Abstract
Background
In patients with craniosynostosis, intracranial pressure (ICP) has been reported to increase even in the absence of overt symptoms. The early and non-invasive detection of intracranial hypertension is important for reducing the risk of abnormal brain development in pediatric patients.
Purpose
To assess whether the apparent diffusion coefficient (ADC) of white matter during the cardiac cycle (ΔADC) would change after surgery to relieve ICP in children with craniosynostosis.
Material and Methods
This prospective study included ten patients diagnosed with craniosynostosis and four normal controls. All ten patients underwent magnetic resonance (MR) examinations before and after surgical treatment. Single-shot diffusion MR imaging (MRI) triggered by an electrocardiogram was performed, with regions of interest (ROIs) placed on frontal white matter and basal ganglia.
Results
In all ten patients, ΔADC values after surgery were higher than those before surgery. This difference was statistically significant (Wilcoxon signed-rank test, P = 0.005).
Conclusion
The change in ΔADC in the frontal white matter before and after surgery in patients with craniosynostosis indicates that it might reflect the change in ICP. Measurements of ΔADC could be a promising tool for non-invasive monitoring of ICP.
Keywords: Craniosynostosis, intracranial pressure, diffusion images, delta ADC
Introduction
The best management of craniosynostosis continues to be debated because the disease varies in severity and type and most patients have no clinically significant symptoms. However, increases in intracranial pressure (ICP) in patients with craniosynostosis can potentially worsen even minimal symptoms (1–5) and can lead to abnormal brain development. Therefore, non-invasive monitoring of ICP is essential for evaluating and treating craniosytosis. The apparent diffusion coefficient (ADC) of white matter changes significantly over the cardiac cycle owing to changes in the arterial inflow (6,7). The difference between the maximum and minimum values of the ADC on a pixel-by-pixel basis through the cardiac cycle was defined as the ΔADC. A number of previous reports showed that ΔADC offered more detailed information than the standard ADC in cases of suspected idiopathic normal-pressure hydrocephalus (iNPH), and hence could facilitate diagnosis (8,9). These reports suggested that an increase in the amount of intracranial water, and therefore in the extracellular space of the brain parenchyma, made ΔADC prominent in iNPH patients. Here we hypothesized that ΔADC also reflects changes in ICP, which typically increases in craniosynostosis patients. The purpose of this study was to determine whether a change in ΔADC from before to after neurosurgical treatment is of diagnostic value in craniosynostosis patients.
Material and Methods
Patients
This prospective study included ten patients diagnosed with primary craniosynostosis at our institution (Juntendo University School of Medicine) between July 2012 and May 2014. The clinical diagnosis of craniosynostosis was made by a neurosurgeon who had more than ten years of experience in diagnosing and treating congenital malformations of the brain and skull (KS and MM). This study was approved by the ethical committee of Juntendo Hospital, Juntendo University and informed consent was obtained from all the participants before imaging examinations. All ten patients underwent magnetic resonance imaging (MRI) both before and after surgical treatment. The ages at preoperative and postoperative MRI exams, period between surgery and postoperative MRI, gender, and type of craniosynostosis for each patient are shown in Table 1. The breakdown by type of craniosynostosis was as follows: trigonocephaly (n = 7) (Figs. 1 and 2); plagiocephaly (n = 2) (Fig. 3); and scaphocephaly (n = 1). The mean ages at the preoperative and postoperative exams were 19.0 ± 14.2 months and 25.4 ± 14.3 months, respectively. MR examinations and delta ADC analyses were also performed in four normal controls (three boys, one girl; age range = 6 months–6 years 2 months; mean age = 3 years 1 month).
Table 1.
Patients’ age and delta ADC preoperatively and postoperatively.
| Patient no. | Gender | Preoperative age | Postoperative age (time from operation) | Type of cranionostosis | ΔADC: preoperative frontal ×10–3 mm2/s | ΔADC: postoperative frontal ×10–3 mm2/s | ΔADC: preoperative BG × 10–3 mm2/s | ΔADC: postoperative BG × 10–3 mm2/s |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 2 y 10 m | 3 y 8 m (7 m) | Trigonocephaly | 0.11 ± 0.02 | 0.16 ± 0.02 | 0.17 ± 0.03 | 0.32 ± 0.08 |
| 2 | Male | 10 m | 1 y 5 m (6 m) | Plagiocephaly | 0.11 ± 0.02 | 0.15 ± 0.04 | 0.14 ± 0.05 | 0.19 ± 0.13 |
| 3 | Male | 5 m | 11 m (6 m) | Scaphocephaly | 0.04 ± 0.01 | 0.32 ± 0.05 | 0.17 ± 0.04 | 0.32 ± 0.08 |
| 4 | Male | 2 y 2 m | 2 y 7 m (3 m) | Trigonocephaly | 0.04 ± 0.002 | 0.07 ± 0.01 | 0.07 ± 0.03 | 0.06 ± 0.01 |
| 5 | Female | 2 m | 6 m (6 m) | Plagiocephaly | 0.11 ± 0.19 | 0.21 ± 0.05 | 0.12 ± 0.08 | 0.19 ± 0.05 |
| 6 | Male | 3 y | 3 y 2 m (6 m) | Trigonocephaly | 0.29 ± 0.09 | 0.33 ± 0.07 | 0.30 ± 0.12 | 0.20 ± 0.06 |
| 7 | Male | 2 y 6 m | 2 y 9 m (3 m) | Trigonocephaly | 0.11 ± 0.01 | 0.16 ± 0.03 | 0.17 ± 0.06 | 0.14 ± 0.07 |
| 8 | Female | 9 m | 1 y 2 m (6 m) | Trigonocephaly | 0.11 ± 0.02 | 0.36 ± 0.07 | 0.09 ± 0.03 | 0.25 ± 0.09 |
| 9 | Male | 4 y 11 m | 5 y 3 m (4 m) | Trigonocephaly | 0.08 ± 0.02 | 0.38 ± 0.03 | 0.17 ± 0.07 | 0.38 ± 0.07 |
| 10 | Male | 3 y 5 m | 3 y 8 m (6 m) | Trigonocephaly | 0.09 ± 0.03 | 0.12 ± 0.01 | 0.14 ± 0.05 | 0.11 ± 0.1 |
Values of delta ADC show mean ± standard deviation. Period between surgery and postoperative MRI, gender, and type of craniosynostosis are also shown.
y, years; m, months.
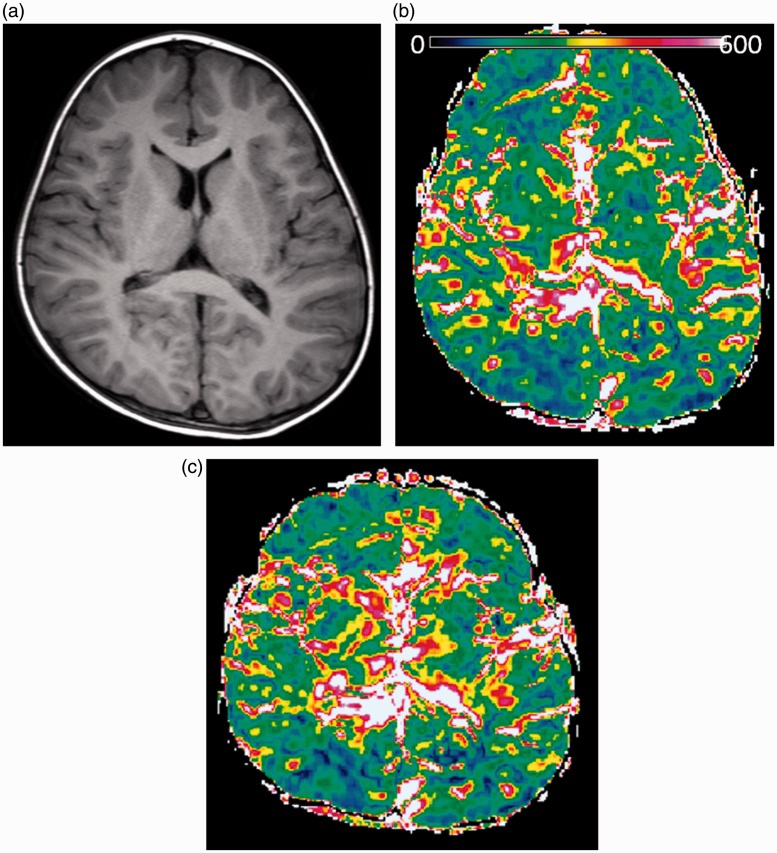
Fig. 1.
Patient 1. A boy with trigonocephaly and mild developmental delay. Preoperative and postoperative MR studies were performed at the age of two years ten months (a, b) and three years eight months (c), respectively. A preoperative T1-weighted axial image shows symmetrical narrowing of the frontal region (a). Color ΔADC maps derived from the images obtained before (b) and after (c) the operation show the changes of the ΔADC in frontal lobes. Postoperative ΔADC values changed from 0.11 ± 0.021 to 0.16 ± 0.017 × 10–3 mm2/s.
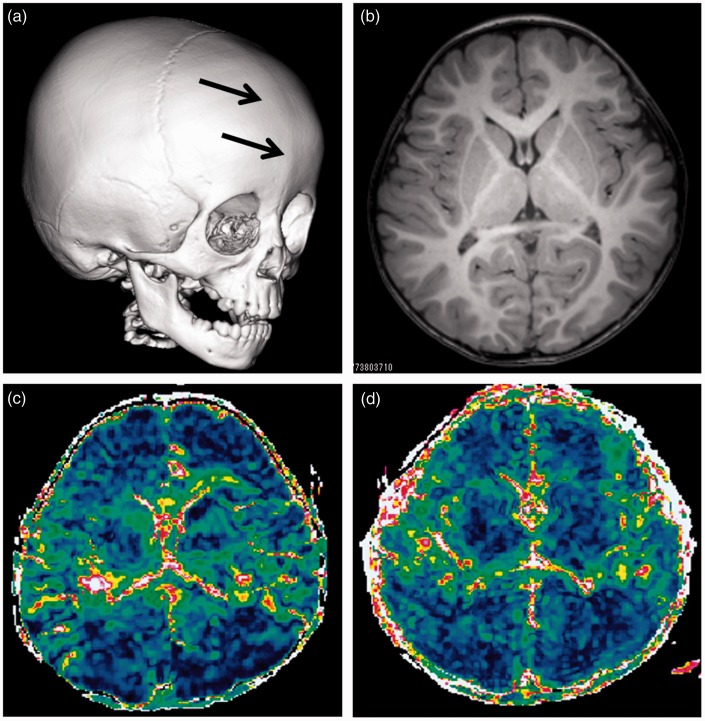
Fig. 2.
Patient 4. A boy with trigonocephaly and a ridge along center of the forehead (prematurely closed suture). Preoperative 3D CT with surface reformation of the calvarium shows a ridge of bone along the closed suture (a). A preoperative T1-weighted axial image shows symmetrical narrowing of the frontal region (b).
MRI was performed preoperatively at the age of two years two months (c) and postoperatively at two years seven months (d), respectively. Color ΔADC maps show changes of the ΔADC in the frontal lobes. ΔADC values changed from 0.04 ± 0.002 to 0.07 ± 0.006 × 10–3 mm2/s.
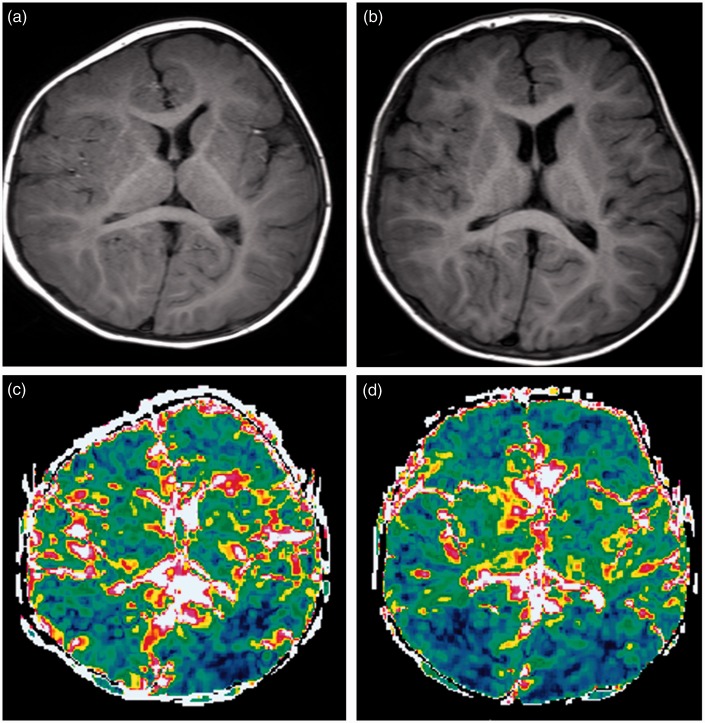
Fig. 3.
Patient 2. A girl with plagiocephaly (right coronal synostosis) and facial asymmetry. MRI was performed preoperatively at ten months (a) and postoperatively at one year five months (b). The right hemicalvarium was smaller than the left (a). After cranioplasaty, the calvarium is almost symmetrical (b). ΔADC maps obtained before (c) and after (d) the operation show changes of the ΔADC predominantly in the right frontal lobe. ΔADC values changed from 0.11 ± 0.020 to 0.15 ± 0.040 × 10–3 mm2/s.
Image acquisition
Images were acquired with a 3T MRI unit (Philips Medical Systems, Best, The Netherlands). Electrocardiogram (ECG)-triggered single-shot diffusion echo-planar imaging (EPI) was performed for b-values of 0 and 1000 mm2/s with the following parameters: repetition time (TR) = two R-R intervals (e.g. TR = 1200 ms at a heart rate of 100 bpm); echo time (TE) = 60.00 ms; flip angle = 90°; section thickness = 5.0 mm; imaging matrix = 256 × 256; field of view (FOV) = 230 × 230 mm; number of signals averaged = 1; cardiac phases = approximately 30 (depending on the heart rate); motion probing gradient (MPG) directions = three separate measures in the x-, y-, and z-axis directions; sensitivity encoding factor = 0.6. Single-shot EPI alone cannot completely eliminate the effect of bulk motion of the brain parenchyma because of the long data-sampling window required for the procedure, and artifacts may remain visible (8). Therefore, single-shot EPI was combined with parallel imaging, half-scan, rectangular FOV, and sensitivity encoding techniques to minimize the bulk motion effect (9). The trigger delays from the R-peak for diffusion-weighted imaging (DWI) were set at regular intervals (30 ms) and 30 phases were acquired.
Procedure for ΔADC analysis
ECG-triggered single-shot diffusion MRI was performed to calculate the ADC map in each cardiac phase. Data were acquired in multiple cardiac phases because ADC maps in different cardiac phases were required to calculate values of ΔADC. The trigger delay was set at regular intervals depending on the heart rate. Multiphase DW images were acquired over multiple heartbeats. Briefly, the ADC was calculated by using the following equation: ADC = –ln(S(b = 1000 mm2/s)/S(b = 0 mm2/s))/1000, where S(b = 1000 mm2/s) = signal intensity at b = 1000 mm2/s and S(b = 0) = signal intensity at b = 0 mm2/s. Moreover, the maximum change in ADC (ΔADC) at each pixel was calculated from images obtained during all cardiac phases using in-house software (http://miyatilab.w3.kanazawa-u.ac.jp/software/index.htm) and the following formula: ΔADC = ADCmax – ADCmin, where ADCmax and ADCmin represent the maximum and minimum ADC values, respectively, during the cardiac cycle (7). The regions of interest (ROIs) were drawn manually in the frontal white matter where all types of craniosynostosis show narrowing of this part. In addition, the ROIs were put on basal ganglia as a part of deep cerebral region. The ROIs were placed at three points in both the right and left frontal lobes and averaged in each patient.
Results
The ΔADC values in the preoperative and postoperative MR exams for each patient are summarized in Table 1. In all ten patients, postoperative ΔADCs were higher than preoperative ΔADCs in frontal lobe. The difference was statistically significant (Wilcoxon signed-rank test, P < 0.005). In basal ganglia, although ΔADCs tended to increase post operation, some patients (patients 4, 6, 7, and 10) decreased imperceptibly. In normal volunteers, mean delta ADC value in the frontal lobe was 0.17 ± 0.03, in the basal ganglia it was 0.24 ± 0.07.
Discussion
In the current study, we demonstrated an increase in ΔADC in the frontal lobe from the preoperative to postoperative state in pediatric craniosynostosis patients. This result was consistent with our hypothesis that changes in the ΔADC reflect changes of the ICP in craniosynostosis patients. ΔADC in basal ganglia also tended to increase after surgery except for four patients who showed relatively subtle decreasing. The frontal lobe might be strongly affected by all three types of craniosynostosis in our study. Therefore, change of ΔADC measured in the frontal lobe would more sensitive than in the basal ganglia. Generally, the indication for surgical treatment is based on the clinical condition. Because most patients with mild craniosynostosis show neither warning signs that ICP is increasing nor symptoms, some parents might hesitate to have their children undergo surgery for an apparently minor cosmetic benefit. The lack of notable symptoms also results in the underestimation of the preoperative incidence of raised ICP in mild craniosynostosis (1,3,4). Renier et al. found that up to six years of age, ICP increased with age in craniosynostosis (5). In addition, in a recent study, a large proportion of patients with craniosynostosis who underwent ICP monitoring showed elevated ICP (44–67%), a finding that held even in patients who were only mildly scaphocephalic (3,4). Whatever the etiology, a high incidence of elevated ICP in scaphocephalic patients with mild deformities that are less likely to indicate surgical treatment is potentially significant. Therefore, early detection of intracranial hypertension is important to reduce the risks of abnormal brain development. However, because ICP monitoring is invasive, measuring ICP in all patients with craniosynostosis including those with mild symptoms may be difficult (7). Our study suggests that ΔADC reflects ICP in pediatric patients with craniosynostosis. If so, then MR examinations could be potentially useful for monitoring ICP.
Previous studies reported that ΔADC was more pronounced in patients with idiopathic normal pressure hydrocephalous (iNPH) than in normal controls (7). In iNPH patients, the amount of intracranial water, and therefore extracellular space, might increase and thereby cause greater fluctuation of water molecules in the brain parenchyma. This could explain the elevated ΔADC in iNPH patients. The authors of previous studies suggested that measurement of ΔADC could facilitate the diagnosis of iNPH non-invasively (7). In the present study, we applied ΔADC calculation to craniosynostosis patients in the preoperative and postoperative states. Although iNPH is pathophysiologically distinct from craniosynostosis, both the diseases cause a change of intracranial water amount affecting molecular mobility in white matter. The reduced intracranial volume in these patients is expected to increase ICP. This condition might reduce the amount and molecular fluctuation of water in the cerebral parenchyma. This would explain why ΔADC increased after surgical treatment.
Some limitations of this study should be acknowledged. First, brain compliance is still undergoing dramatic changes in the first two years of life due to myelin formation, and we did not include ΔADC data from normal infants as controls for these temporal changes. We showed ΔADC at a single point in a small number of normal volunteers. In fact, ΔADC values were quite variable between individuals and might be of equivocal diagnostic value. However, a comparison between preoperative and postoperative treatment in the same patient is valuable to evaluate the success of the treatment. Second, we could not compare ΔADC values with observed ICP and clinical outcomes assessed with gold standard method. In future studies, to test whether ΔADC measurements can provide a reliable indication of the need for treatment, ΔADC values should be compared between age-matched normal controls and patients along with ICP and long-term prognosis. Therefore, the preliminary data presented in this study will require confirmation in a larger cohort and a comparison of preoperative and postoperative outcome.
In conclusion, the change in ΔADC in the frontal white matter before and after surgery in patients with craniosynostosis indicates that it might reflect the change in ICP. Measurements of ΔADC could be a promising tool for non-invasive monitoring of ICP.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (221S0003).
References
- 1.Bristol RE, Lekovic GP, Rekate HL. The effects of craniosynostosis on the brain with respect to intracranial pressure. Semin Pediatr Neurol 2004; 11: 262–267. [DOI] [PubMed] [Google Scholar]
- 2.Tamburrini G, Caldarelli M, Massimi L, et al. Intracranial pressure monitoring in children with single suture and complex craniosynostosis: a review. Childs Nerv Syst 2005; 21: 913–921. [DOI] [PubMed] [Google Scholar]
- 3.Wall SA, Thomas GP, Johnson D, et al. The preoperative incidence of raised intracranial pressure in nonsyndromic sagittal craniosynostosis is underestimated in the literature. J Neurosurg Pediatr 2014; 14: 674–681. [DOI] [PubMed] [Google Scholar]
- 4.Morritt DG, Yeh FJ, Wall SA, et al. Management of isolated sagittal synostosis in the absence of scaphocephaly: a series of eight cases. Plast Reconst Surg 2010; 126: 572–580. [DOI] [PubMed] [Google Scholar]
- 5.Renier D, Sainte-Rose C, Marchac D, et al. Intracranial pressure in craniostenosis. J Neurosurg 1982; 57: 370–377. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Miyati T, Kasai H, et al. Bulk motion-independent analyses of water diffusion changes in the brain during the cardiac cycle. Radiol Phys Technol 2009; 2: 133–137. [DOI] [PubMed] [Google Scholar]
- 7.Ohno N, Miyati T, Mase M, et al. Idiopathic normal-pressure hydrocephalus: temporal changes in ADC during cardiac cycle. Radiology 2011; 261: 560–565. [DOI] [PubMed] [Google Scholar]
- 8.Skare S, Andersson JL. On the effects of gating in diffusion imaging of the brain using single shot EPI. Magn Reson Imaging 2001; 19: 1125–1128. [DOI] [PubMed] [Google Scholar]
- 9.Norris DG. Implications of bulk motion for diffusion-weighted imaging experiments: effects, mechanisms, and solutions. J Magn Reson Imaging 2001; 13: 486–495. [DOI] [PubMed] [Google Scholar]