Abstract
Background:
Adjuvant therapy after curative resection is associated with survival benefit in stage III pancreatic cancer. We analyzed the factors affecting the outcome of adjuvant therapy in stage III pancreatic cancer and compared overall survival with different modalities of adjuvant treatment.
Methods:
This is a retrospective study of patients with stage III pancreatic cancer listed in the National Cancer Database (NCDB) who were diagnosed between 2004 and 2012. Patients were stratified based on adjuvant therapy they received. Unadjusted Kaplan-Meier and multivariable Cox regression analysis were performed.
Results:
We analyzed a cohort included 1731 patients who were recipients of adjuvant therapy for stage III pancreatic cancer within the limits of our database. Patients who received adjuvant chemoradiation had the longest postdiagnosis survival time, followed by patients who received adjuvant chemotherapy, and finally patients who received no adjuvant therapy. On multivariate analysis, advancing age and patients with Medicaid had worse survival, whereas Spanish origin and lower Charlson comorbidity score had better survival.
Conclusions:
Our study is the largest trial using the NCDB addressing the effects of adjuvant therapy specifically in stage III pancreatic cancer. Within the limits of our study, survival benefit with adjuvant therapy was more apparent with longer duration from date of diagnosis.
Keywords: Pancreatic cancer, stage III, adjuvant therapy
Introduction
Pancreatic cancer is currently one of the more life-threatening cancers among solid organ malignancies.1 Pancreatic cancer is the third leading cause of cancer-related death in the United States. In 2017, it has been estimated that there will be 53 670 new cases of pancreas cancer and 43 090 pancreatic cancer–related deaths, which is an increase compared with previous years, reflecting the high prevalence of advanced disease.2
Survival has been shown to be slightly improved with localized pancreatic malignancies, as surgical resection is the only curative treatment modality at present. Most patients are diagnosed at late stages when the disease has extended beyond the pancreas, and surgical resection is no longer an option. Despite continuing advancements in the management of pancreatic cancer, the 5-year overall survival has been estimated to be as low as 4%.3
Adjuvant chemotherapy with single-agent gemcitabine has been the standard of care for many years for locally advanced disease following resection.4
Surgical resection is currently the only curative therapy for pancreatic adenocarcinoma. Postoperative adjuvant therapy may be limited to chemotherapy alone or induction chemotherapy followed by radiation. The addition of radiation therapy to chemotherapy for adjuvant therapy has remained a controversial topic, with the benefits of radiation therapy still debated.
Per National Comprehensive Cancer Network (NCCN) guidelines, those with locally advanced disease should receive first-line therapy with chemotherapy alone, chemotherapy followed by radiation or stereotactic body radiation therapy, or chemoradiation alone. The first-line chemotherapy regimens in patients with good performance status include single-agent chemotherapy with gemcitabine alone or multi-agent chemotherapy with 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (Folfirinox) or other combination gemcitabine regimens (eg. gemcitabine-paclitaxel).
A randomized trial in Europe in 2004 demonstrated that adjuvant chemotherapy had a significant impact on improving survival, whereas radiation therapy provided no additional survival benefit.5 The type of adjuvant chemotherapy, whether fluorouracil-based chemotherapy or gemcitabine monotherapy, did not differ in its impact on overall survival.6
The Gastrointestinal Tumor Study Group (GITSG) conducted a small randomized trial in which combined chemotherapy and radiation showed significantly greater median survival in participants who received adjuvant chemotherapy compared with those who did not (21 vs 9 months, respectively).7 A previous analysis of the National Cancer Database (NCDB) that included 11 526 patients diagnosed between 1998 and 2002 showed significant survival benefit of adjuvant chemoradiation treatment. In this multivariable analysis, combined chemoradiation was associated with 21% lower risk of death compared with no adjuvant treatment. There was no difference in risk among those receiving chemotherapy only vs those not receiving any form of adjuvant treatment.8
Given that the studies mentioned above showed varying results regarding adjuvant therapy, the purpose of our study was to use the NCDB to more clearly establish the association of adjuvant chemotherapy or adjuvant chemoradiation on patient survival following resection in stage III pancreatic cancer after adjusting for patient demographic and clinical factors.
Methods
This is a retrospective study of patients with stage III pancreatic cancer listed in the NCDB who were diagnosed between 2004 and 2012. The NCDB is a clinical oncology database sourcing data collected from more than 1500 Commission on Cancer–accredited facilities in the United States and Puerto Rico. This database evaluates cancer diagnoses from American College of Surgeons (ACOS)-certified hospitals, accounting for 70% of all newly diagnosed cancer cases.9
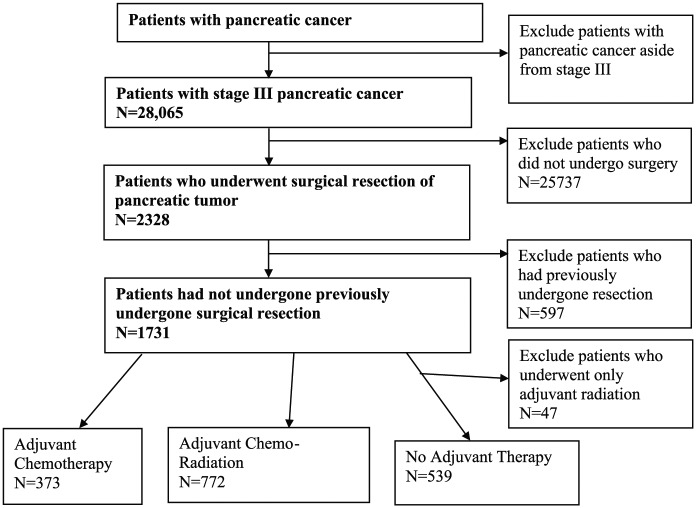
A total of 28,065 patients with stage III pancreatic cancer were identified within the NCDB. We excluded 25,737 patients who had not undergone surgical resection of the pancreatic tumor and another 597 patients who had previously undergone surgical resection. Our final analysis cohort included 1731 patients (Figure 1). Our primary outcome was overall patient survival, defined as the number of months from the patient’s date of diagnosis to either their date of death, when they were lost to follow-up, or date of study end (December 31, 2012); the NCDB does not collect cancer-specific survival. Our primary independent variable was whether a patient received adjuvant chemotherapy, adjuvant radiation, adjuvant chemoradiation, or no adjuvant therapy. Type of surgery was also noted (Table 1). Patient-level covariates included age, biological sex, race (white vs not white), Hispanic ethnicity, primary payer (uninsured, private, Medicare, Medicaid, or other government), Charlson/Deyo score, and socioeconomic indicators that included median income and the proportion of individuals who did not graduate high school for the zip code of a patient’s area of residence.
Figure 1.
Flowchart illustrating inclusion/exclusion criteria of individuals in the study.
Table 1.
Percentage of different types of surgical procedures that were performed in the individuals included in the study.
| Type of surgery | No. (%) |
|---|---|
| Local excision, NOS | 85 (5.1) |
| Partial pancreatectomy | 183 (10.9) |
| Local or partial pancreatectomy and duodenectomy | 94 (5.6) |
| Without distal/partial gastrectomy | 117 (7.0) |
| With Whipple | 692 (41.1) |
| Total pancreatectomy | 45 (2.7) |
| With subtotal gastrectomy or duodenectomy | 128 (7.6) |
| Extended pancreatoduodenectomy | 118 (7.0) |
| Pancreatectomy, NOS | 43 (2.6) |
| Surgery, NOS | 179 (10.6) |
Abbreviation: NOS, not otherwise specified.
We were also able to obtain descriptive data on the subtypes based on the location of the tumor as listed in Table 2. A variety of surgical procedures were performed on patients in this study. The most common type of surgery was the Whipple procedure (41.1%). Partial pancreatectomy and extended pancreatoduodenectomy, among other surgical procedures, were also used for excision in these patients.
Table 2.
Anatomic site of origin of pancreatic cancer.
| Primary site | No. (%) |
|---|---|
| Head | 1066 (63.3) |
| Body | 160 (9.5) |
| Tail | 160 (9.5) |
| Duct | 13 (0.8) |
| Islets of Langerhans | 1 (0.1) |
| Other Specified Parts | 18 (1.1) |
| Overlapping lesion | 111 (6.6) |
| Not specified | 155 (9.2) |
Statistical Analysis
Between-therapy differences for continuous variables were evaluated using the Kruskal-Wallis test with post hoc Mann-Whitney tests, whereas differences in categorical variables were evaluated using the χ2 tests with post hoc Fisher exact tests. Unadjusted between-group differences in survival were evaluated using the Kaplan-Meier method with post hoc log-rank tests. Multivariable Cox regression models were estimated to evaluate between-group differences in risk of death after adjusting for the patient-level covariates described previously. Prior to estimating the final Cox regression models, the functional form for patient age at diagnosis was evaluated using smoothed martingale residuals; the proportionality of hazards assumption was evaluated graphically using log-negative-log survival curves and statistically using interactions with time. For all Cox regression models, a robust sandwich covariance matrix was used to account for the nesting of patients within facilities; this marginal Cox regression modeling approach was considered appropriate for the NCDB data (in lieu of a shared frailty, aka, mixed-effects Cox regression models), given that these data represent a near population of newly diagnosed cancer cases. As such, all estimated hazard ratios represent the populated-averaged effect across all patients and facilities. All statistical analyses were conducted using SAS (version 9.4, SAS Institute, Cary, NC, USA), with P < .05 used to indicate statistical significance in the Cox regression models; all post hoc tests were Tukey-Kramer adjusted.
Results
Patient characteristics
A total of 1731 patients with stage III pancreatic cancer who underwent initial surgical treatment were identified from the NCDB. Because only 47 patients received adjuvant radiation therapy, they were excluded from the analysis, resulting in a final sample of 1684 patients. Descriptive statistics for demographic and clinical variables are presented in Table 3 for the entire sample, as well as for each therapy group separately. Approximately 22.2% received adjuvant chemotherapy, 45.8% received adjuvant chemoradiation, and 32.0% received no adjuvant therapy. In general, patients were white (85.7%), had income higher than $48 000/y (60.0%), had Medicare insurance (49.3%), and were free of comorbidities (70.8%). Relative to either adjuvant therapy group, patients not receiving adjuvant therapy were older, thereby having a higher rate of Medicare insurance. There was also a higher rate of female patients and patients who lived in areas in which fewer individuals had high school diplomas.
Table 3.
Descriptive statistics for demographic and clinical variables.
| All patients (n = 1684) | Chemotherapy (n = 373) | Chemoradiation (n = 772) | No adjuvant therapy (n = 539) | P | |
|---|---|---|---|---|---|
| Age, y, mean | 64.5 | 64.5 | 62.1 | 67.7 | <.001 |
| Months of follow-up, mean | 18.9 | 20.1 | 22.5 | 12.8 | <.001 |
| White, % | 85.7 | 84.2 | 86.5 | 85.5 | .564 |
| Hispanic, % | 4.7 | 4.6 | 4.8 | 4.6 | .982 |
| Female, % | 44.7 | 44.5 | 41.2 | 49.9 | .008 |
| Primary payer | <.001 | ||||
| Not insured, % | 3.2 | 3.2 | 3.4 | 2.8 | |
| Private, % | 41.7 | 42.4 | 47.8 | 32.5 | |
| Medicaid, % | 4.5 | 4.8 | 5.4 | 3.0 | |
| Medicare, % | 49.3 | 48.8 | 51.6 | 60.7 | |
| Government, % | 1.4 | 0.8 | 1.8 | 1.1 | |
| Charlson/Deyo score | .109 | ||||
| 0, % | 70.8 | 70.2 | 73.7 | 67.0 | |
| 1, % | 22.8 | 22.5 | 20.9 | 25.8 | |
| 2, % | 6.4 | 7.2 | 5.4 | 7.2 | |
| Median income, $ | .324 | ||||
| <38 000, % | 17.7 | 16.4 | 17.5 | 18.9 | |
| 38 000–47 999, % | 22.3 | 22.5 | 20.3 | 24.9 | |
| 48 000–62 999, % | 26.3 | 28.2 | 26.2 | 25.2 | |
| >63 000, % | 33.7 | 33.0 | 36.0 | 31.0 | |
| No HSD, % | .001 | ||||
| >21, % | 16.0 | 17.2 | 15.2 | 16.5 | |
| 13–20.9, % | 25.9 | 25.7 | 21.6 | 32.1 | |
| 7–12.9, % | 32.6 | 31.9 | 36.0 | 28.2 | |
| <7, % | 25.5 | 25.2 | 27.2 | 23.2 | |
| Died, % | 83.7 | 83.1 | 82.0 | 86.6 | .076 |
Abbreviation: HSD, high school diploma.
Unadjusted survival estimates
Median length of follow-up across all patients was approximately 13.0 (interquartile range = 7.1-23.7) months. A total of 1410 deaths were observed during follow-up. Within each therapy group, 467 (86.6%) patients who received no adjuvant therapy died, 310 (83.1%) patients who received adjuvant chemotherapy died, and 633 (82.0%) patients who received adjuvant chemoradiation died. Figure 2 presents Kaplan-Meier curves stratified by therapy group; Table 4 presents the percentage of patients alive and the number of patients remaining at risk at 6, 12, 36, and 60 months postdiagnosis. The overall log-rank test was statistically significant, , P < .001, with significant between-group differences in survival observed between all groups (all adjusted P’s < .001). Specifically, patients receiving adjuvant chemotherapy had median survival of 14.3 (95% confidence interval [CI] = 13.1-17.3) months, patients receiving adjuvant chemoradiation had median survival of 17.2 (95% CI = 16.1-18.3) months, and patients not receiving adjuvant therapy had median survival of 6.8 (95% CI = 6.1-7.9) months.
Figure 2.
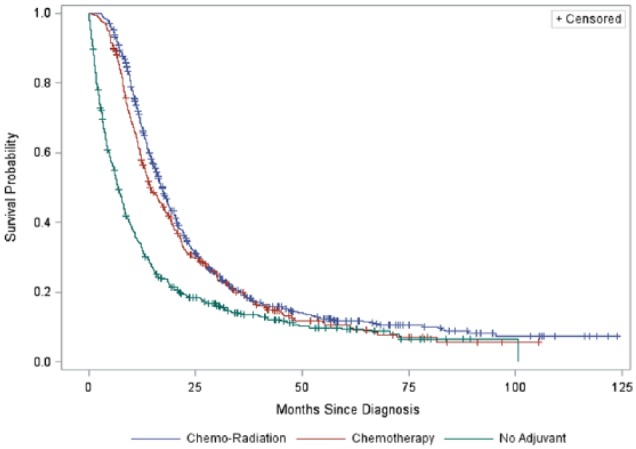
Kaplan-Meier survival curve by therapy group.
Table 4.
Final multivariable Cox regression model—adjusted between-therapy differences at 6-month intervals.
| HR | 95% CI for HR |
||
|---|---|---|---|
| Lower | Upper | ||
| 6 months postdiagnosis | |||
| Chemotherapy vs no adjuvant | 0.38 | 0.31 | 0.47 |
| Chemoradiation vs no adjuvant | 0.34 | 0.28 | 0.40 |
| Chemotherapy vs chemoradiation | 1.12 | 0.95 | 1.33 |
| 12 months postdiagnosis | |||
| Chemotherapy vs no adjuvant | 0.58 | 0.49 | 0.68 |
| Chemoradiation vs no adjuvant | 0.53 | 0.47 | 0.60 |
| Chemotherapy vs chemoradiation | 1.09 | 0.95 | 1.25 |
| 36 months postdiagnosis | |||
| Chemotherapy vs no adjuvant | 3.18 | 1.87 | 5.41 |
| Chemoradiation vs no adjuvant | 3.33 | 2.02 | 5.49 |
| Chemotherapy vs chemoradiation | 0.96 | 0.76 | 1.20 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Estimate = log hazard. All effects in this table are also adjusted for the patient demographic and clinical covariates presented in Table 3. The reference group for all categorical covariates is indicated by the category after the “vs.” For example, specifically at 6 months postdiagnosis, patients receiving chemotherapy were 62% less likely to die than patients receiving no adjuvant therapy.
Adjusted survival estimates
The functional form of age at diagnosis was linear. A violation of the proportionality of hazards assumption was observed only in patients not receiving adjuvant therapy and patients who received either adjuvant chemotherapy or adjuvant chemoradiation. This violation indicated that the risk of death changed as the number of months following the pancreatic cancer diagnosis increased (therapy-by-time interactions had P < .001); however, the risk of death was proportional between the 2 adjuvant therapy groups (P = .488). Given our focus on between-therapy differences, we retained the therapy-by-time interactions in the final marginal Cox regression model.
Table 4 presents adjusted hazard ratios between therapy groups at 6, 12, and 36 months postdiagnosis, controlling for the patient-level covariates. We chose to present results only as far as 36 months postdiagnosis because the number of patients remaining at risk beyond 36 months was small. Results indicated that the risk of death was similar for patients receiving chemotherapy or chemoradiation at all postdiagnosis intervals (all P’s > .05). Results also indicated that patients receiving no adjuvant therapy had a significantly higher risk of death at both at 6 and 12 months postdiagnosis relative to patients who received adjuvant chemotherapy or adjuvant chemoradiation. At 36 months postdiagnosis, the risk of death was significantly higher for the adjuvant therapy groups; however, these estimates are imprecise, given the wide confidence intervals resulting from the small number of patients remaining at risk. Regarding the patient-level covariates, Table 5 presents adjusted hazard ratios for all covariates included in the final marginal Cox regression model (also controlling for therapy). Given that the proportional hazards assumption was tenable for each covariate, the risk estimate (or hazard) for each covariate was assumed constant throughout the entire length of follow-up. In general, patients had lower risk of death if they were younger, women, had fewer comorbidities, and lived in areas with higher median incomes. Specifically, women were 11% less likely to die compared with men, those with no comorbidities were 25% less likely to die compared with those with 2 or more comorbidities, and those living in areas with a median income of less than $38 000 were 26% more likely to die compared with patients living in areas with a median income of more than $63 000. Furthermore, each year older a patient was at diagnosis was associated with a 1% increased likelihood of dying.
Table 5.
Final multivariable Cox regression model—adjusted covariate effects.
| Covariate | HR | 95% CI for HR |
|
|---|---|---|---|
| Lower | Upper | ||
| Age | 1.01 | 1.01 | 1.02 |
| White vs not white | 1.04 | 0.89 | 1.22 |
| Hispanic vs not Hispanic | 1.08 | 0.83 | 1.41 |
| Female vs male | 0.89 | 0.79 | 0.99 |
| Primary payer | |||
| Not insured vs private | 1.01 | 0.73 | 1.40 |
| Not insured vs Medicaid | 0.74 | 0.50 | 1.12 |
| Not insured vs Medicare | 0.90 | 0.64 | 1.26 |
| Not insured vs government | 0.87 | 0.52 | 1.46 |
| Private vs Medicaid | 0.74 | 0.56 | 0.97 |
| Private vs Medicare | 0.89 | 0.77 | 1.03 |
| Private vs government | 0.86 | 0.53 | 1.41 |
| Medicaid vs Medicare | 1.21 | 0.91 | 1.60 |
| Medicaid vs government | 1.17 | 0.69 | 1.99 |
| Medicare vs government | 0.97 | 0.60 | 1.58 |
| Charlson/Deyo score | |||
| 0 vs 1 | 0.85 | 0.75 | 0.97 |
| 0 vs 2 | 0.75 | 0.60 | 0.94 |
| 1 vs 2 | 0.89 | 0.70 | 1.13 |
| Zip code median income, $ | |||
| <38 000 vs >63 000 | 1.26 | 1.01 | 1.57 |
| 38 000-47 999 vs >63 000 | 0.98 | 0.83 | 1.17 |
| 48 000-62 999 vs >63 000 | 1.10 | 0.93 | 1.30 |
| <38 000 vs 38 000-47 999 | 1.14 | 0.94 | 1.39 |
| <38 000 vs 38 000-47 999 | 1.28 | 1.07 | 1.55 |
| 48 000-62 999 vs 38 000-47 999 | 0.89 | 0.75 | 1.06 |
| Zip code no HSD, % | |||
| <7 vs 7-12.9 | 0.89 | 0.78 | 1.02 |
| <7 vs 13-20.9 | 0.90 | 0.74 | 1.08 |
| <7 vs >21 | 0.99 | 0.77 | 1.26 |
| 7-12.9 vs 13-20.9 | 1.01 | 0.86 | 1.18 |
| 7-12.9 vs >21 | 1.10 | 0.89 | 1.37 |
| 13-20.9 vs >21 | 1.10 | 0.91 | 1.33 |
Abbreviations: CI, confidence interval; HSD, high school diploma; HR, hazard ratio.
Bold values indicate HRs statistically significant at P < .05. All effects in this table are also adjusted for a patient’s therapy group, as presented in Table 3. The reference group for all categorical covariates is indicated by the category after the “vs.” For example, white patients were nonsignificantly 4% more likely to die relative to non-white patients (ie, [1.04-1]*100).
Discussion
The purpose of this study was to evaluate the association between adjuvant chemotherapy, adjuvant chemoradiation, and no adjuvant therapy on survival in a large sample of patients with stage III pancreatic cancer who had undergone surgical resection. Our results indicated that patients who received adjuvant chemoradiation had the longest postdiagnosis survival time, followed by patients who received adjuvant chemotherapy, and finally patients who received no adjuvant therapy. Although median survival favored patients receiving adjuvant chemoradiation, an important finding of our study was the similar adjusted hazard ratio between patients receiving either adjuvant chemotherapy or adjuvant chemoradiation.
Adjuvant therapy has shown increased survival relative to no adjuvant therapy in several previous studies. Using the NCDB, Kooby et al8 evaluated the effect of adjuvant therapy in patients with pancreatic cancer diagnosed at any stage between 1998 and 2002. They found that patients receiving adjuvant chemoradiation had a 21% lower risk of death relative to patients not receiving adjuvant therapy and no difference in risk of death for patients who received adjuvant chemotherapy. They did not compare risk of death between the 2 adjuvant therapy groups. In addition, results of a randomized controlled trial by Chauffert et al based on 119 patients found a significant difference in median overall survival favoring chemotherapy over chemoradiation (8.6 vs 13.0 months, respectively). It is noted that eligibility criteria for this trial included those who had ductal adenocarcinoma with no evidence of distant metastasis, and chemotherapy was restricted to patients who received only gemcitabine, as compared with our study, which includes all patients who received chemotherapy irrespective of the agent.10 Finally, the Eastern Cooperative Oncology Group (ECOG) conducted a trial of 72 patients that compared chemoradiation to chemotherapy in patients with localized unresectable adenocarcinoma, finding that median survival was 11.1 months among patients who received chemoradiation and 9.2 months among patients who received chemotherapy.11
By contrast, adjuvant chemoradiation has been shown to be no more effective than chemotherapy in several studies. For example, in an open-label trial by Hammel et al, which enrolled 449 patients with locally advanced pancreatic cancer between 2008 and 2011, median survival among patients who received chemotherapy and chemoradiation was estimated to be similar at 16.5 and 15.2 months, respectively. Furthermore, a meta-analysis by Liao et al compared the 5 different modalities of adjuvant treatment in pancreatic adenocarcinoma. After careful selection, 10 articles (including 9 randomized trials) were included in the meta-analysis. Results indicated that, when compared with no adjuvant therapy, patients who received fluorouracil had 38% decreased risk of death, whereas no decreased risk of death was observed with chemoradiation.12
Regarding the results of the patient-level covariates, there were clear demographic differences between the differing treatment groups that may have affected the type of treatment administered. The standard treatment of care for pancreatic cancer at stage III would include some form of adjuvant therapy, but 32% patients in our study received no treatment. The lack of adjuvant therapy in these patients could be attributed to their initial health status, as patients who received no adjuvant therapy tended to be older than patients that received adjuvant therapy. Adjuvant therapy in older patients may have been less favorable because of presumed morbidity and mortality. For example, age has been shown to affect choice of treatment, usually leaning toward less aggressive treatment13; however, more aggressive treatment, including chemotherapy, can provide benefit to elderly patients as well.14 Frakes et al15 showed that patients with pancreatic cancer more than 70 years old were less likely to receive adjuvant therapy compared with younger patients but had similar postoperative complications and mortality. Despite the impact of comorbidities and age on survival, the benefit of providing adjuvant therapy to these patients may outweigh the risks. Closely related to age is the effect of primary payer insurance. Specifically, 32.5% of patients without adjuvant therapy had private insurance, whereas at least 40% of patients receiving adjuvant therapy had private insurance. Studies have also shown the impact of insurance on treatment choice for various cancers.16,17 Loehrer et al examined the rates of pancreatic cancer admission and surgical resection before and after the Massachusetts health care reform of 2006. The reform mandated that residents obtain some form of health care insurance and provided insurance for those well below the poverty line. Results showed that after the reform, pancreatic cancer admission rates increased by 15% and surgical resection rates increased by 67%.18
Our study is not without limitations. First, this was a retrospective study; therefore, we were unable to control which variables were measured in patients.2 Second, our analysis was limited by the unavailability of certain demographic covariates, such as body mass index and family history of pancreatic cancer, within the limits of the database. Finally, there were no data regarding the effect of adjuvant therapy on patient quality of life or the toxicity suffered, so disease-free survival could not be estimated. Our analysis was also restricted in terms of identifying and comparing the different chemotherapeutic regimens used for pancreatic cancer (eg, single-agent vs multi-agent chemotherapy regimens).
National Cancer Database accounts for 70% of newly diagnosed cancer cases and provides data only from ACOS-certified hospitals; there may be certain groups (eg, patients in rural hospitals) that are underrepresented in our study due to these restrictions. However, in comparison with the Surveillance, Epidemiology, and End Results (SEER) program, the NCDB provides access to more cases and provides access to hospitals across the country, whereas the SEER gathers information from 28% of the US population.2
Conclusions
From our analysis of data from the NCDB, we can conclude that the benefit of adjuvant therapy among patients with stage III pancreatic cancer was observed only during the early phase of treatment. Currently, it is unclear what exactly contributed to the change in survival trends between treatment groups over time. In general, in the first year, patients receiving adjuvant chemoradiation or chemotherapy have lower risk of death relative to patients who receive no adjuvant therapy; the effect of different modalities of adjuvant chemoradiation and adjuvant chemotherapy was found to be comparable with respect to survival.
Acknowledgments
This study was published as an abstract in the proceedings of American Society of Cancer Oncology (ASCO) annual meeting in 2016.
Footnotes
Peer review:Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 313 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.T.S. reports receiving payment for lectures from Bristol Myers and Celgene in the past.
Author Contributions: Conception or design of the work - MK. Data collection - RW, MK. Data analysis and interpretation - RW, MK. Drafting the article - MK, AA. Critical revision of the article - PTS, MK. Final approval of the version to be published - ML, AA, RW, PTS.
References
- 1. Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA A Cancer J Clin. 2013;63:318–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://seer.cancer.gov/statfacts/html/pancreas.html.
- 3. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 5. Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. [DOI] [PubMed] [Google Scholar]
- 6. Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–1585. [DOI] [PubMed] [Google Scholar]
- 7. Nava HR, Panahon A, Penetrante R, et al. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer. 1987;59:2006–2010. [DOI] [PubMed] [Google Scholar]
- 8. Kooby DA, Gillespie TW, Liu Y, et al. Impact of adjuvant radiotherapy on survival after pancreatic cancer resection: an appraisal of data from the National Cancer Data Base. Ann Surg Oncol. 2013;20:3634–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chauffert B, Mornex F, Bonnetain F. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann. 2008;9:1592–1599. [DOI] [PubMed] [Google Scholar]
- 11. Loehrer PJ, Feng Y, Cardenes H, et al. Gemcitabine alone versus gem-citabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao WC, Chien KL, Lin YL, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:1095–1103. [DOI] [PubMed] [Google Scholar]
- 13. Mor V, Masterson-Allen S, Goldberg R. Relationship between age at diagnosis and treatments. J Am Ger Soc. 1985;33:585–589. [DOI] [PubMed] [Google Scholar]
- 14. Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. [DOI] [PubMed] [Google Scholar]
- 15. Frakes JM, Strom T, Springett GM, et al. Resected pancreatic cancer outcomes in the elderly. J Geriatr Oncol. 2015;6:127–132. [DOI] [PubMed] [Google Scholar]
- 16. Azzopardi J, Walsh D, Chong C, Taylor C. Surgical treatment for women with breast cancer in relation to socioeconomic and insurance status. Breast J. 2014;20:3–8. [DOI] [PubMed] [Google Scholar]
- 17. Groth SS, Al-Refaie WB, Zhong W, et al. Effect of insurance status on the surgical treatment of early-stage non-small cell lung cancer. Ann Thorac Surg. 2016;95:1221–1226. [DOI] [PubMed] [Google Scholar]
- 18. Loehrer AP, Chang DC, Hutter MM, et al. Health insurance expansion and treatment of pancreatic cancer: does increased access lead to improved care? J Am Coll Surg. 2015;221:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]