Abstract
Euptox A (9-oxo-10, 11-dehydroageraphorone), the main toxin isolated from Eupatorium adenophorum, is known to induce immunotoxicity in animals. However, the precise mechanism underlying the effects of Euptox A on splenocytes is unclear. Here, we aimed to investigate the molecular mechanisms underlying the effect of Euptox A in mouse spleens after its intragastric administration and found that Euptox A exhibits proautophagic effects in splenocytes. Euptox A markedly arrested the splenocytes in the G0/G1 phase, which was accompanied by inhibition of the expression of the positive regulators CDK4, CDK2, cyclin D1, PCNA, and E2F1, and promotion of the expression of the negative regulators p53, p21 Waf1/Cip1, p27 Kip1, and Chk1. We also found that Euptox A did not markedly induce splenocyte apoptosis, but induced autophagy while increasing the subcellular localization of punctate LC3, ratio of LC3-II/LC3-I, and Beclin 1 levels, and decreasing p62 levels. Euptox A also significantly inhibited p-PI3K, p-p38 MAPK, p-Akt, and p-mTOR expression, but increased PTEN and p-AMPK expression. These results indicated that Euptox A induced splenocyte autophagy by inhibiting the PI3K/Akt/mTOR pathway, suppressing p38 MAPK expression, and activating AMPK. These findings provide new insights into the mechanisms involved in spleen toxicity caused by Euptox A in mice.
Keywords: apoptosis, autophagy, cell cycle arrest, E. adenophorum, 9-oxo-10, 11-dehydroageraphorone, PI3K/Akt/mTOR pathway
Introduction
Euptox A (9-oxo-10, 11-dehydroageraphorone), a cadenine sesquiterpene, is the main toxin extracted from Eupatorium adenophorum.1 E. adenophorum is an invasive weed and an important destructive exotic species, representing a major threat to the economy and ecology of some regions of the world. A large number of reports have indicated that regular ingestion of E. adenophorum causes livestock poisoning.2 Feeding goats E. adenophorum can cause goat spleen injury and induce apoptosis in a large number of cells in the spleen.3 In addition, the intragastric administration of different concentrations of the E. adenophorum extract results in obvious pathological changes in the mouse spleen such as white pulp atrophy and splenic nodule destruction.4
Euptox A accounts for a large proportion of E. adenophorum toxins.5 It not only significantly causes pathological changes in hepatic lobules and hepatocytes but also increases the spleen index of mice and induces immunotoxicity.6 As the largest peripheral lymphoid tissue in the body, the spleen is of vital importance in immune function.7 Therefore, it is necessary to study the mechanism underlying the toxicity of Euptox A in mouse spleens.
Autophagy is a complicated process through which damaged, dysfunctional, or superfluous organelles and proteins are degraded to maintain cellular metabolism, viability, and homeostasis.8 Autophagy often occurs when tissues or cells are deprived of nutrition or exposed to stress.9 The mTOR kinase plays a critical role in regulating autophagy progression, and diverse signaling pathways are involved in the regulation of mTOR signaling. These pathways include positive regulators of mTOR such as PI3K/Akt and p38 MAPK, which suppress autophagy, and negative regulators of mTOR such as AMPK and p53, which promote autophagy.10,11
Our hypothesis was that Euptox A inhibits growth and promotes the autophagy of splenocytes and thus causes spleen cell toxicity. Therefore, in the present study, we aimed to investigate the cytotoxic effects of Euptox A on splenocytes and to elucidate the possible mechanisms involved in Euptox A-induced splenic toxicity in mice. Our findings indicate that Euptox A exhibits splenic toxicity by inducing G1 arrest and autophagy through the p38 MAPK- and PI3K/Akt/mTOR-mediated pathways and that it has potential for use as an antitumor drug used to kill apoptosis-resistant cells.
Materials and Method
Extraction and Purification of Euptox A
E. adenophorum was collected from Xichang City of Sichuan Province, Southwest China, in July, 2015. Fifty grams milled leaves were mixed with 100 ml water. The mixture contained Euptox A, coumarin, and gallotannic acid, and volatile oil was ultrasonic extracted by carbinol and hexyl acetate for 30 min at 40C. To separate Euptox A from the extraction, the sample was purified by silica column chromatography method and silica gel thin-layer chromatography and then used to analyze the existence of Euptox A in the final extraction. According to the high-performance liquid chromatography determination result, the purity of the toxin we had extracted was over 96%.
Animal Model
The animal model was established in accordance with several previous reports.1 Adult mice (8 weeks, 25–30 g) were obtained from the animal experimental center of Sichuan Agricultural University. The animals were maintained in an air-conditioned room (22C ± 3C) on a 12-hr light/dark cycle with free water and food available. The animals were randomly divided into 4 groups with 18 animals in each group: control group and treatment groups. The control group received 0.9% normal saline, whereas treatment groups received 200, 400, and 800 mg/kg Euptox A via intragastric gavage daily for 30 days.6,12 After the experiment, mice were anesthetized with an overdose of chloral hydrate (10% solution) and sacrificed to obtain the spleen tissues. All methods were carried out in accordance with the approved guidelines and all animal experimental protocols were approved by the Animal Care and Use Committee of Sichuan Agricultural University, China.
Cell Cycle Detection Through Flow Cytometry
The spleens were immediately removed and minced using scissors to form a cell suspension that was filtered through a 300-mesh nylon screen. The cells were washed twice with cold phosphate-buffered saline (PBS; pH 7.2–7.4) and subsequently suspended in PBS (cat. no 51-66121E; BD Biosciences, San Jose, CA) at a concentration of 1 × 106 cells/ml. A total of 500 µl of the solution was transferred to a 5-ml culture tube, followed by centrifugation (200 × g). After the cell suspension was permeabilized with 1 ml of 0.25% Tritonx-100 for 20 min at 4C, the cells were washed with PBS, and 5µl of propidium iodide (PI; cat. no. 51-66211E; BD Biosciences) was subsequently added. The cells were then gently vortexed and incubated for 30 min at 4C in the dark. Finally, 500 µl of PBS was added to each tube, and the cell cycle phases were analyzed through flow cytometry (FCM; BD FACSCalibur; BD Biosciences) within 45 min.
Annexin-V/PI Apoptosis Detection by FCM
The spleens were immediately removed and minced using scissors to form a cell suspension that was filtered through a 300-mesh nylon screen, washed twice with cold PBS, and then suspended in cells in 1× binding buffer at a concentration of 1 × 106 cells/ml. Then 100 µl of the solution was transferred to a 5 ml culture tube, and 5 µl of Annexin V–fluorescein isothiocyanate (FITC) and 5 µl of PI was added to it. The cells were gently vortexed and incubated for 15 min at room temperature (25C) in the dark. Then 400 µl of 1× binding buffer was added to each tube and analyzed by FCM (BD FACSCalibur) within 1 hr.
Determination of DNA Fragmentation by Agarose Gel Electrophoresis
Both control and Euptox A administration splenocytes were collected and washed with PBS. DNA extraction was performed according to previous studies.13 After dissolving in the Tris–EDTA buffer, DNA was subjected to 2% agarose gel electrophoresis for DNA fragmentation analysis.
Mitochondrial Membrane Potential Assay
The transmembrane potential, ΔΨm was analyzed using a JC-1 Mitochondrial Potential Detection Kit (Biotium Inc; Hayward, CA). The cell suspension was filtered through a 300-mesh nylon mesh, washed twice with cold PBS, and stained by 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazolcarbocyanine iodide (JC-1; Molecular Probes, Biotium Inc., Hayward, CA) in PBS for 15 min at room temperature in the dark, followed by flow cytometric analysis.
Transmission Electron Microscopy
The ultrastructure of splenocytes was observed by transmission electron microscopy (TEM). After the end of intragastric administration, the cell pellet was fixed with 40 g/l glutaraldehyde in 0.1 M sucrose with 0.2 M sodium cacodylate buffer (pH 7.4) overnight at 4C. Washed with sodium cacodylate buffer, the cells were re-fixed with 10 g/l osmium tetroxide for 1.5 hr. Following routine dehydration, epoxy resin embedding and ultrathin section, the specimens were stained with 20 g/l uranyl acetate–lead citrate and observed with an H700 transmission electron microscope (Hitachi; Tokyo, Japan).
Immunocytochemistry and Immunohistochemistry
For immunocytochemistry (IC), the splenocytes were fixed with 3% paraformaldehyde for 15 min at 37C. Next, the permeabilization step was carried out with chilled methanol (100%) for 10 min at −20C and then cells were incubated in a blocking solution containing 5% bovine serum albumin (BSA) and 1% Triton-X 100 for 1 hr at 37C. Primary antibodies were diluted in 5% BSA [rabbit pAb against LC3 (Abcam) at 1:1000 (Cell Signal Technology Inc., Beverly, MA)] and incubated for 2 hr at room temperature and then FITC-conjugated anti-rabbit IgG was added at 1:1000 dilutions for 1 hr at 37C. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (1 µg/ml) for 10 min at 37°C. Fluorescence images were captured by LSM 700 confocal laser scanning microscope (Carl Zeiss; Oberkochen, Germany).
The method of immunohistochemistry (IHC) was applied according to the report by Fang et al.14 In brief, the spleen paraffin sections were dewaxed in xylene, rehydrated through a graded series of ethanol solutions, and washed in distilled water, and the PBS and endogenous peroxidase activity was blocked by incubation with 3% H2O2 in methanol for 15 min to quench endogenous peroxidase activity. The sections were treated with microwave heating for 15 min in a 0.01 M, pH 6.0, sodium citrate buffer to facilitate antigen retrieval. The sections were saturated with normal 10% goat sera for 30 min and then incubated for 17 hr at 4C with primary rabbit antibodies [anti–p-mTOR (Ser2448; 1:20), anti–p-Akt (Ser473; 1:25), anti–p-PI3K (1:25), anti-LC3 (1:100), anti–p-62(1:100), anti-PTEN (1:100)]. After washing in PBS, the sections were incubated with 1% biotinylated goat anti-rabbit IgG secondary antibody (Cell Signal Technology Inc.) for 1 hr at 37C, and then the strept avidin-1 biotin complex (Cell Signal Technology Inc.) was applied for 30 min at 37C. To visualize the immunoreaction, sections were immersed in diaminobenzidine hydrochloride (Cell Signal Technology Inc.). Finally, the sections were lightly counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene, and mounted. Negative controls for the specificity of immunohistochemical reactions were performed by replacing the primary antibody with IgG of nonimmunized rabbit. Then the images were acquired with a digital microscope camera system (Nikon DS-Ri1; Nikon, Japan).
Western Blot Analysis
Splenocytes were harvested and washed with ice-cold PBS, then lysed with the ice-cold radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology; Beijing, China) with 1 mmol/l phenylmethylsulfonyl fluoride. Protein concentrations were calculated by BCA assay kits (Pierce; Rockford, IL). In all, 20 µg of total cellular protein was subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore; Atlanta, GA). The membranes were blocked with 5% defatted milk powder at room temperature for 1 hr and then immunoblotting was performed with the diluted (1:1000) primary antibodies (see Table 1) at 4C overnight, followed by horseradish peroxidase–conjugated secondary antibodies (dilution: 1:5000) at room temperature for 1 hr. Following each step, the membranes were washed five times with PBS–Tween 20 for 3 min. Finally, the blots were developed using the enhanced chemiluminescence system.
Table 1.
Antibodies Used in the Study.
| Target | Host, Clonality | Clone/Cat. No | Source |
|---|---|---|---|
| CDK2 | Rabbit, polyclonal | PB0562 | Boster |
| phospho(p)-CDK2 | Rabbit, polyclonal | BM4590 | Boster |
| cyclin D1 | Rabbit, polyclonal | BA0770 | Boster |
| p-cyclin D1 | Rabbit, polyclonal | BM4272 | Boster |
| p53 | Rabbit, polyclonal | A00001 | Boster |
| p21 Waf1/Cip1 | Rabbit, polyclonal | BM4382 | Boster |
| Bcl-2 | Rabbit, polyclonal | D17C4 | Cell Signaling Technology |
| Bcl-xl | Rabbit, polyclonal | 54H6 | Cell Signaling Technology |
| p-PI3K | Rabbit, polyclonal | 4228S | Cell Signaling Technology |
| PI3K | Rabbit, polyclonal | 4249S | Cell Signaling Technology |
| p-p38 MAPK | Rabbit, polyclonal | 8690S | Cell Signaling Technology |
| p38 MAPK | Rabbit, polyclonal | 7257s | Cell Signaling Technology |
| p-Akt (Ser473) | Rabbit, polyclonal | 4060S | Cell Signaling Technology |
| Akt | Rabbit, polyclonal | 4691S | Cell Signaling Technology |
| p-mTOR | Rabbit, polyclonal | 5536S | Cell Signaling Technology |
| mTOR | Rabbit, polyclonal | 2983S | Cell Signaling Technology |
| Beclin 1 | Rabbit, polyclonal | 3495S | Cell Signaling Technology |
| LC3-I/II | Rabbit, polyclonal | 12741S | Cell Signaling Technology |
| p62 | Rabbit, polyclonal | 8025S | Cell Signaling Technology |
| PTEN | Rabbit, polyclonal | 9188S | Cell Signaling Technology |
| p-AMPK | Rabbit, polyclonal | 2537S | Cell Signaling Technology |
| AMPK | Rabbit, polyclonal | 4150S | Cell Signaling Technology |
| 4EBP1 | Rabbit, polyclonal | 9644S | Cell Signaling Technology |
| p-4EBP | Rabbit, monoclonal | 2855S | Cell Signaling Technology |
| p-P70S6K | Rabbit, monoclonal | 9204S | Cell Signaling Technology |
Statistical Analysis
All data are expressed as the means ± SD of three independent experiments. Statistical analyses were performed to compare the experimental groups with the control group through one-way analysis of variance (ANOVA), followed by the Tukey–Kramer multiple comparison test with an equal sample size. All statistical analyses were performed using a commercially available statistical software package (SPSS18.0; SPSS Inc., Chicago, IL).
Results
Euptox A Induces G1 Arrest in Splenocytes
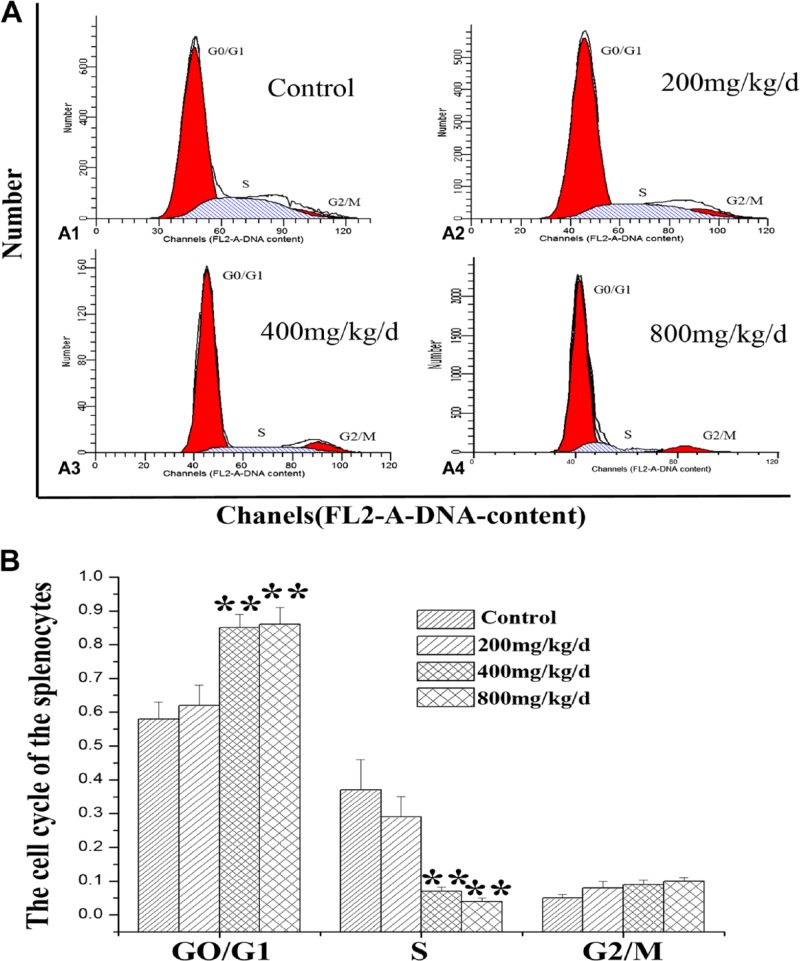
After intragastric administration of Euptox A, we examined its effect on the cell cycle distribution of splenocytes using FCM and found that the proportion of splenocytes in the G1 phase was remarkably increased in the treatment groups. As shown in Fig. 1A and B, the G0/G1 phase ratio of cells significantly increased from 52% (control) to 62.3%, 84.9%, and 85.6% in the groups treated with 200, 400, and 800 mg/kg/day of Euptox A, respectively. Consistent with this result, a slight decrease in the number of S phase cells and the G2/M phase ration of cells was also observed. These data suggested that Euptox A inhibited splenocyte growth in the mice through the inhibition of the G1 to S phase transition in the cell cycle.
Figure 1.
DNA histogram of the splenocytes cell cycle. (A) Mice were treated with different doses of Euptox A for 30 days. Subsequently, the DNA histogram of the splenocyte cell cycle was analyzed through flow cytometry with PI staining. (B) The G0/G1%, S%, and G2 + M% phases of the splenocytes were analyzed using flow cytometry. The flow cytometric histograms are representative of three separate experiments. The data are presented as the means ± SD of three independent experiments. *p<0.05 and **p<0.01, compared with the control group. Abbreviation: PI, propidium iodide.
Euptox A Induces G1 Arrest by Modulating Key Cell Cycle Regulators
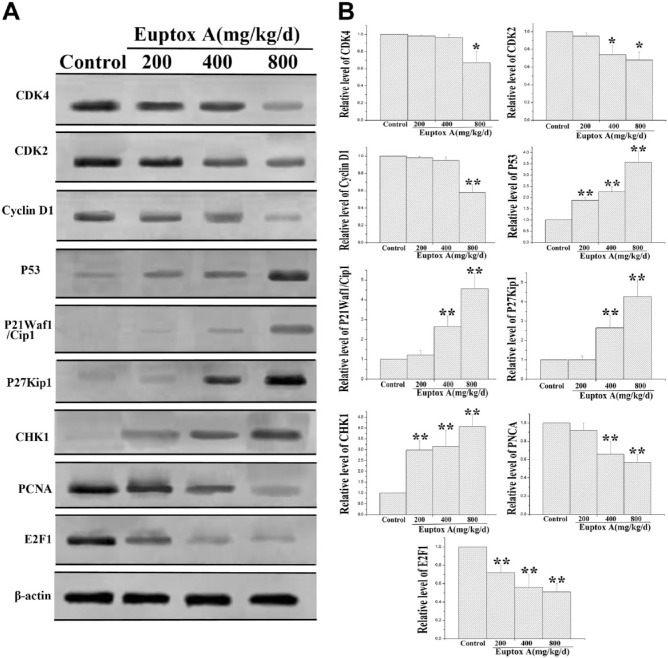
To investigate the mechanism underlying the Euptox A–induced cell cycle arrest, western blotting was used to examine the expression of key cell cycle regulating proteins—p53, p21 Waf1/Cip1, CDK2, CDK4, cyclin D1, p27 Kip1, Chk1, PCNA, and E2F1—in the mouse splenocytes. As shown in Fig. 2, Euptox A significantly inhibited the expression of the positive regulators of mTOR in the splenocytes: the expression of CDK4, CDK2, cyclin D1, PCNA, and E2F1 was reduced in a dose-dependent manner. Compared with the control group, the group treated with 800 mg/kg/day of Euptox A showed a 32.4% and 38.5% decrease in the expression level of CDK4 and cyclin D1 (Fig. 2A and B), respectively. CDK2 expression was also decreased by 28.5% and 30.7% in the groups treated with 400 and 800 mg/kg/day Euptox A (Fig. 2A and B), respectively, and the level of PCNA was decreased by 31.5% and 41.5% (Fig. 2A and B), respectively. E2F1 expression was decreased by 28.0%, 45.4%, and 48.7% in the groups treated with Euptox A at concentrations of 200, 400, and 800 mg/kg/day, respectively (Fig. 2A and B). However, the expression of the negative regulators of cell cycle progression was significantly increased in a dose-dependent manner in the treatment groups. Compared with the control group, the groups treated with 200, 400, and 800 mg/kg/day Euptox A showed a 1.8-, 2.23-, and 3.6-fold increase in p53 expression (Fig. 2A and B), respectively. Similarly, the groups treated with 400 and 800 mg/kg/day of Euptox A showed a 2.61- and 4.59-fold increase and 2.65- and 4.23-fold increase in the levels of p21 Waf1/Cip1 and p27 Kip1 (Fig. 2A and B), respectively. The administration of Euptox A at 200, 400, and 800 mg/kg/day resulted in a 2.95-, 3.19-, and 4.02-fold increase in CHK1 expression (Fig. 2A and B). Taken together, these results suggested that Euptox A caused the G1 arrest of splenocytes by decreasing the expression of CDK4, CDK2, cyclin D1, PCNA, and E2F1 and increasing the expression of p53, p21 Waf1/Cip1, p27 Kip1, and Chk1.
Figure 2.
Effect of Euptox A on the expression levels of key regulators of cell cycle in the splenocytes. Splenocytes were treated with Euptox A at concentrations of 200, 400, and 800 mg/kg/day for 30 days and then protein samples of cells were subjected to a western blotting assay. (A) Representative blots for CDK4, CDK2, cyclin D1, p53, p27 Kip1, p21 Waf1/Cip1, Chk1, PCNA, and E2F1, and (B) bar graphs showing the relative expression level of CDK4, CDK2, cyclin D1, p53, p27 Kip1, p21 Waf1/Cip1, Chk1, PCNA, and E2F1 in the splenocytes. All data are presented with the means ± SD and mean values of three independent experiments. *p<0.05, compared with the control group; **p<0.01, compared with the control group.
Euptox A Did Not Induce Apoptosis
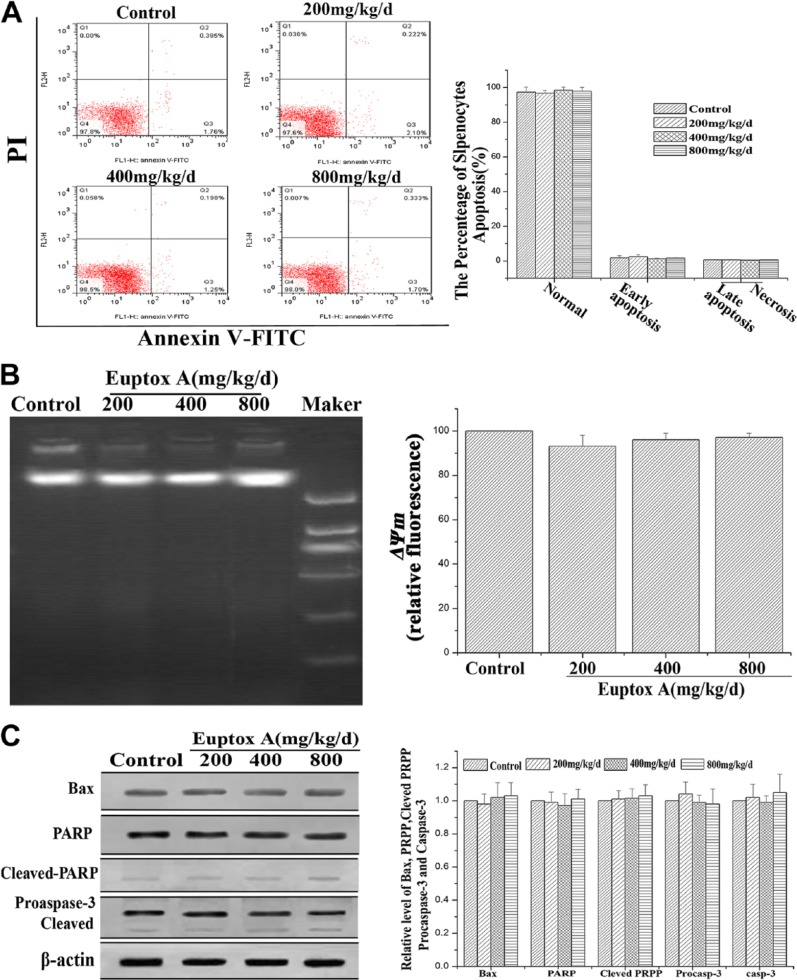
To further test the toxicity of Euptox A in the splenocytes, the effect of Euptox A on programmed cell death was examined. We first examined the apoptosis-inducing effect of Euptox A in the splenocytes by flow cytometric analysis. As shown in Fig. 3A, there was no significant change in the percentage of apoptotic splenocytes with increasing dose of Euptox A. Then, we examined ΔΨm and DNA fragmentation by FCM and agarose gel electrophoresis, respectively, but could not observe a decrease in ΔΨm and the DNA ladder (Fig. 3B). Moreover, we measured poly(ADP-ribose) polymerase (PARP), caspase-3, and Bax expression via western blotting and found that Euptox A did not induce any marked change in the expression of these proteins (Fig. 3C). These results indicated Euptox A did not induce apoptosis in the splenocytes.
Figure 3.
Euptox A administration does not induce apoptosis in splenocytes. (A) The scattergram of apoptotic splenocytes. The splenocytes were analyzed by flow cytometry for Annexin V and PI staining. Euptox A did not significantly induce apoptosis in splenocytes. (B) In Euptox A-treated splenocytes, there was no DNA ladder. DNA isolated from splenocytes was subjected to 2% agarose gel electrophoresis, followed by visualization of bands and photography. (C) Flow cytometry and JC-1 measure the effect of Euptox A on ΔΨm. Data are presented with the means ± SD and mean values of three independent experiments. *p<0.05, compared with the control group; **p<0.01, compared with the control group. Abbreviation: PI, propidium iodide.
Euptox A Triggered Autophagy in the Splenocytes
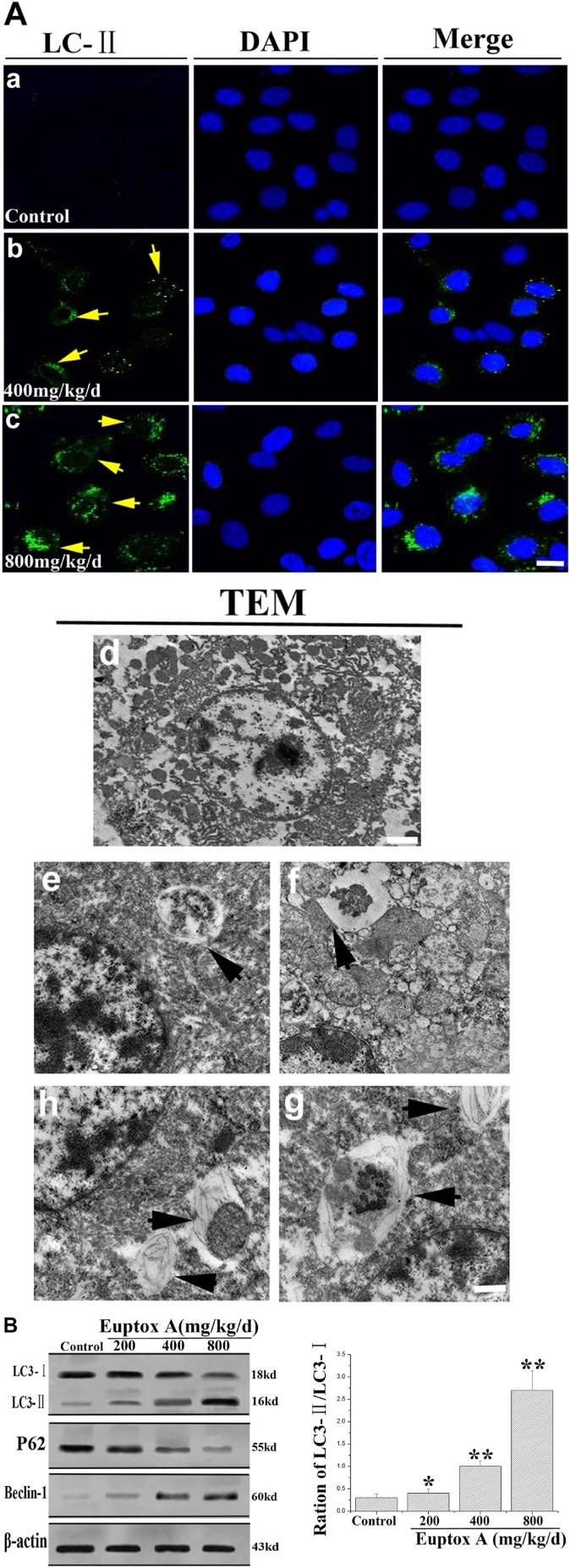
To confirm autophagy induction by Euptox A, LC3 immunostaining, DAPI, and TEM were conducted to detect autophagy. As shown in Fig. 4A, in the experimental groups, the results of LC3-II immunostaining showed that Euptox A promoted subcellular localization of punctate LC3-II and the LC3-II puncta formation was increased in the splenocytes. In addition, characteristic ultrastructural morphology representative of autophagy was also observed by TEM; the untreated cells had normal nuclei, cytoplasm, and organelles, whereas the Euptox A–treated cells showed a high number of autophagosomes of various sizes (Fig. 4A). We further measured changes in the expression of three major autophagy-related markers (LC3, Beclin 1, and P62) using western blotting and found that Beclin 1 and LC3-II were significantly upregulated, but LC3-I and P62 were markedly downregulated in the treatment groups (Fig. 4B). These results indicated that autophagy was triggered in the splenocytes after Euptox A treatment.
Figure 4.
The autophagy was activated by Euptox A. (A) Morphological observation of autophagy in splenocytes. Splenocytes were stained with LC3-II (yellow arrow) antibodies. Nuclei were stained with DAPI (blue; bar = 10 µm): the characteristic ultrastructural morphology of autophagy in splenocytes such as autophagic vacuoles (black arrow; bar = 2 µm). (B) The representative blots show the expression levels of LC3-I, LC3-II, Beclin 1, and p62 in splenocytes treated with Euptox A. β-actin was used as the internal control. Bar graph shows the ratio of LC3-II/LC3-I. All data are presented with the means ± SD and mean values of three independent experiments. *p<0.05, compared with the control group; **p<0.01, compared with the control group (Bars a–c = 10 µm; d = 2 µm; e–h = 0.5µm).
Euptox A Decreased the Activation of PI3K/AKT/mTOR Signal and Increased Autophagy in the Splenocytes
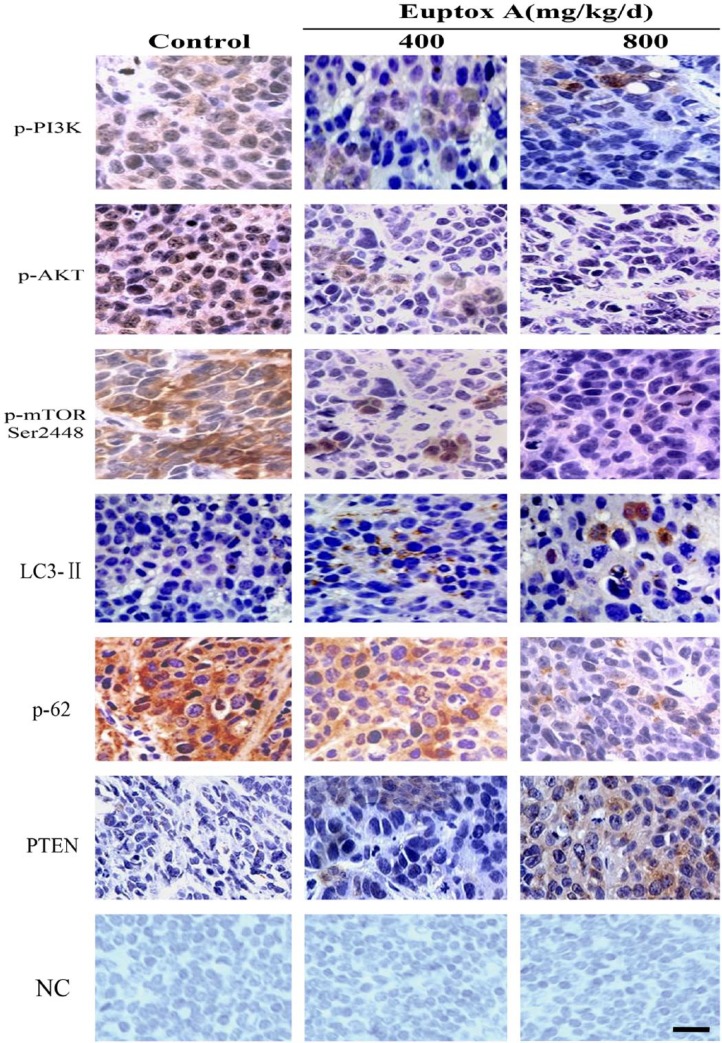
To further confirm that Euptox A induced autophagy in the splenocytes, we performed histochemical analyses to evaluate the effect of Euptox A treatment on the level of autophagy and the activation of PI3K/AKT/mTOR signals in both the control and the Euptox A–treated cells. As shown in Fig. 5, Euptox A inhibited the expression of p-PI3K, p-AKT, and p-mTOR, but increased the expression of PTEN. Moreover, it upregulated the expression of the autophagy-related protein LC3-II and downregulated the autophagy substrate, p62 (Fig. 5). Together, these results suggested Euptox A induces autophagy in splenocytes by inhibiting PTEN/AKT/mTOR signaling.
Figure 5.
Expression of LC3-II, p62, p-PI3K, p-AKT, p-mTOR, and PTEN protein in the splenocytes. Scale bar = 20 µm. Abbreviation: NC, negative control.
Euptox A Induces Autophagy by Regulating the PI3K/Akt/mTOR Axis, and the AMPK and p38 MAPK Signaling Pathways in Splenocytes
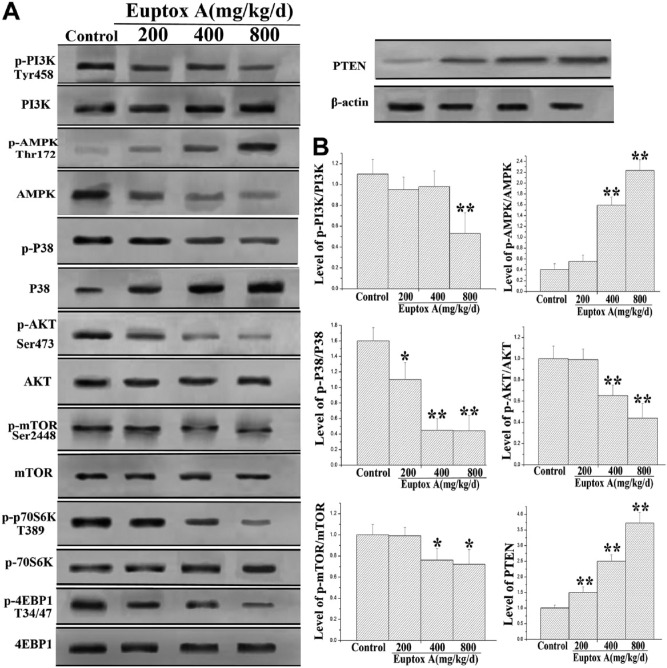
After confirming that Euptox A induced autophagy in the splenocytes, we tried to elucidate the mechanisms underlying the Euptox A-induced autophagy. To this end, we tested the phosphorylation levels of PI3K, p38 MAPK, and AMPK by western blotting. These proteins are the upstream signaling molecules of the protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway, which plays an important role in the regulation of autophagy.8,15 As shown in Fig. 5, Euptox A decreased the phosphorylation level of PI3K and p38 MAPK but increased the expression of total PI3K and p38 MAPK. Moreover, treatment with Euptox A at concentrations of 200, 400, and 800 mg/kg/day resulted in a 14.0%, 11.2%, and 53.2% decrease in the p-PI3K/PI3K ratio (Fig. 6A and B), respectively, and a 31.2%,71.2%, and 71.3% decrease in the p-p38/p38 ratio (Fig. 6A and B). Euptox A also increased the level of p-AMPK and reduced the level of AMPK, which resulted in an increase in the p-AMPK/AMPK ratio. The p-AMPK/AMPK ratio was increased by 1.4-, 4.0-, and 5.6-fold on treatment with Euptox A at concentrations of 200, 400, and 800 mg/kg/day, respectively (Fig. 6A and B). Akt is a downstream effector of PI3K, which is an upstream regulator of mTORC1, whereas p70 S6 kinase (P70S6K) and 4E binding protein 1 (4EBP1) are the downstream substrates of mTORC1. On measuring the levels of these proteins, we found that there was a concentration-dependent decline in the phosphorylation levels of Akt with Euptox A treatment, but the expression of total Akt was not significantly altered (Fig. 6A and B). Compared with the control group, the groups treated with 200, 400, and 800 mg/kg/day of Euptox A showed a 1.1%, 36.5%, and 57.5% decrease in the p-Akt/Akt ratio, respectively (Fig. 6A and B). We then evaluated the phosphorylation levels of mTOR at Ser2448 after Euptox A treatment and found that Euptox A obviously decreased the phosphorylation level of mTORC1 as well as the phosphorylation levels of its downstream effectors including p70S6K and 4EBP1 (Fig. 6A and B); this result indicated that the activity of the mTORC1 pathway was dramatically reduced on Euptox A treatment. PTEN is a negative regulator of the Akt/mTOR and MAPK signaling pathways, which play a key role in cell death.16 In this study, we found that Euptox A significantly increased the expression of PTEN (Fig. 6A and B). Together, these findings suggested that Euptox A induced splenocyte autophagy by inhibiting the PI3K/Akt/mTOR and p38 MAPK pathways and by suppressing AMPK activation.
Figure 6.
Effect of Euptox A on the expression or phosphorylation levels of key autophagy-regulating molecules in splenocytes. (A) Representative blots show the protein levels of PI3K, Akt, and mTOR, and the phosphorylation of PI3K, Akt T37/46, AMPK, p38 MAPK, mTOR S2448, p70S60K, and 4EBP1 in splenocytes administered with Euptox A. (B) Bar graphs show the ratio of p-PI3K/PI3K, p-AMPK/AMPK, p-Akt/Akt, p-p38 MAPK/p38 MAPK, p-mTOR/mTOR, and the expression levels of PTEN in splenocytes. β-Actin was used as the internal control. Data are presented with the means ± SD and mean values of three independent experiments. *p<0.05, compared with the control group; **p<0.01, compared with the control group.
Discussion
Euptox A is the main component in E. adenophorum and induces diarrhea or even death of livestock.17,18 Euptox A has been found to show hepatotoxicity in rats19 and immunotoxicity in mice.6 Therefore, in this study, we aimed to explore the mechanisms underlying the effects of Euptox A on mouse splenocytes.
Cell cycle control is a major regulatory mechanism in the process of cell growth, and many cytotoxic substances cause cell death by cell cycle arrest at the G0/G1, S, or G2/M phases.20,21 The binding of activated CDK4/6 to cyclin D1 leads to its dissociation from the E2F complex and promotes entry into the S phase of the cell cycle.20 The inhibition of cyclin D1 can induce G1 phase arrest and can result in apoptosis or autophagy.22,23 Besides, the association of CDK2 with cyclin E/A regulates the G1/S transition and modulates cellular events in the S phase.24 There are also many other upstream regulators that alter the activity of the CDK4/6–cyclin D1 complex. p53 is a critical tumor suppressor and a positive regulator of cell cycle arrest.25 Generally, G1 DNA damage or chromosomal instability promotes the phosphorylation of p53, thus activating the transcriptional ability of p53. p53 then downregulates p21, which binds to and inhibits PCNA, ultimately serving to inactivate the CDK4/6–cyclin D1 complex and suppressing G1/S transition.26,27 In this study, we found that the splenocytes were marked arrested in the G1 phase; this result is consistent with that observed previously on feeding goats with whole grass of E. adenophorum.3 We then examined the levels of the key regulators of cell cycle checkpoints including CDK2, CDK4, cyclin D1, E2F1, p53, p21, and PCNA in the splenocytes. p53 and p21 expressions were significantly increased, and CDK2, CDK4, E2F1, and PCNA expressions were remarkably decreased in the splenocytes on treatment with Euptox A. In addition, other key factors such as p27 Kip1 and Chk1 also play important roles in the regulation of the cell cycle. As a proliferation inhibitor, p27 Kip1 prevents G1/S phase transition by binding to and suppressing CDK2 activity.28 Chk1 acts as a DNA damage checkpoint signaling protein and has been reported to induce G1 phase arrest by inhibiting Cdc25A activity.29 In the present study, we found that treatment with Euptox A markedly increased the p27 Kip1 and Chk1 levels in the splenocytes. Collectively, these results suggested that Euptox A treatment arrested the splenocytes at the G1 phase by upregulating the expression of the antiproliferative regulators P53, P21, p27 Kip1, and Chk1 and inhibiting the expression of the pro-proliferative modulators CDK2, CDK4, cyclin D1, PCNA, and E2F1.
Autophagy is a self-digestive process that allows the elimination of toxic metabolites, intracellular pathogens, and damaged proteins and organelles, thus making energy and amino acids necessary for vital functions during metabolic stress. Regular organization needs high biosynthetic activity, which requires cellular basal autophagy.30 Nevertheless, excessive autophagy can destroy and directly lead to cell death.31 In this study, Euptox A induced splenic toxicity, which was reflected by autophagy. We used immunostaining and immunohistochemistry to detect the localization of punctate LC3, p62, and TEM to observe the autophagosomes using previously described methods.32 The increased subcellular localization of punctate LC3 and p62, and the existence of autophagosomes in the present study confirmed that Euptox A induces autophagosome formation in the splenocytes. Beclin 1 and LC3-II are the two specific markers of autophagy, and both of them are closely involved in autophagic processes.33 In our study, the changes in the p62, LC3-II, and Beclin 1 levels proved that Euptox A induced autophagy without interfering with autophagic flux in the splenocytes. Generally, autophagy and apoptosis occur through similar regulatory pathways and even share initiator and effector molecules. Autophagy and apoptosis can cooperate in a balanced interplay to allow cells to decide which route to take, thus influencing the fate of the cell.34 The occurrence of autophagy is usually accompanied by apoptosis.35 However, in this study, FCM, DNA ladder, and western blotting assays showed that Euptox A did not induce splenocyte apoptosis in the mice. Together, our results indicated that the mechanism of action of Euptox A in the splenocytes did not involve apoptosis. However, the result is inconsistent with the observations made in a previous study in goats fed E. adenophorum. This difference in the results may be because the excessive autophagy in our study resulted in the removal of damaged debris or denatured subcellular constituents and eliminated damaged organelles and dangerous pathogens, which are pro-apoptotic factors; moreover, the degradation of damaged mitochondria blocks apoptotic pathways by preventing mitochondrial outer membrane permeabilization and the subsequent release of pro-apoptotic molecules such as cytochrome c and Smac/Diablo.36 In our study, we also found that caspase-3 and PARP were not activated, which might be another reason why Euptox A did not induce apoptosis. When the tissues of animals are exposed to environmental toxins, apoptosis occurs and causes tissue damage.37,38 In our study, we did not find a large number of apoptotic cells among the splenocytes, but found a large number of autophagocytes. Such occurrence of autophagy and inhibition of apoptosis has been observed previously in some other similar studies. For example, Yu Jinhua et al. found that pseudolaric acid B could induce the apoptosis of mouse fibrosarcoma and inhibit apoptosis.39 In addition, Chao Nie et al. showed that procyanidins can induce autophagy in human gastric cancer cells but do not induce apoptosis.40 In addition, Liao Fei showed that Euptox A has good antitumor activity.41 Based on these data, we believe that Euptox A has the potential for use as a drug that promotes autophagy (similar to temozolomide) to kill apoptosis-resistant tumor cells by autophagic death. However, the interplay between apoptosis and autophagy in splenocytes subjected to Euptox A is still not clear. Further studies are needed to fully elucidate the relationship between apoptosis and autophagy.
The PI3K/Akt/mTOR signaling pathway is a central pathway that plays a role in autophagy through the regulation of cell growth, motility, protein synthesis, cell metabolism, cell survival, and cell death in response to various stimuli.42 In general, Akt phosphorylation is upregulated following the activation of PI3K, and mTOR can then integrate upstream activating signals through the PI3K/Akt pathway and become phosphorylated to inhibit autophagy.43 In our study, we found that Euptox A downregulated the phosphorylation level of PI3K, Akt, mTOR, and p38 MAPK, while promoting the expression of PTEN, which is a negative regulator of the PI3K/Akt/mTOR pathway. Euptox A also promoted autophagy via the activation of AMPK, which is one of the main mTOR regulators and can directly communicate with mTORC1 by phosphorylating raptor, resulting in 14-3-3 binding and the allosteric inhibition of mTORC1.44 These findings suggest that Euptox A-induced autophagy occurs via suppression of the PI3K/Akt/mTOR and p38 MAPK signaling pathways and the activation of the AMPK signaling pathway.
Previous studies have shown that autophagy is most active in the G0/G1 phases of the cell cycle and that autophagy can regulate cell proliferation by contributing to cell cycle arrest.45 Specific regulators of the cell cycle have also been shown to affect autophagy; for example, CDK1 and p27 overexpression is sufficient to induce autophagy.46 Therefore, we speculate that Euptox A-induced autophagy indirectly inhibits the proliferation of splenocytes by regulating cell cycle arrest. However, further studies are needed to clarify this hypothesis.
In conclusion, the results of the present study showed that Euptox A induces G1 arrest in splenocytes and promotes autophagy by activating PTEN and AMPK and inhibiting the PI3K/Akt/mTOR and p38 MAPK signaling pathways. These findings provide new insights into the mechanisms underlying Euptox A–induced splenic toxicity in mice. Moreover, our finding indicating that Euptox A induces autophagy without inducing apoptosis suggests its potential for use as a drug to kill apoptosis-resistant tumor cells. Obviously, there is still much for us to unveil the complete mechanisms underlying Euptox A–caused growth inhibition and splenocyte autophagy.
Footnotes
Author Contributions: YH designed the study and drafted the manuscript; QM and LH performed the immunohistochemistry; QM and JW carried out the molecular genetics studies; and YZ, RX, YCZ, ZCZ, ZR, JD, ZJZ, GP, XN, and YW contributed to the analysis of the experimental data. All authors have read and approved the final manuscript.
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Science and Technology Support Program of Sichuan Province (grant 035Z1125), Special Fund for Agroscientific Research in Public Interest (grant 201203062), and the Chang-jiang Scholars and the Innovative Research Team in University (grant IRT0848).
Contributor Information
Quan Mo, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Liwen Hu, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Jiahua Weng, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Yong Zhang, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Yancheng Zhou, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Ruiguang Xu, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Zhicai Zuo, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Junliang Deng, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Zhihua Ren, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Zhijun Zhong, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Guangneng Peng, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Xiang Nong, College of Life Science, Leshan Normal University, Leshan, China.
Yahui Wei, Key Laboratory of Resource Biology and Biotechnology in Western China, School of Life Science, Northwest University, Xi’an, China.
Yanchun Hu, Key laboratory of Animal Disease and Human Health of Sichuan Province, College of Veterinary Medicine, Sichuan Agricultural University, Chengdu, China.
Literature Cited
- 1. Seawright AA, Oelrichs PB, Ng JC, Nolan CC, Jukes R, Davis A. P3B42—GSH-dependent biliary tract toxicity in the mouse caused by 9-oxo-10, 11-dehydroageraphorone (euptox). Toxicol Lett. 1998;95:162. [Google Scholar]
- 2. Oelrichs PB, Calanasan CA, Macleod JK, Seawright AA, Ng JC. Isolation of a compound from Eupatorium adenophorum (Spreng.) [Ageratina adenophora (Spreng.)] causing hepatotoxicity in mice. Nat Toxins. 1995;3:350–4. [DOI] [PubMed] [Google Scholar]
- 3. He Y, Quan M, Hu Y, Chen W, Luo B, Lei W, Yan Q, Xu R, Zhou Y, Zuo Z. E. adenophorum induces cell cycle arrest and apoptosis of splenocytes through the mitochondrial pathway and caspase activation in saanen goats. Sci Rep. 2015;5:15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiu H, LI C, LI H, Meng L, Wang R, Zhang Z. Effects of Eupatorium adenophorum spreng extract on apoptosis of splenocytes in mice. Chin J Radiol Health. 2013;22(6):751–3. [Google Scholar]
- 5. Bohlmann F, Gupta RK. Six cadinene derivatives from Ageratina adenophora. Phytochemistry. 1981;20:1432–3. [Google Scholar]
- 6. Ouyang CB, Liu XM, Liu Q, Bai J, Li HY, Li Y, Wang QX, Yan DD, Mao LG, Cao A. Toxicity assessment of cadinene sesquiterpenes from Eupatorium adenophorum in mice. Nat Prod Bioprospect. 2014;5:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altamura M, Caradonna L, Amati L, Pellegrino NM, Urgesi G, Miniello S. Splenectomy and sepsis: the role of the spleen in the immune-mediated bacterial clearance. Immunopharmacol Immunotoxicol. 2001;23:153–61. [DOI] [PubMed] [Google Scholar]
- 8. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qiang L, Wu C, Ming M, Viollet B, He YY. Autophagy controls p38 activation to promote cell survival under genotoxic stress. J Biol Chem. 2013;288:1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang G, Yue Z, Talloczy Z, Hagemann T, Cho W, Messing A, Sulzer DL, Goldman JE. Autophagy induced by Alexander disease-mutant GFAP accumulation is regulated by p38/MAPK and mTOR signaling pathways. Hum Mol Genet. 2008;17:1540–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katoch R, Sharma OP, Dawra RK, Kurade NP. Hepatotoxicity of Eupatorium adenophorum to rats. Toxicon. 2000;38:309–14. [DOI] [PubMed] [Google Scholar]
- 13. Li Z, Xu X, Yong H, Li D, Wang Z, Yu G, Dan X, Wei L, Tong D. Swainsonine activates mitochondria-mediated apoptotic pathway in human lung cancer A549 cells and retards the growth of lung cancer xenografts. Int J Biol Sci. 2012;8:394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang J, Cui H, Peng X, Chen Z, He M, Tang L. Developmental changes in cell proliferation and apoptosis in the normal duck thymus. Anat Histol Embryol. 2011;40:457–65. [DOI] [PubMed] [Google Scholar]
- 15. Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. [DOI] [PubMed] [Google Scholar]
- 16. Mester J, Eng C. When overgrowth bumps into cancer: the PTEN-opathies. Am J Med Genet C Semin Med Genet. 2013;163:114–21. [DOI] [PubMed] [Google Scholar]
- 17. Bordoloi MJ, Shukla VS, Sharma RP. Absolute stereochemistry of the insect antifeedant cadinene from Eupatorinm adenophorum. Tetrahedron Lett. 1985;26:509–10. [Google Scholar]
- 18. Sahoo A, Singh B, Sharma OP. Evaluation of feeding value of Eupatorium adenophorum in combination with mulberry leaves. Livest Sci. 2011;136:175–83. [Google Scholar]
- 19. Kaushal V, Dawra RK, Sharma OP, Kurade NP. Hepatotoxicity in rat induced by partially purified toxins from Eupatorium adenophorum (Ageratina adenophora). Toxicon. 2001;39:615–9. [DOI] [PubMed] [Google Scholar]
- 20. Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hengartner MO. Hengartner MOThe biochemistry of apoptosis. Nature. 2000;407:770–6. [DOI] [PubMed] [Google Scholar]
- 22. Choi YJ, Saez B, Anders L, Hydbring P, Stefano J, Bacon NA, Cook C, Kalaszczynska I, Signoretti S, Young RA, Scadden DT, Sicinski P. D-cyclins repress apoptosis in hematopoietic cells by controlling death receptor Fas and its ligand FasL. Dev Cell. 2014;30:255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo Y, Hurwitz J, Massagué J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995;375:159–61. [DOI] [PubMed] [Google Scholar]
- 24. Resnitzky D, Reed SI. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wesierska-Gadek J, Wojciechowski J, Ranftler C, Schmid G. Role of p53 tumor suppressor in ageing: regulation of transient cell cycle arrest and terminal senescence. J Physiol Pharmacol. 2005;56:15–28. [PubMed] [Google Scholar]
- 26. Chen X, Bargonetti J, Prives C. p53, through p21 (WAF1/CIP1), Induces Cyclin D1 Synthesis. Cancer Res. 1995;55:4257–63. [PubMed] [Google Scholar]
- 27. Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 2003;13:65–70. [DOI] [PubMed] [Google Scholar]
- 29. Reinhardt HC, Yaffe MB. Kinases that control the cell cycle in response to DNA damage: chk1, Chk2, and MK2. Curr Opin Cell Biol. 2009;21:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. [DOI] [PubMed] [Google Scholar]
- 31. Codogno P. Shining light on autophagy. Nat Rev Mol Cell Biol. 2014;15:153. [DOI] [PubMed] [Google Scholar]
- 32. Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–502. [DOI] [PubMed] [Google Scholar]
- 33. Codogno P. Shining light on autophagy. Nat Rev Mol Cell Biol. 2014;15:153. [DOI] [PubMed] [Google Scholar]
- 34. El-Khattouti A, Selimovic D, Haikel Y, Hassan M. Crosstalk between apoptosis and autophagy: molecular mechanisms and therapeutic strategies in cancer. J Cell Death. 2013;6:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thorburn A. Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis. 2008;13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lennon SV, Martin SJ, Cotter TG. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif. 1991;24:203–14. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Bai C, Guan H, Chen R, Wang X, Wang B, Jin H, Piao F. Subchronic exposure to arsenic induces apoptosis in the hippocampus of the mouse brains through the Bcl-2/Bax pathway. J Occup Health. 2015;57:212–21. [DOI] [PubMed] [Google Scholar]
- 39. Jing HY, Liu CY, Gui BZ, Li YZ, Ming HY, Wen YZ, Xian YM, Xiao FY. Pseudolaric acid B induced cell cycle arrest, autophagy and senescence in murine fibrosarcoma L929 cell. Int J Med Sci. 2013;10:707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nie C, Zhou J, Qin X, Shi X, Zeng Q, Liu J, Yan S, Zhang L. Reduction of apoptosis by proanthocyanidin-induced autophagy in the human gastric cancer cell line MGC-803. Oncol Rep. 2016;35:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liao F, Hu Y, Wu L, Tan H, Luo B, He Y, Qiao Y, Mo Q, Wang Y, Zuo Z. Induction and mechanism of HeLa cell apoptosis by 9-oxo-10, 11-dehydroageraphorone from Eupatorium adenophorum. Oncol Rep. 2015;33:1823–7. [DOI] [PubMed] [Google Scholar]
- 42. Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10:143–53. [DOI] [PubMed] [Google Scholar]
- 43. Polivka J, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142:164–75. [DOI] [PubMed] [Google Scholar]
- 44. Shang L, Wang X. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy. 2011;7:924–6. [DOI] [PubMed] [Google Scholar]
- 45. Eskelinen EL, Prescott AR, Cooper J, Brachmann SM, Wang L, Tang X, Backer JM, Lucocq JM. Inhibition of autophagy in mitotic animal cells. Traffic. 2002;3:878–93. [DOI] [PubMed] [Google Scholar]
- 46. Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–24. [DOI] [PubMed] [Google Scholar]