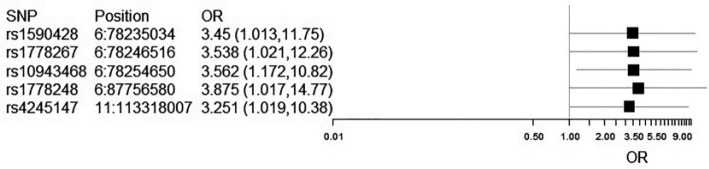
We found that there were several SNPs associated with GOS or with mRS scores at 12 months post SAH (Tables 4 and 5). For GOS associations, these SNPs were located in the serotonin receptor genes 1B and 1E and in the dopamine receptor D2. The listed alleles of these SNPs confer a dose-dependent risk of poor functional outcomes after SAH (OR of 1 or greater). These OR were statistically significant for p ⩽ 0.05, with confidence intervals shown by Forest plot (Figure 2). However, these p values do not survive Bonferroni correction of p = 1.28 × 10−3.
Table 4.
SNPs associated with poor GOS scores.
| Chromosome | SNP | Gene name | Gene symbol | Allele | OR | SE | L95 | U95 | p |
|---|---|---|---|---|---|---|---|---|---|
| 6 | rs1590428 | 5-hydroxytryptamine (serotonin) receptor 1E | HTR1E | A | 3.88 | 0.68 | 1.02 | 14.77 | 0.05 |
| 6 | rs1778267 | 5-hydroxytryptamine (serotonin) receptor 1B | HTR1B | T | 3.56 | 0.57 | 1.17 | 10.82 | 0.03 |
| 6 | rs10943468 | 5-hydroxytryptamine (serotonin) receptor 1B | HTR1E | G | 3.54 | 0.64 | 1.02 | 12.26 | 0.05 |
| 6 | rs1778248 | 5-hydroxytryptamine (serotonin) receptor 1B | HTR1E | A | 3.45 | 0.63 | 1.01 | 11.75 | 0.05 |
| 11 | rs4245147/rs4936271 | Dopamine receptor D2 | DRD2 | A | 3.25 | 0.59 | 1.02 | 10.38 | 0.05 |
rs4245147 and rs4936271 are in perfect linkage disequilibrium.
Table 5.
SNPs associated with poor mRS scores.
| Chromosome | SNP | Gene name | Gene symbol | Allele | OR | SE | L95 | U95 | p |
|---|---|---|---|---|---|---|---|---|---|
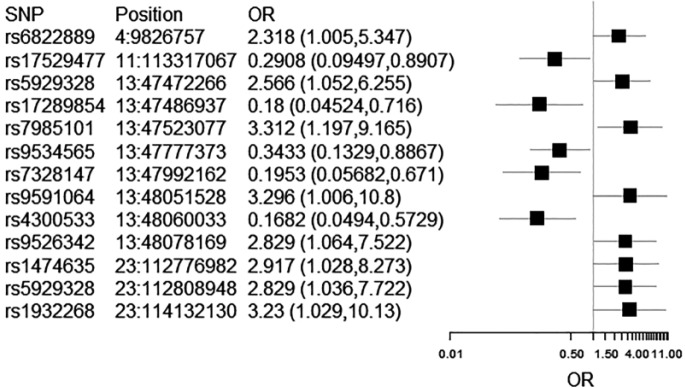
| 13 | rs7985101 | 5-hydroxytryptamine (serotonin) receptor 2A | HTR2A | G | 3.31 | 0.52 | 1.20 | 9.17 | 0.02 |
| 13 | rs9591064 | 5-hydroxytryptamine (serotonin) receptor 2A | HTR2A | G | 3.30 | 0.61 | 1.01 | 10.80 | 0.05 |
| 23 | rs1932268 | 5-hydroxytryptamine (serotonin) receptor 2C | HTR2C | G | 3.23 | 0.58 | 1.03 | 10.13 | 0.05 |
| 23 | rs1474635 | 5-hydroxytryptamine (serotonin) receptor 2C | HTR2C | T | 2.92 | 0.53 | 1.03 | 8.27 | 0.04 |
| 13 | rs9526342 | 5-hydroxytryptamine (serotonin) receptor 2A | HTR2A | T | 2.83 | 0.50 | 1.06 | 7.52 | 0.04 |
| 23 | rs5929328 | 5-hydroxytryptamine (serotonin) receptor 2C | HTR2C | T | 2.83 | 0.51 | 1.04 | 7.72 | 0.04 |
| 13 | rs5929328 | 5-hydroxytryptamine (serotonin) receptor 2C | HTR2C | C | 2.57 | 0.45 | 1.05 | 6.26 | 0.04 |
| 4 | rs6822889 | Dopamine receptor D5 | DRD5 | C | 2.32 | 0.43 | 1.01 | 5.35 | 0.05 |
| 13 | rs9534565 | 5-hydroxytryptamine (serotonin) receptor 2A | HTR2A | A | 0.34 | 0.48 | 0.13 | 0.89 | 0.03 |
| 11 | rs17529477 | Dopamine receptor D2 | DRD2 | T | 0.29 | 0.57 | 0.09 | 0.89 | 0.03 |
| 13 | rs7328147 | 5-hydroxytryptamine (serotonin) receptor 2A | HTR2A | A | 0.20 | 0.63 | 0.06 | 0.67 | 0.01 |
| 13 | rs17289854/ rs17289889 | 5-hydroxytryptamine (serotonin) receptor 2A | HTR2A | A | 0.18 | 0.70 | 0.05 | 0.72 | 0.01 |
| 13 | rs4300533 | 5-hydroxytryptamine (serotonin) receptor 2A | HTR2A | G | 0.17 | 0.63 | 0.05 | 0.57 | 0.004 |
N. B. rs17289854 and rs17289889 are in perfect linkage disequilibrium.
Figure 2.
Forest plot of confidence intervals for SNPs associated with GOS scores at 12 months. As rs4245147 and rs4936271 are in perfect LD and have identical statistics, only rs4245147 is shown.
Different SNPs were significantly associated with poor mRS scores at 12 months. SNPs in the serotonin receptor genes 2A and 2C, and dopamine receptor D5, conferred a risk of poor functional outcome (OR greater than 1). Other SNPs within the same serotonin genes, and within the dopamine receptor gene D2, were protective for better functional outcomes after aSAH (OR less than 1). Again, these SNPs were significant at p ⩽ 0.05, but did not survive Bonferroni correction (p = 1.28 × 10−3). The Forest plots for confidence intervals are shown in Figure 3.
Figure 3.
Forest plot of confidence intervals for SNPs associated with mRS scores at 12 months. As rs17289854 and rs17289889 are in perfect LD and have identical statistics, only rs4245147 is shown.
Discussion
While exploratory, this work adds to the body of literature on genetic contributions to functional outcomes post aSAH so that the results may be replicated in larger samples. Our findings are similar to prior work that shows that certain demographic and clinical variables are significant for the prediction of functional outcomes after aSAH.21–23 In our sample, we found that age was significant for associations with poor GOS. We also found that admission GCS, HH, and Fisher scores were significantly associated with mRS and GOS functional outcomes. These clinical associations are not surprising as the level of impairment from the injury at admission can often predict the level of impairment at 1 year after stroke. Furthermore, we are also in agreement with the literature that the treatment method (either endovascular coiling or neurosurgical clipping) is not associated with functional outcomes at 12 months. But unlike some other studies, we did not find that sex or the side of aneurysm was significant for associations with long-term outcomes.
We show here that genes within the DA, serotonergic, and BDNF pathways do have associations with functional outcomes. This hints at a potential biological explanation for the improvements in outcomes when medications with these effects are given, and is despite the fact that these associations do not survive multiple testing correction. In our work, SNPs in serotonin receptors 1B and 1E showed uncorrected associations with poor GOS scores at 12 months, while SNPs in serotonin receptors 2A and 2C, and dopamine receptor D5 showed uncorrected associations with poor mRS scores at 12 months. SNPs within dopamine receptor D2 showed uncorrected associations with both measures for poor functional outcomes, although the associations were for different specific variants for each instrument.
We are supported in this inquiry by biological plausibility of these pathways. The serotonin receptor family is a common target for studies in vasoconstriction. Vasospasm (vasoconstriction) is a major complication post aSAH. The serotonin receptor 1B is particularly important for cerebral vasoconstriction, and messenger RNA (mRNA) levels for this receptor have been shown to be increased in animal models of aSAH.24 This upregulation is then also associated with DCI, which in humans is known to produce poor functional outcomes.25
While compelling, little is known about the functionality of the HTR1B SNP rs1590428. Further functional investigation may reveal this to be the mechanism behind expression changes, which could potentially be used clinically as early indicators of vasospasm leading to DCI. The serotonin receptor 1E is also present in cerebral arteries26 although little is known about its precise functionality. Variations in the serotonin receptor 2A and serotonin receptor 2C are known targets for SSRIs,27 which have been shown to improve functional outcomes when given post-stroke.8 But more work should be done in replicating these associations, and then characterizing which of the serotonergic genetic changes are the most important for functional outcomes post aSAH. This would allow the most precise targeting for pharmacological therapy.
Here, we have also shown that variations in DRD2 and DRD5 may be associated with disability and recovery outcomes. But the precise nature of this association remains unclear. Dopamine has been previously shown to be important for mental health outcomes after aSAH.3 It could be that more disabled, or less fully recovered, patients are more likely to also have poor mental health outcomes. This is commonly seen clinically and is supported in the literature.28
It is also possible that vasospasm post aSAH could be a mediator responsible for these associations. Similarly to serotonin, dopamine has been shown to play a role in vasospasm/constriction, although these studies have not yet been replicated in human subjects.29 There is also evidence that antidepressants given after stroke can improve motor function, which is thought to be a result of dopamine modulation.30 Work should be done to investigate this relationship.
Although our pilot work is supportive for DA and serotonergic associations, our results disagree with other works on BDNF and associations with long-term outcomes. For instance, the BDNF SNP rs11030119 was independently associated with poor mRS scores at 7 years after stroke,4 and the BDNF rs6265 AA genotype was related to poor mRS at 3 months after stroke.5 But these associations were not seen in our group, possibly due to a different time period for the outcome measurements or variations in sample size/demographics. Furthermore, our sample may exhibit different minor allele frequencies than those in previous work, which can reduce power to detect these outcomes.
Limitations
While exciting, this work has certain limitations. Most importantly, this work represents an exploratory pilot sample focused on long-term outcomes of aSAH survivors in a limited number of subjects. Those persons who did not survive the initial hemorrhagic event, or who passed away at some time prior to 12 months, were not included in this substudy analysis. Therefore, we can only draw conclusions regarding factors predictive of disability for the survivors and cannot make any claims regarding whether such factors would be predictive of mortality. Future work should be done to determine what factors are predictive of mortality as compared to long-term disability for this group.
Furthermore, this work is limited by other potential confounders when considering long-term outcomes. For instance, complications during the hospital course or during recovery and the use of rehabilitation services were not considered in this analysis. We were limited in the availability of such data given the purely retrospective substudy nature of this work. Our subjects were originally recruited as a part of a larger parent project, and so, these data were not available for this analysis. Larger replications of this work should include such factors.
We were also limited by the quality of genotype data. While we were powered to detect common variants of large effect (MAF > 0.15, >90% power to detect variants with an OR > 2.5), we were statistically underpowered to fully address issues like multiple testing or subgroup sampling. The work was meant to determine feasibility and effect sizes design considerations to conduct these types of associations in a larger group.
Furthermore, this analysis comprised self-identified Caucasian subjects, with this ancestral background verified by PCA. This limits generalization to populations of different ancestral background. Alternate racial and ethnic groups may have changes in allele frequencies that make these associations more or less readily seen.
Finally, we were limited in our data collection methods to only consider SNPs with probe identifiers on the Affymetrix genome-wide human SNP array 6.0 (Affymetrix, Santa Clara, CA, USA). While this microarray covers 906,600 SNPs, it does not include coverage for some known size-based polymorphisms (such as variable number tandem repeats (VNTRs)).
Conclusion
Despite these limitations, our work suggests that there is genetic evidence for a relationship between genetic factors and functional outcomes post stroke. In the future, these results should be longitudinally replicated in larger and more diverse sample sizes. After further investigation and confirmation, this information could then be used to identify patients early in the acute period who may have poor outcomes, allowing for targeted early intervention.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the University of Pittsburgh (REN15110020/IRB980950).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Nursing Research (5 T32 NR009759, R01NR004339).
Informed consent: Written informed consent was obtained from all subjects before the study or written informed consent was obtained from legally authorized representatives before the study. Both of these statements are true due to the critical nature of subarachnoid hemorrhage.
References
- 1. McKinney JS, Cheng JQ, Rybinnik I, et al. Comprehensive stroke centers may be associated with improved survival in hemorrhagic stroke. J Am Heart Assoc 2015; 4: e001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke 2010; 41: e519–e536. [DOI] [PubMed] [Google Scholar]
- 3. Stanfill A, Elijovich L, Baughman B, et al. A review and conceptual model of dopaminergic contributions to poststroke depression. J Neurosci Nurs 2016; 48: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stanne TM, Tjarnlund-Wolf A, Olsson S, et al. Genetic variation at the BDNF locus: evidence for association with long-term outcome after ischemic stroke. PLoS ONE 2014; 9: e114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao J, Wu H, Zheng L, et al. Brain-derived neurotrophic factor G196A polymorphism predicts 90-day outcome of ischemic stroke in Chinese: a novel finding. Brain Res 2013; 1537: 312–318. [DOI] [PubMed] [Google Scholar]
- 6. Ruscher K, Kuric E, Wieloch T. Levodopa treatment improves functional recovery after experimental stroke. Stroke 2012; 43: 507–513. [DOI] [PubMed] [Google Scholar]
- 7. Cramer SC. Drugs to enhance motor recovery after stroke. Stroke 2015; 46: 2998–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chollet F, Tardy J, Albucher JF, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 2011; 10: 123–130. [DOI] [PubMed] [Google Scholar]
- 9. Li W, Ling S, Yang Y, et al. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol Lett 2014; 35: 104–109. [PubMed] [Google Scholar]
- 10. Lindvall P, Runnerstam M, Birgander R, et al. The Fisher grading correlated to outcome in patients with subarachnoid haemorrhage. Br J Neurosurg 2009; 23: 188–192. [DOI] [PubMed] [Google Scholar]
- 11. Jennett B, Snoek J, Bond MR, et al. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 1981; 44: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krause-Titz UR, Warneke N, Freitag-Wolf S, et al. Factors influencing the outcome (GOS) in reconstructive cranioplasty. Neurosurg Rev 2016; 39: 133–139. [DOI] [PubMed] [Google Scholar]
- 13. Jennett B, Teasdale G, Braakman R, et al. Predicting outcome in individual patients after severe head injury. Lancet 1976; 1: 1031–1034. [DOI] [PubMed] [Google Scholar]
- 14. Van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 15. Murthy SB, Moradiya Y, Dawson J, et al. Perihematomal edema and functional outcomes in intracerebral hemorrhage: influence of hematoma volume and location. Stroke 2015; 46: 3088–3092. [DOI] [PubMed] [Google Scholar]
- 16. Kim H, Crago E, Kim M, et al. Cerebral vasospasm after sub-arachnoid hemorrhage as a clinical predictor and phenotype for genetic association study. Int J Stroke 2013; 8: 620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38: 904–909. [DOI] [PubMed] [Google Scholar]
- 19. R Core Team. R: a language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing, 2014. [Google Scholar]
- 20. Ramos E, Chen G, Shriner D, et al. Replication of genome-wide association studies (GWAS) loci for fasting plasma glucose in African-Americans. Diabetologia 2011; 54: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katati MJ, Santiago-Ramajo S, Perez-Garcia M, et al. Description of quality of life and its predictors in patients with aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis 2007; 24: 66–73. [DOI] [PubMed] [Google Scholar]
- 22. Scharbrodt W, Stein M, Schreiber V, et al. The prediction of long-term outcome after subarachnoid hemorrhage as measured by the Short Form-36 Health Survey. J Clin Neurosci 2009; 16: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 23. Alajbegovic A, Djelilovic-Vranic J, Nakicevic A, et al. Post stroke depression. Med Arch 2014; 68: 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ansar S, Edvinsson L. Equal contribution of increased intracranial pressure and subarachnoid blood to cerebral blood flow reduction and receptor upregulation after subarachnoid hemorrhage. Laboratory investigation. J Neurosurg 2009; 111: 978–987. [DOI] [PubMed] [Google Scholar]
- 25. Povlsen GK, Johansson SE, Larsen CC, et al. Early events triggering delayed vasoconstrictor receptor upregulation and cerebral ischemia after subarachnoid hemorrhage. BMC Neurosci 2013; 14: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klein MT, Teitler M. Distribution of 5-ht(1E) receptors in the mammalian brain and cerebral vasculature: an immunohistochemical and pharmacological study. Br J Pharmacol 2012; 166: 1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Artigas F. Serotonin receptors involved in antidepressant effects. Pharmacol Ther 2013; 137: 119–131. [DOI] [PubMed] [Google Scholar]
- 28. Matsuzaki S, Hashimoto M, Yuki S, et al. The relationship between post-stroke depression and physical recovery. J Affect Disord 2015; 176: 56–60. [DOI] [PubMed] [Google Scholar]
- 29. Pyne-Geithman GJ, Caudell DN, Cooper M, et al. Dopamine D2-receptor-mediated increase in vascular and endothelial NOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in vitro. Neurocrit Care 2009; 10: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siepmann T, Kepplinger J, Zerna C, et al. The effects of pretreatment versus de novo treatment with selective serotonin reuptake inhibitors on short-term outcome after acute ischemic stroke. J Stroke Cerebrovasc Dis 2015; 24: 1886–1892. [DOI] [PubMed] [Google Scholar]