Abstract
Availability of normative patient outcome data may assist in designing experiments and estimating sample sizes. The purpose of this review was to determine normative ranges for colonic transit time (CTT), Patient Assessment of Constipation-Symptoms (PAC-SYM), and Patient Assessment of Constipation-Quality of Life (PAC-QOL) in adults diagnosed with functional constipation per Rome III guidelines. Pooled estimates were derived from random-effects meta-analysis. Meta-regression was used to explore sources of heterogeneity among studies. A total of 24 studies (3786 patients) were included in the review. In 10 studies with 1119 patients, pooled CTT was 58 hours (95% confidence interval [CI]: 50-65 hours). Publication bias was not evident (Egger P = .51); heterogeneity was high (I2 = 92%, P < .001). In meta-regression, geographical location explained 57% of the between-study variance, with CTT significantly longer in studies conducted in Europe (71 hours) compared with Asia (49 hours) or the Americas (44 hours). In 9 studies with 2061 patients, pooled PAC-SYM was 1.70 (95% CI: 1.58-1.83). Publication bias was not evident (Egger P = .44). Heterogeneity was high (I2 = 90%, P < .001); however, no study or patient factor influenced PAC-SYM in meta-regression. In 12 studies with 1805 patients, pooled PAC-QOL was 1.97 (95% CI: 1.70-2.24). Publication bias was not evident (Egger P = .28); heterogeneity was high (I2 = 98%, P < .001). In meta-regression, age explained 52% of the between-study variance, with older age associated with lower PAC-QOL scores. Overall, in adults diagnosed with functional constipation per Rome III criteria, significant heterogeneity in CTT, PAC-SYM, and PAC-QOL exists among studies. Variability among studies may be explained by geography and patient factors.
Keywords: Colonic transit, constipation, functional, meta-analysis, patient assessment, systematic review, Rome III
Introduction
Functional constipation (FC) is a common disorder of colonic or anorectal function affecting 14% of adults worldwide.1 Functional constipation is responsible for a large economic burden2 and decreased quality of life3 in affected individuals. Although the Rome III Diagnostic Criteria4 were developed to improve standardization of diagnosis among functional gastrointestinal disorders, patients with FC present a range of different symptoms, some of which overlap with other functional bowel disorders.5 Variability in patient symptomatology may be problematic when designing clinical trials focused on FC therapies because estimating baseline symptom severity with reasonable accuracy is necessary for power analysis and sample size calculations. In accordance with International Council for Harmonisation E6 guidance,6 normative test values for outcomes should be established prior to conducting a clinical trial. We previously published a systematic review and meta-analysis that established reference ranges for stool frequency and form values in patients with FC.7 Colonic transit time (CTT), Patient Assessment of Constipation-Symptoms (PAC-SYM), and Patient Assessment of Constipation-Quality of Life (PAC-QOL) are also common end points in FC clinical trials. An integral component of power analysis calculations in clinical trials is accurate estimation of the pretreatment mean and variability for an outcome. Furthermore, factors that may introduce variation to these estimates should be identified to appropriately refine study designs and appropriate inclusion criteria. Therefore, the objective of this systematic review and meta-analysis was to determine normative ranges for CTT, PAC-SYM, and PAC-QOL in adults diagnosed with FC per Rome III criteria.
Methods
Literature search
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).8 We systematically searched Medline (including in-process citations), Embase, and Scopus databases for studies, regardless of study design, that reported CTT, PAC-SYM, or PAC-QOL in adults diagnosed with FC using Rome III criteria. Search terms included “bowel function,” “chronic constipation,” “constipat*,” “functional constipation,” “functional gastrointestinal disorder,” “idiopathic constipation,” and “Rome III.” We also manually searched the Directory of Open Access Journals, Google Scholar, and the reference lists of included papers and other relevant meta-analyses. Searches were restricted to papers published since 2006, which coincides with development of the Rome III guidelines. The final search was conducted in April 2017.
Study selection and data extraction
Two researchers independently selected studies for inclusion in the review. Disagreements were resolved by consensus. Titles and abstracts were screened to exclude all non-English papers, review articles, commentaries, letters, case reports, and obviously irrelevant manuscripts. Full texts of the remaining manuscripts were retrieved and reviewed. Studies were included that enrolled patients with FC per Rome III criteria and reported CTT, PAC-SYM, or PAC-QOL. Studies were excluded if patients were less than 18 years of age; FC was secondary to disease, surgery, or medication use; Rome III diagnostic criteria were not applied; or Rome III diagnostic criteria were modified to additionally include CTT thresholds for study inclusion. An initial database was developed, pilot tested, and refined to maintain consistency with outcomes reported in the literature. Data were extracted from eligible peer-reviewed articles by one researcher and then verified by a second researcher. Data extraction discrepancies were resolved by consensus. Baseline outcome data were extracted from longitudinal studies. In studies with multiple groups, baseline data from each group were pooled into a single estimate for each outcome.
Outcomes
Main outcomes were CTT, PAC-SYM, and PAC-QOL. Colonic transit time was preferentially extracted from studies that used radiopaque markers or wireless motility capsule. Studies that reported transit geometric mean at specific time intervals were excluded given different units of measure. The PAC-SYM is a 12-question survey that comprised 3 subscales (stool symptoms, rectal symptoms, and abdominal symptoms) that measures the severity of constipation symptoms over the past 2 weeks.9 For each question, patients are asked to rate symptom severity as absent (0), mild (1), moderate (2), severe (3), and very severe (4). The range of possible scores on this questionnaire is 0 to 48, with higher scores indicative of greater symptom severity. The PAC-QoL is a 28-question survey that measures the impact that constipation has on daily life over the past 2 weeks. The questions comprised 4 subscales (worries and concerns, physical discomfort, psychosocial discomfort, and satisfaction) and an overall scale.10 For each question, patients are asked to rate the impact of constipation on quality of life using a 0 to 4 scale, where higher scores represent a greater burden. The range of possible scores on this questionnaire is 0 to 112, with higher scores indicative of a greater burden of constipation on quality of life. The PAC-SYM and PAC-QOL total scores were normalized to a common 0 to 4 scale for analysis purposes.
Data analysis
For each outcome, the pooled estimate and 95% confidence interval (CI) were calculated using a random-effects model given the a priori assumption that outcome estimates among studies were heterogeneous. A forest plot was used to illustrate the individual study findings and the random-effects meta-analysis results. Publication bias was visually assessed with funnel plots (not shown) and quantitatively assessed using the Egger regression test.11 We assessed heterogeneity with the I2 statistic, which reflects the amount of heterogeneity among studies in relation to sampling variation; I2 values of ≤25%, 50%, and ≥75% represent low, moderate, and high heterogeneity, respectively.12 A random-effects meta-regression using the method of Knapp and Hartung13 was undertaken to examine the impact of moderator variables on outcome estimates using regression-based techniques. P values were 2-sided with a significance level <.05. Statistical analyses were performed using Comprehensive Meta-Analysis version 3.3 (Biostat, Englewood, NJ, USA).
Results
Study selection
After screening 426 records for eligibility, 24 studies representing 3786 unique patients were included in the meta-analysis.5,14-36 The most common reasons for study exclusion were attributable to absence of main outcome reporting or Rome III FC diagnosis. A flow diagram of study identification and selection is shown in Figure 1.
Figure 1.
Study flow diagram. FC indicates functional constipation.
Study and patient characteristics
Median patient characteristic values were age 47 (range: 23-76) years, 80% women (range: 0%-100%), body mass index 24 (range: 23-27) kg/m2, and 8 years symptom duration (range: 1-17 years), with the latter 2 variables reported inconsistently among studies. Functional constipation was diagnosed by a physician in 15 (63%) studies or via questionnaire only in 9 (37%) studies. Colonic transit was assessed by the Bouchoucha method37 in 5 studies, the Metcalf method38 in 2 studies, custom radiopaque marker ingestion protocols in 2 studies, and wireless motility capsule in 1 study (Table 1).
Table 1.
Study and patient characteristics.
| Study | Country | N | Age, y | Female, % | BMI, kg/m2 | Symptom duration, y | Physician diagnosis | Reported outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CTT | PAC-SYM | PAC-QOL | ||||||||
| Abbott et al14 | US | 100 | 46 | 75 | — | — | No | No | No | Yes |
| Bazzocchi et al15 | Italy | 29 | 40 | 86 | — | — | Yes | Yesa | No | No |
| Bellini et al16 | Italy | 549 | 53 | 79 | [24] | [>10] | Yes | No | Yes | Yes |
| Belvaux et al17 | France | 11 | [49] | 100 | [25] | [>10] | Yes | Yesa | Yes | Yes |
| Bouchoucha et al18 | France | 151 | 43 | 74 | 25 | [15] | Yes | Yesa | No | No |
| Camilleri et al19 | US | 158 | 43 | 87 | — | — | No | Yesa | No | No |
| Choopani et al20 | Iran | 35 | 48 | 91 | 27 | — | No | No | Yes | No |
| Da et al21 | China | 67 | 37 | 81 | — | 10 | No | No | No | Yes |
| Dupont et al22 | France | 244 | 42 | 100 | 24 | — | No | No | No | Yes |
| Fateh et al23 | Iran | 60 | 23 | 0 | — | 1 | No | No | Yes | No |
| Gürsen et al24 | Turkey | 50 | 39 | 92 | 24 | 8 | Yes | No | No | Yes |
| Iqbal et al25 | UK | 20 | 39 | 80 | — | 6 | Yes | No | Yes | Yes |
| Jiang et al26 | China | 126 | 51 | 72 | — | — | No | No | Yes | Yes |
| Neri et al27 | Italy | 878 | 50 | 80 | 24 | 17 | Yes | No | Yes | No |
| Park et al28 | Korea | 88 | [56] | [55] | — | — | Yes | Yesb | No | No |
| Polymeros et al29 | Greece | 39 | 56 | 87 | 24 | — | Yes | Yesa | No | No |
| Rao et al30 | US | 27 | 71 | 52 | — | — | Yes | Yesc | No | No |
| Ruiz-López and Coss-Adame31 | Mexico | 25 | 51 | 76 | — | — | No | No | No | Yes |
| Saberi et al32 | Iran | 52 | 37 | 81 | — | [3] | Yes | Yesa | No | No |
| Shekhar et al5 | UK | 11 | 38 | 100 | 23 | — | Yes | Yesd | No | No |
| Wong et al33 | US | 231 | 76 | 70 | — | — | Yes | No | No | Yes |
| Yiannakou et al34 | Internationale | 370 | 58 | 0 | — | 9 | Yes | No | Yes | Yes |
| Zhang et al35 | China | 553 | 42 | 48 | — | 5 | Yes | Yesd | No | No |
| Zhang et al36 | China | 12 | 60 | 67 | — | — | No | No | Yes | Yes |
Abbreviations: CTT, colonic transit time; PAC-QOL, Patient Assessment of Constipation-Quality of Life; PAC-SYM, Patient Assessment of Constipation-Symptoms.
The values in brackets represent estimated values.
Assessed by Bouchoucha method.37
Assessed by Metcalf method.38
Assessed by wireless motility capsule.
Assessed by custom radiopaque marker ingestion protocol.
European study.
Colonic transit time
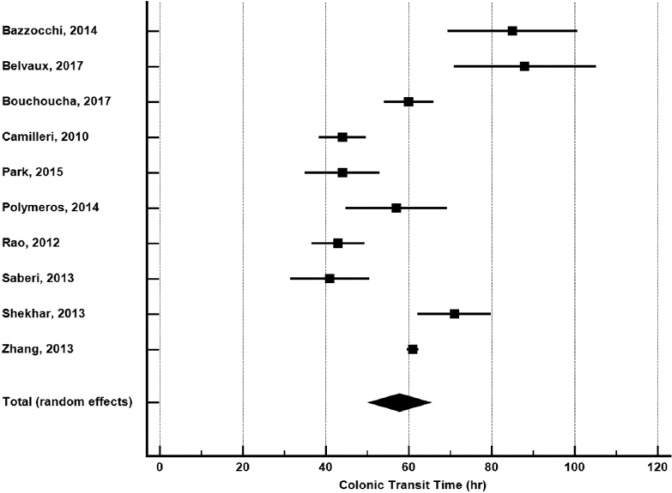
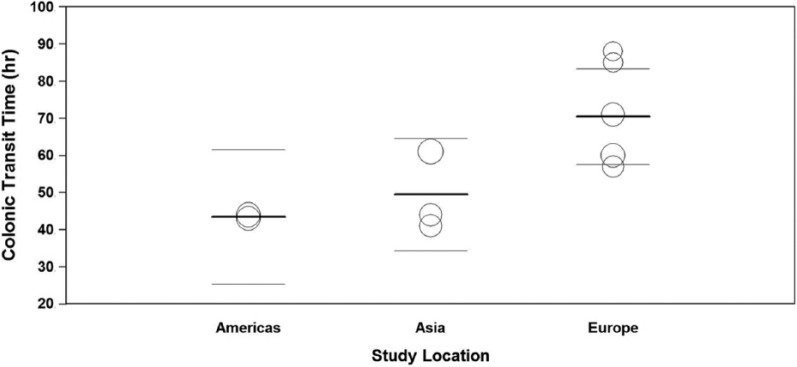
In 10 studies with 1119 patients,5,15,17-19,28-30,32,35 pooled CTT was 58 hours (95% CI: 50-65 hours) (Figure 2). Publication bias was not evident (Egger P = .51). Heterogeneity in CTT was high among studies (I2 = 92%, P < .001). In meta-regression, geographical location explained 57% of the between-study variance (Figure 3). Pooled CTT was significantly longer in studies performed in Europe (71 hours) compared with Asia (49 hours) or the Americas (44 hours). No other study or patient factor influenced CTT (Table 2).
Figure 2.
Colonic transit time in adults with functional constipation. Colonic transit time estimates from random-effects meta-analysis. The mean and 95% confidence interval are plotted for each study. The pooled estimate is represented by the diamond apex (58 hours) and the 95% confidence interval is represented by the diamond width (50-65 hours). Publication bias: Egger P = .51. Heterogeneity: I2 = 92%, P < .001.
Figure 3.
Meta-regression of relationship between geographic location and colonic transit time in adults with functional constipation. Percentage of variance in transit time explained by geography = 57%, P = .04. Pairwise comparisons: Europe vs Asia, P = .04; Europe vs Americas, P = .02. Open circles represent values of individual studies where circle size is proportional to the study weight in the random-effects model. Thick lines represent the pooled mean in each group. Thin lines represent the 95% confidence interval of the pooled mean in each group.
Table 2.
Meta-regression of study-related and patient-related factors on colonic transit time and patient assessment of constipation.
| Covariate | Unit of measure | CTT |
PAC-SYM |
PAC-QOL |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercepta | Slope(95% CI)b | P | Intercepta | Slope(95% CI)b | P | Intercepta | Slope(95% CI)b | P | ||
| Geography | Americas | 43.5 | Reference | — | — | — | 1.487 | Reference | ||
| Asia | 43.5 | 6.0(−17.6 to 29.6) | .57 | 1.622 | Reference | 1.487 | 0.772(−0.255 to 1.800) | .12 | ||
| Europe | 43.5 | 27.0(4.8–49.2) | .02 | 1.622 | 0.203(−0.286 to 0.692) | .36 | 1.487 | 0.549(−0.441 to 1.538) | .24 | |
| Physician diagnosis | Yes vs no | 44.0 | 16.2(−23.6 to 55.9) | .38 | 1.622 | 0.203(−0.286 to 0.692) | .36 | 1.868 | 0.213(−0.617 to 1.044) | .58 |
| Sample size | Per 10 patients | 59.6 | −0.1(−0.9 to 0.7) | .80 | 1.786 | −0.002(−0.011 to 0.006) | .58 | 2.167 | −0.001(−0.037 to 0.012) | .28 |
| Female proportion | Per 1% | 25.3 | 0.4(−0.2 to 1.0) | .14 | 1.577 | 0.003(−0.004 to 0.009) | .41 | 1.729 | 0.003(−0.014 to 0.020) | .67 |
| Age | Per 1 y | 83.0 | −0.5(−1.7 to 0.7) | .34 | 1.869 | −0.003(−0.027 to 0.021) | .79 | 4.038 | −0.041(−0.068 to −0.015) | <.01 |
Abbreviations: CI, confidence interval; CTT, colonic transit time; PAC-QOL, Patient Assessment of Constipation-Quality of Life; PAC-SYM, Patient Assessment of Constipation-Symptoms.
Intercept represents estimated outcome value when covariate value = 0.
Slope represents the magnitude of change in estimated outcome value per unit increase in covariate value.
— indicates no available studies to perform meta-regression.
Patient Assessment of Constipation-Symptoms
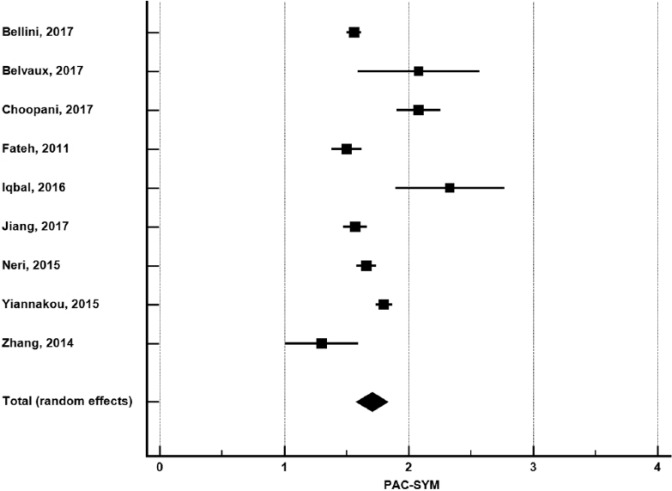
In 9 studies with 2061 patients,16,17,20,23,25-27,34,36 pooled PAC-SYM was 1.70 (95% CI: 1.58-1.83) (Figure 4). Publication bias was not evident (Egger P = .44). Heterogeneity in PAC-SYM was high among studies (I2 = 90%, P < .001). In meta-regression, no study or patient factor influenced PAC-SYM (Table 2).
Figure 4.
PAC-SYM in adults with functional constipation. PAC-SYM (Patient Assessment of Constipation-Symptoms) estimates from random-effects meta-analysis. The mean and 95% confidence interval are plotted for each study. The pooled estimate is represented by the diamond apex (1.70) and the 95% confidence interval is represented by the diamond width (1.58-1.83). Publication bias: Egger P = .44. Heterogeneity: I2 = 90%, P < .001.
Patient Assessment of Constipation-Quality of Life
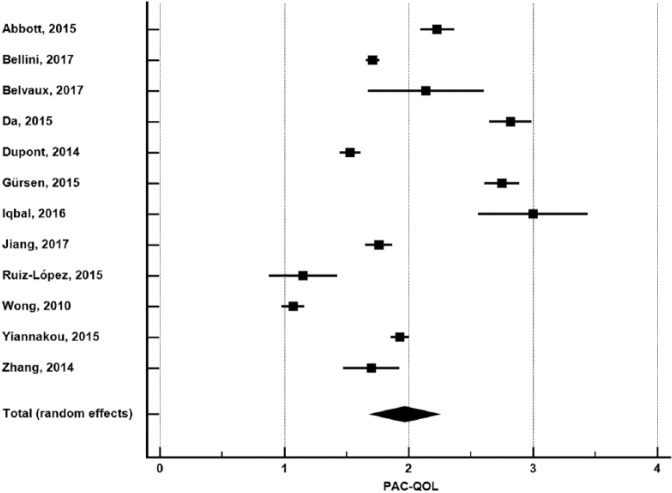
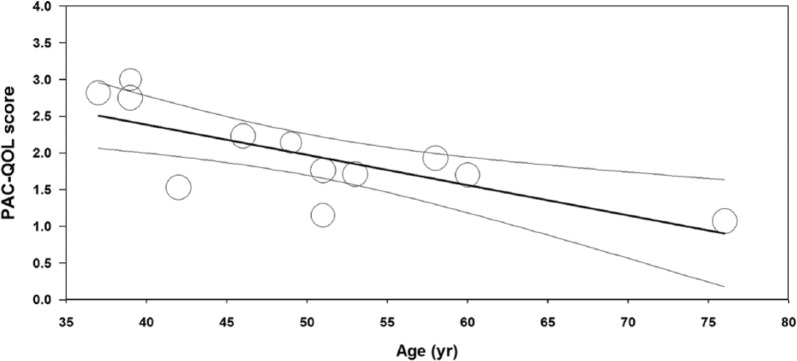
In 12 studies with 1805 patients,14,16,17,21,22,24-26,31,33,34,36 pooled PAC-QOL was 1.97 (95% CI: 1.70-2.24) (Figure 5). Publication bias was not evident (Egger P = .28). Heterogeneity in PAC-QOL was high among studies (I2 = 98%, P < .001). In meta-regression, age explained 52% of the between-study variance where older age was associated with lower PAC-QOL scores (Figure 6). No other study or patient factor influenced PAC-QOL (Table 2).
Figure 5.
PAC-QOL in adults with functional constipation. PAC-QOL (Patient Assessment of Constipation-Quality of Life) estimates from random-effects meta-analysis. The mean and 95% confidence interval are plotted for each study. The pooled estimate is represented by the diamond apex (1.97) and the 95% confidence interval is represented by the diamond width (1.70-2.24). Publication bias: Egger P = .28. Heterogeneity: I2 = 98%, P < .001.
Figure 6.
Meta-regression of relationship between age and PAC-QOL score in adults with functional constipation. Percentage of variance in PAC-QOL (Patient Assessment of Constipation-Quality of Life) score explained by age = 52%, P < .01. Open circles represent values of individual studies where circle size is proportional to the study weight in the random-effects model. Thick lines represent the regression line of best fit. Thin lines represent the 95% confidence interval of the regression line.
Discussion
To our knowledge, this is the first meta-analysis that reports pooled CTT, PAC-SYM, or PAC-QOL values in patients diagnosed with FC per Rome III guidelines. The main findings of this systematic review and meta-analysis were that despite the use of consistent diagnostic guidelines for FC, significant heterogeneity in CTT, PAC-SYM, and PAC-QOL exists among studies. In our analysis, CTT was significantly associated with geography and PAC-QOL was inversely associated with patient age; no covariates were associated with PAC-SYM.
While the pooled mean for CTT was 58 hours, the reported mean in individual studies ranged from 41 to 88 hours. The finding that CTT was notably higher in European studies should be interpreted cautiously. On one hand, it is plausible that the association of geography on CTT is influenced by factors that were not measured in this review such as ethnicity, diet, activity, and stress levels. Another plausible explanation for this finding is due to cultural or geographic differences in interpretation and reporting of patient symptoms. On the other hand, the stability of these estimates may be questionable given the inclusion of only 5 European studies and 5 non-European studies that reported CTT.
A limitation of the Rome III guidance for FC is that there is no delineation of rapid, normal, and slow transit. In fact, in the American Gastroenterological Association technical review on constipation, the subset of patients with slow-transit constipation is not considered to be truly functional.39 Furthermore, accurate diagnosis is complicated by the significant overlap of symptoms in those with FC and irritable bowel syndrome.40 Clinical trials of FC treatments that use CTT as a primary end point may benefit by additionally stipulating minimum CTT thresholds for study entry. Although such a design may limit generalizability of findings, it could exclude patients with normal transit and presumably little potential for further improvement.
Pooled mean patient assessment values were 1.7 for PAC-SYM and 2.0 for PAC-QOL. For reference, these questionnaires are scored from 0 to 4, where a higher value corresponds to worse symptom severity and constipation-specific quality of life, respectively. These data suggest that patient assessment total scores may not accurately reflect the burden of FC. Given the heterogeneous nature of FC and that the patient assessment questionnaires comprise multiple domains, these total scores may have limited responsiveness to therapy in patients who exhibit worse scores in some, but not all, subdomains of these questionnaires. As with CTT, the responsiveness of PAC-SYM and PAC-QOL to treatment may be somewhat blunted given that the pooled total scores do not suggest severe patient burden.
The results of this meta-analysis may be of benefit in the design of future clinical trials on FC. Power analyses and sample size calculations must consider not only the anticipated effect size of treatment but also the baseline mean and the variability around the mean. Our results suggest that patients diagnosed with FC per Rome III guidelines present with only modest CTT delays and constipation-specific complaints using standard questionnaires. Responsiveness of these outcomes may be somewhat limited unless patient entry guidelines are tailored to select those with more severe baseline symptoms.
This meta-analysis was associated with several limitations. Unreported confounding factors such as temporal symptoms, psychological issues, stress levels, diet, hydration, physical activity, and medical history may have influenced outcomes. The results presented here are applicable to adults diagnosed with FC using Rome III guidelines; however, generalizability of these results to constipated adults diagnosed using other methods is unknown. The number of included studies reporting each outcome was minimally sufficient to reliably assess publication bias and sources of heterogeneity. We also excluded body mass index and symptom duration as covariates in meta-regression due to insufficient reporting. Furthermore, subgroup analysis and meta-regression results should be considered exploratory and hypothesis-generating. For these reasons, the estimates within may be somewhat unstable and the conclusions prone to change with inclusion of future studies. Finally, we did not conduct a formal risk of bias assessment because data were extracted from cross-sectional studies as well as baseline data from interventional studies, for which no assessment tools are available.
Conclusions
In adults diagnosed with FC per Rome III criteria, significant heterogeneity in CTT, PAC-SYM, and PAC-QOL exists among studies. Variability among studies may be explained by geography and patient factors.
Acknowledgments
The authors thank David Fay, PhD, for assistance with literature review and data extraction verification.
Footnotes
Peer Review:Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 167 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financed by Danisco Sweeteners OY (Kantvik, Finland), a subsidiary of DuPont Nutrition and Health.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contribution: LM, AI, and AO contributed to conception and design; analysis and interpretation of the data; critical revision of the article for important intellectual content; and final approval of the article. LM contributed to drafting of the article.
References
- 1. Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–1591; quiz 1581, 1592. [DOI] [PubMed] [Google Scholar]
- 2. Nellesen D, Yee K, Chawla A, Lewis BE, Carson RT. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm. 2013;19:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belsey J, Greenfield S, Candy D, Geraint M. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31:938–949. [DOI] [PubMed] [Google Scholar]
- 4. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 5. Shekhar C, Monaghan PJ, Morris J, et al. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterology. 2013;145:749–757; quiz e713-e744. [DOI] [PubMed] [Google Scholar]
- 6. Food and Drug Administration. Guidance for industry: E6 good clinical practice: consolidated guidance. http://www.fda.gov/downloads/Drugs/./Guidances/ucm073122.pdf. Published 1996. Accessed September 7, 2016.
- 7. Miller LE, Ibarra A, Ouwehand AC, Zimmermann AK. Normative values for stool frequency and form using Rome III diagnostic criteria for functional constipation in adults: systematic review with meta-analysis. Ann Gastroenterol. 2017;30:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 9. Frank L, Kleinman L, Farup C, Taylor L, Miner P., Jr. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34:870–877. [DOI] [PubMed] [Google Scholar]
- 10. Marquis P, De La Loge C, Dubois D, McDermott A, Chassany O. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005;40:540–551. [DOI] [PubMed] [Google Scholar]
- 11. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. [DOI] [PubMed] [Google Scholar]
- 14. Abbott R, Ayres I, Hui E, Hui KK. Effect of perineal self-acupressure on constipation: a randomized controlled trial. J Gen Intern Med. 2015;30:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bazzocchi G, Giovannini T, Giussani C, Brigidi P, Turroni S. Effect of a new synbiotic supplement on symptoms, stool consistency, intestinal transit time and gut microbiota in patients with severe functional constipation: a pilot randomized double-blind, controlled trial. Tech Coloproctol. 2014;18:945–953. [DOI] [PubMed] [Google Scholar]
- 16. Bellini M, Usai-Satta P, Bove A, et al. Chronic constipation diagnosis and treatment evaluation: the “CHRO.CO.DI.T.E.” study. BMC Gastroenterol. 2017;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Belvaux A, Bouchoucha M, Benamouzig R. Osteopathic management of chronic constipation in women patients. Results of a pilot study [published online ahead of print February 15, 2017]. Clin Res Hepatol Gastroenterol. doi: 10.1016/j.clinre.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 18. Bouchoucha M, Devroede G, Bon C, Bejou B, Mary F, Benamouzig R. Is-it possible to distinguish irritable bowel syndrome with constipation from functional constipation? Tech Coloproctol. 2017;21:125–132. [DOI] [PubMed] [Google Scholar]
- 19. Camilleri M, Thorne NK, Ringel Y, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil. 2010;22:874–882, e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choopani R, Ghourchian A, Hajimehdipoor H, Kamalinejad M, Ghourchian F. Effect of Descurainia sophia (L.) Webb ex Prantl on adult functional constipation: a prospective pilot study [published online ahead of print January 1, 2017]. J Evid Based Complementary Altern Med. doi: 10.1177/2156587217703018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Da N, Wang X, Liu H, et al. The effectiveness of electroacupuncture for functional constipation: a randomized, controlled, clinical trial. Evid Based Complement Alternat Med. 2015;2015:670963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dupont C, Campagne A, Constant F. Efficacy and safety of a magnesium sulfate-rich natural mineral water for patients with functional constipation. Clin Gastroenterol Hepatol. 2014;12:1280–1287. [DOI] [PubMed] [Google Scholar]
- 23. Fateh R, Iravani S, Frootan M, Rasouli MR, Saadat S. Synbiotic preparation in men suffering from functional constipation: a randomised controlled trial. Swiss Med Wkly. 2011;141:w13239. [DOI] [PubMed] [Google Scholar]
- 24. Gürsen C, Kerem Gunel M, Kaya S, Kav T, Akbayrak T. Effect of connective tissue manipulation on symptoms and quality of life in patients with chronic constipation: a randomized controlled trial. J Manipulative Physiol Ther. 2015;38:335–343. [DOI] [PubMed] [Google Scholar]
- 25. Iqbal F, Thomas GP, Tan E, et al. Transcutaneous sacral electrical stimulation for chronic functional constipation. Dis Colon Rectum. 2016;59:132–139. [DOI] [PubMed] [Google Scholar]
- 26. Jiang Y, Tang YR, Xie C, Yu T, Xiong WJ, Lin L. Influence of sleep disorders on somatic symptoms, mental health, and quality of life in patients with chronic constipation. Medicine (Baltimore). 2017;96:e6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neri L, Conway PM, Basilisco G, et al. ; Laxative Inadequate Relief Survey (LIRS) Group. Confirmatory factor analysis of the Patient Assessment of Constipation-Symptoms (PAC-SYM) among patients with chronic constipation. Qual Life Res. 2015;24:1597–1605. [DOI] [PubMed] [Google Scholar]
- 28. Park SY, Park HB, Lee JM, et al. Relevance of colonic gas analysis and transit study in patients with chronic constipation. J Neurogastroenterol Motil. 2015;21:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polymeros D, Beintaris I, Gaglia A, et al. Partially hydrolyzed guar gum accelerates colonic transit time and improves symptoms in adults with chronic constipation. Dig Dis Sci. 2014;59:2207–2214. [DOI] [PubMed] [Google Scholar]
- 30. Rao SS, Coss-Adame E, Valestin J, Mysore K. Evaluation of constipation in older adults: radioopaque markers (ROMs) versus wireless motility capsule (WMC). Arch Gerontol Geriatr. 2012;55:289–294. [DOI] [PubMed] [Google Scholar]
- 31. Ruiz-López MC, Coss-Adame E. Quality of life in patients with different constipation subtypes based on the Rome III criteria. Rev Gastroenterol Mex. 2015;80:13–20. [DOI] [PubMed] [Google Scholar]
- 32. Saberi H, Asefi N, Keshvari A, Agah S, Arabi M, Asefi H. Measurement of colonic transit time based on radio opaque markers in patients with chronic idiopathic constipation; a cross-sectional study. Iran Red Crescent Med J. 2013;15:e16617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wong RK, Palsson OS, Turner MJ, et al. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am J Gastroenterol. 2010;105:2228–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yiannakou Y, Piessevaux H, Bouchoucha M, et al. A randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy, safety, and tolerability of prucalopride in men with chronic constipation. Am J Gastroenterol. 2015;110:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang C, Guo L, Guo X, Li G, Guo X. Short and long-term efficacy of combining Fuzhengliqi mixture with acupuncture in treatment of functional constipation. J Tradit Chin Med. 2013;33:51–59. [DOI] [PubMed] [Google Scholar]
- 36. Zhang N, Huang Z, Xu F, et al. Transcutaneous neuromodulation at posterior tibial nerve and ST36 for chronic constipation. Evid Based Complement Alternat Med. 2014;2014:560802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bouchoucha M, Devroede G, Arhan P, et al. What is the meaning of colorectal transit time measurement? Dis Colon Rectum. 1992;35:773–782. [DOI] [PubMed] [Google Scholar]
- 38. Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. [DOI] [PubMed] [Google Scholar]
- 39. Bharucha AE, Pemberton JH, Locke GR., III American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siah KT, Wong RK, Whitehead WE. Chronic constipation and constipation-predominant IBS: separate and distinct disorders or a spectrum of disease? Gastroenterol Hepatol (N Y). 2016;12:171–178. [PMC free article] [PubMed] [Google Scholar]