Introduction
About atopic dermatitis
Atopic dermatitis (AD), commonly known as eczema, is a chronic, pruritic inflammatory skin disease associated with a profound physical and psychosocial burden, which can contribute to a reduced quality of life.1 Prevalence of AD is as high as 20% in children and 10% in adults.2-4 AD usually begins in early childhood but can persist into or begin in adulthood.5 It often fluctuates between states of relative quiescence and flares, although some patients have chronically active disease.6,7 AD is often found in individuals with a personal or family history of atopy, including allergic rhinitis, asthma and AD.1
The etiology of AD is multifactorial. The current understanding of AD’s pathophysiology centres on skin barrier defects and the dysregulation of the immune system on a genetically and environmentally susceptible background.1 The interplay between these mechanisms leads to the signs and symptoms of AD: pruritus, dry skin, edema, excoriations, lichenification and oozing. AD’s presentation may vary depending on the patient’s age, disease severity and chronicity.1
AD treatment focuses first on the daily practice of basic skin care in addition to topical anti-inflammatory agents, phototherapy and systemic immunomodulators.8-10 Novel targeted therapeutic options are in the development pipeline.1 The primary goals of AD management involve the treatment and prevention of AD flares and repair and maintenance of the skin barrier.10-12 In these guidelines, we will discuss in detail different elements of AD assessment and management.
Understanding the burden of AD
AD has a profound negative physical, psychosocial and economic impact on affected patients and their families, contributing to impaired quality of life in children and adults.1,13,14 The itch associated with AD drives much of this burden, leading to discomfort and poor sleep. Other impacts of AD include bullying and embarrassment related to the appearance of the skin, avoidance of specific activities such as sports and specific career choices.14 The self-reported impact on quality of life has also been found to be comparable to that experienced in families of children with diabetes and or cystic fibrosis.15
The economic impact of AD is significant for individual patients and society. In 2004 in the United States, direct medical costs of AD (medical services and health products) were estimated to be about $1 billion, costs due to lost productivity $619 million and costs attributable to quality- of-life impairment $2.6 billion.16 Those estimates did not take into account the costs of over-the-counter medications, which can be very expensive for patients and their families.17 Given the increasing prevalence of AD and rising health care costs in general, these numbers are likely to have increased substantially since then.
Role of community pharmacists
Pharmacists, as primary care providers, are in a prime position to support both patients and physicians as trusted and easily accessible members of the health care team in the management of AD (Box 1). Pharmacists are often a first point of entry into the health care system, as patients with AD may approach a pharmacist first for nonprescription remedies. In addition, pharmacists often have cultivated a long-standing rapport with their patients and thus can reassess patients’ understanding of their disease and treatment plan and reinforce nonpharmacologic measures and treatment compliance. Pharmacists are trained in the management of medication-related concerns and play a key role in the ongoing optimization and monitoring of prescribed AD therapy’s safety, effectiveness, drug interactions and adherence.
Box 1. Pharmacists’ responsibilities for patients with atopic dermatitis.
- Improving adherence through patient education
- Atopic dermatitis (AD) patient-friendly education: highlight the appropriate use of AD treatment, goals of therapy, AD triggers to avoid, when to see the physician and review of myths and misunderstandings in AD management
- Safety of topical corticosteroid (TCS): reassure patients that appropriate use of TCS can safely relieve symptoms without adverse effects
- Safety of topical calcineurin inhibitor (TCI): reassure patients that, to date, there is no substantial evidence to suggest TCIs are associated with increased cancer risk (including lymphomas) or systemic immunosuppression
- Basic skin care: reinforce the regular use of moisturizers and the avoidance of aggravating factors
- Pharmacists should work with their patients to create SMART (Specific, Measurable, Attainable, Relevant and Time-based) goals as a means of providing patients with a structured framework to set treatment objectives for themselves in managing their AD.
- AD therapy optimization and monitoring
- Pharmacists should provide ongoing optimization and monitoring of prescribed AD therapy’s safety, effectiveness, drug interactions and adherence to avoid potentially serious complications and poor treatment outcomes.
- Recommending appropriate moisturizer
- Pharmacists should tailor moisturizer recommendations based on patient and disease factors including tolerability, cost, mechanism of action, absence of sensitizing agents or fragrances, degree of AD severity and affected location.
- Improving communication of instructions between the prescriber and the patient: the fingertip unit system
- Pharmacists represent a key pillar in the health care delivery system and are prime candidates to advocate for the integration of the fingertip unit measurement with topical medications as a means of accurately conveying how much product the prescribing physician would like their patients to use and ensure dosing consistency.
- Community health literacy
- Pharmacists should lead a variety of educational activities for community members, such as hosting free educational talks within the pharmacy or at a community centre, geared toward educating the public about the basics of AD care and destigmatizing AD within the community.
- Connecting patients living with AD with patient-support organizations
- Pharmacists should connect patients to available community patient-support organizations, such as the Eczema Society of Canada, where they can gain access to further reputable, practical and patient-friendly information on AD.
Diagnosis and assessment
AD is a clinical diagnosis based on patient history, associated clinical signs and the skin lesion’s morphology and distribution.18 The skin lesions in eczema involve erythematous, xerotic (dry), scaly papules and plaques that can be excoriated, lichenified or oozing depending on the degree of pruritus, chronicity and presence of a superimposed bacterial infection (Appendix 1, available in the online version of the article). The distribution of the lesions changes from childhood to adulthood. In infants, lesions tend to affect the facial and extensor regions. In older children, lesions tend to affect the flexor, periocular and neck regions. In adults, lesions tend to follow a similar distribution pattern to older children, in addition to hand eczema being more common.18 There may also be a personal or first-degree family history of atopic disease including AD, asthma and allergic rhinitis.18
Management of AD: Daily skin care and prevention of AD flares
Pharmacists should
recommend appropriate moisturizers based on patient and disease factors and
engage in AD therapy optimization and monitoring.
General daily skin care for AD includes the maintenance of the skin barrier with liberal use of emollients and avoidance of aggravating factors.10,19 A defective skin barrier is increasingly recognized as a primary pathogenic factor in AD.1 A defective skin barrier allows moisture to leave the skin and allows allergens and irritants to penetrate into the skin.1 Barrier defects can be genetic, with mutations in epidermal barrier proteins such as filaggrin, or secondary to environmental factors or inflammation.20,21 Rehydrating the skin and restoring the skin’s barrier function is accomplished with the regular application of unscented moisturizers throughout the day.22 This includes application of moisturizers immediately following baths or showers.8,23,24 Bathing or showering should be short and use warm (not hot) water, and cleaning should be done with the least amount of nonirritating soaps or nonsoap cleansers (pH balanced to the skin).8 The importance of the use of moisturizers in AD management cannot be overemphasized as this has been found to reduce the incidence of AD flares and the need for topical corticosteroid (TCS) use.22,25,26 There is evidence in the literature that daily moisturizer use, compared with no moisturizer use, extended the flare-free period and can also improve eczema severity scores in actively inflamed skin.27,28
Altogether, the different types of moisturizers work to improve the skin’s hydration status through a variety of mechanisms that replenish water content and restore the skin’s ability to absorb, retain and redistribute water content. In the literature, emollients soften the skin and are often included in various moisturizer products.19
Moisturizers are formulated with varying amounts of different active ingredients, including occlusive agents, emollients and humectants. Moisturizers are categorized as occlusive emollient moisturizers or humectants and are produced in various vehicles such as cream, ointment and lotions.19
Looking closer at the active ingredients in moisturizers, occlusive agents, such as petrolatum, work by mitigating the loss of water from the stratum corneum with a layer of oil.19 Emollient agents, such as ceramides and linoleic acid, enhance the skin’s flexibility and suppleness by filling in cracks and bolstering the structural integrity in the affected skin. Humectants, such as urea and glycerine, work by drawing water from the environment and concentrating water over the area of application for water absorption and redistribution.19
The choice of occlusive or humectant moisturizer and type of vehicle are dependent on patient and disease factors including tolerability, cost, mechanism of action, absence of sensitizing agents or fragrances, degree of AD severity and affected location on the body.8 Each moisturizing product has its unique composition of active ingredients, with variations in hydrophobic and hydrating properties. Occlusive emollient ointments are generally preferable, but patients may find these cosmetically unacceptable because of their greasy texture. In that case, occlusive emollient creams are a suitable alternative. For many patients, creams will sting upon application compared with ointments when applied to open or fissured areas of AD. Lotions are less preferable in the management and prevention of AD because of their higher water-to-oil content. Moreover, the benefit of topical ceramides over other moisturizers is not well established, given the lack of head-to-head studies at this time. Despite these considerations, patient preferences differ, and the best moisturizer is the product the patient or caregiver will use liberally and regularly. With all moisturizers, there is a risk of adverse reactions that may include irritant reactions, contact allergy and folliculitis.29 Humectants, like urea, can be irritating to the affected skin and consequently may not be a tolerable option for the patient.12 Appropriate moisturizing and skin care are paramount strategies in the effective management of AD. In the marketplace, there is a cornucopia of moisturizers available from which the patients can choose and pharmacists can recommend and provide patient education. The Seal of Acceptance program is an Eczema Society of Canada program in which patient society members with sensitive skin review moisturizers and cleansers for tolerability and absence of skin irritation. Products then undergo a formulation review by dermatologists, selected products are shared with the public and the product may display the seal on its packaging.
In addition to moisturizing, recognizing and avoiding irritants when possible is important for basic skin care.10,11 Patients with AD generally have more sensitive skin, and irritants can trigger or exacerbate their AD. To avoid aggravating factors, one must first identify potential AD triggers through careful history taking.10,11 On top of these potential patient-specific AD triggers, there are also known physical (e.g., wool), environmental (e.g., excessive dry heating) and chemical (e.g., solvents, detergents, perfumes) irritants that should generally be avoided in AD patients.10-12 Whether taking showers or baths, the use of nonirritating and mildly acidic soaps or nonsoap cleansers is preferable. Also, the application of moisturizers to AD affected and nonaffected skin, within 3 minutes following bathing and at minimum twice a day, is recommended for optimal hydration.12
AD flares: Management of pruritus and inflammation
AD’s symptoms are relapsing in nature. While some patients have chronic persistently active disease, most have symptoms that wax and wane from various presentations of active disease to periods of remission.7 Each AD patient’s course of disease differs, and therapy should therefore be individualized considering both patient (age, adherence) and disease factors (extent, distribution, acute flare or chronic disease).1,7
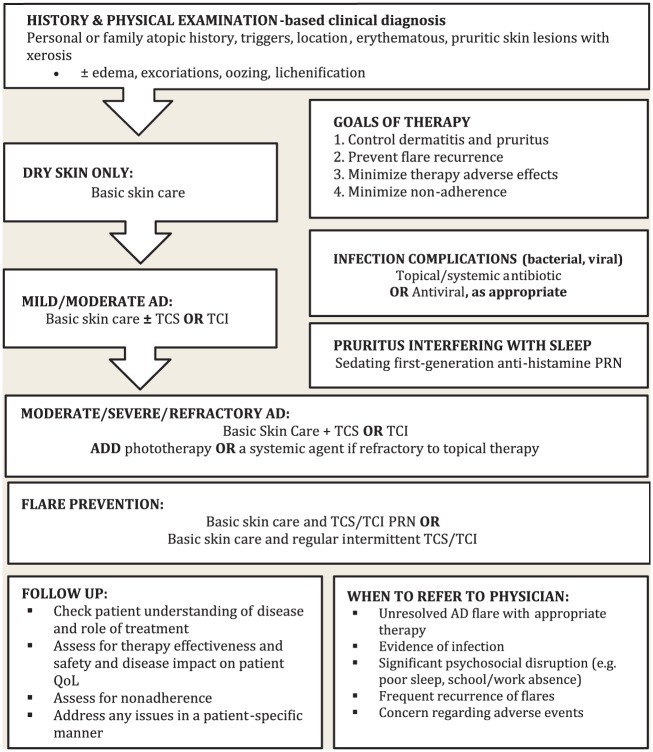
Factors that aid in the identification of acute AD flares include the worsening of AD symptoms (pruritus, pain, limiting day-to-day activities), the relapse of skin lesions previously in remission and the start of new AD lesions.1,4,10,11 AD flare management focuses on daily skin care and inflammation control (Figure 1).
Figure 1.
Algorithm summary of atopic dermatitis management
PRN, as needed; QoL, quality of life; TCS, topical corticosteroids; TCI, topical calcineurin inhibitors; systemic agents: glucocorticoid, cyclosporine, methotrexate, azathioprine, or mycophenolate mofetil.
Topical and systemic agents are used to address the inflammatory component of AD (Table 1). Use of each agent is dependent on AD severity and response to other agents. One must aim to achieve optimal effectiveness while minimizing adverse effects and satisfying patient preferences.
Table 1.
Outline of available treatments in atopic dermatitis
| Topical | Phototherapy and systemic therapy |
|---|---|
|
Class: Corticosteroids
Examples*: Low-potency • Hydrocortisone acetate 1% cream/ointment • Desonide 0.05% cream/ointment Mid-potency • Betamethasone valerate 0.05% or 0.1% cream/ointment • Mometasone furoate 0.1% cream • Hydrocortisone valerate 0.2% cream/ointment High-potency • Fluocinonide 0.05% cream/ointment/gel • Mometasone furoate 0.1% ointment • Desoximetasone 0.25% cream/ointment/gel Very-high potency • Betamethasone dipropionate glycol 0.05% ointment • Clobetasol propionate 0.05% cream/ointment • Halobetasol propionate 0.05% cream/ointment |
Class: Corticosteroids
Example: Prednisone Class: Calcineurin inhibitor Example: Cyclosporine A Class: Anti-metabolites Examples: Methotrexate Azathioprine Mycophenolate mofetil |
|
Class: Calcineurin inhibitors
Examples: Tacrolimus 0.03% ointment Tacrolimus 0.1% ointment Pimecrolimus 1% cream |
Phototherapy
Example: • Narrow-band UVB (311 nm) • Broad-band UVB • Psoralen + UVA photochemotherapy |
For a comprehensive list of topical corticosteroids, organized by potency, available in Canada and available dosage forms, contact the Eczema Society of Canada for the “Topical Treatments in Atopic Dermatitis—Health Care Provider Resource Chart.”
Topical anti-inflammatory treatment
Topical agents are the mainstay of treatment for mild-moderate AD and include TCS and topical calcineurin inhibitors (TCI).8,11 TCS are effective agents in addressing the pruritus and inflammation of AD and work by inducing inhibitory proteins that decrease inflammatory mediator activity.4 Although they are often prescribed for twice-daily use, evidence suggests that once-daily application is just as effective and may minimize the risk of adverse events.30 TCS are classified by potency (low, mid, high and very high), and the selection of a specific TCS for a given patient is dependent on multiple factors, including the body location of the lesional skin and lesion severity (e.g., erythema, lichenification; Table 1). Identifying the location where the TCS is applied on the body is crucial in maximizing TCS effectiveness and reducing the likelihood of adverse effects.8 For example, the skin on the face and the skin folds is thin, and AD lesions in these areas may be treated with a low-potency TCS to minimize the likelihood of adverse effects.8
In general, anti-inflammatory treatment for AD consists of a low-potency TCS for the face and folds and a mid-potency TCS for other areas of the body once to twice daily to any actively inflamed areas.8 For particularly thick or recalcitrant lesions or for lesions on the palms and soles, higher potency TCS may be used for enhanced penetration.4,8 As AD is a chronic disease, putting time limits on the use of TCS is generally impractical and can result in undertreatment. However, if a specific lesion does not improve after 2 to 4 weeks of daily, adequate application of a TCS, the patient should consult his or her physician to see if the diagnosis or treatment plan should be reconsidered.
TCS adverse effects include skin atrophy, striae, rosacea and perioral dermatitis and, if systemic absorption occurs, hypothalamic-pituitary-adrenal axis suppression.31 In addition, periocular use can result in cataract and glaucoma development.32 The risk of TCS adverse effects is associated with the TCS potency, the type of skin where the TCS is applied and the frequency and duration of TCS use.4 It must be emphasized that the risk of adverse events when TCS are used properly is low, and the harm associated with undertreatment is generally greater than the potential for adverse events.8 Prescribers and pharmacists should properly educate their patients on the relative benefits and risks associated with their use, with an emphasis on reassurance regarding their safety profile, as steroid phobia in the patient population is quite common and can negatively affect compliance.33
TCI are an effective alternative to TCS therapy.4,11 TCI are nonsteroidal immunomodulators working to reduce AD inflammation through the inhibition of T cells and inflammatory cytokine production.4 There are 2 TCI available on the market: tacrolimus and pimecrolimus. In terms of anti-inflammatory action, tacrolimus 0.1% is considered equivalent to a mid-potency TCS.34 Tacrolimus 0.03% was not found to be as effective as a mid-potency TCS but was found to be more effective than a low-potency TCS.34 The clinical role of pimecrolimus 1% remains unclear, as it was found to be less effective than mid- and high-potency TCS and there is an absence of key head-to-head studies with low-potency corticosteroids.8,34 It should be noted that the indications of TCI are age specific. Pimecrolimus cream 1% and tacrolimus 0.03% ointment are indicated for use in patients 2 years of age or older, while tacrolimus 0.1% ointment is indicated for use only in adult patients.4 However, they are frequently used off label in younger patients.35
Although they are considered second-line agents, TCI have a favourable safety profile without the risks of skin atrophy and hypothalamic-pituitary-adrenal (HPA) axis suppression associated with TCS, and as such, they are frequently used off label as steroid-sparing therapy.4 TCI are a particularly good option in the periocular region given the potential for TCS to cause cataracts and glaucoma if used in those areas. Evidence suggests that prolonged use of TCI is safe, with pimecrolimus up to 4 years and tacrolimus up to 2 years.36,37 Adverse effects of TCI include transient itching and burning sensations that tend to occur when TCI is applied to an oozing, open barrier lesion with active eczema.4 Because of concerns of a potential increased risk of malignancy associated with calcineurin inhibitors, a black-box warning was issued for TCI. Since then, there has been no evidence suggesting a link between TCI and malignancy.38
For patients with frequent flares in predictable areas, basic skin care may be inadequate, and a more proactive approach with intermittent application of TCS or TCI to those areas even during periods of remission may be recommended.10,26 Studies have found that this proactive approach with either TCS or TCI has extended the time to first relapse when compared with vehicle.39-46 In addition, closer histologic examination of areas of past active disease has revealed persistent subclinical inflammation and an altered skin barrier, providing a further rationale for this proactive approach.47 The success of a proactive approach should be carefully evaluated. Presently, there is a paucity of studies comparing the proactive use of TCS and TCI head to head; thus, clinicians have little direct evidence with which to choose one approach over the other.10 Furthermore, the intermittent dosing schedule varies in studies of proactive topical anti-inflammatories, including instructions for 2 times weekly, 3 times weekly, 2 consecutive days per week, once-daily and twice-daily applications.10 Thus, a consensus for a specific dosing regimen in a proactive approach remains to be determined.10 Whether an AD patient should pursue such a proactive approach to preventing AD flares is dependent on his or her treating clinician’s clinical experience, the patient’s disease severity and pattern and patient choice after he or she has been fully informed regarding the risks and benefits of this approach.
Phototherapy and systemic treatment
For patients with moderate-to-severe disease refractory to topical therapy, consideration should be given to the use of phototherapy or systemic immunomodulatory agents. Narrow-band UVB (wavelength = 311 nm) is the most commonly used type of phototherapy for AD and is typically administered 2 to 3 times per week. Narrow-band UVB is a safe option that can provide relief from the signs and symptoms of AD.9 Potential adverse effects of phototherapy include erythema and burns, as well as photoaging and increased skin cancer risk.
If phototherapy is contraindicated, not successful or not available, systemic agents should be considered. These must be used cautiously by practitioners well versed in their use, as they are each associated with potential serious adverse effects and require regular clinical and laboratory monitoring.9 Currently available systemic agents include systemic corticosteroids, cyclosporine A, methotrexate, azathioprine and mycophenolate mofetil.48 Other than systemic corticosteroids, these are all used off label for AD.
The use of systemic corticosteroids for AD is controversial. While they provide quick, effective relief of AD lesions, they are associated with severe AD flares upon withdrawal.49 Further, long-term use of systemic corticosteroids is associated with significant adverse events, including HPA suppression, weight gain, hypertension, glucose intolerance, emotional lability and decreased bone density.9,50,51 As such, in recent guidelines published by the American Academy of Dermatology, it was recommended that systemic corticosteroids generally be avoided in adults and children with AD.9 Further, those guidelines suggest they be limited to short-term use as a bridge to other systemic agents or phototherapy.
Cyclosporine A is a calcineurin inhibitor that controls T-cell–mediated inflammation by preventing the transcription of inflammatory cytokines.4 Cyclosporine A has been found to be effective in the management of severe and refractory AD in adults and children.48 However, the risk of serious adverse effects, including hypertension, renal dysfunction and an increased risk of infection, limit it to short-term use.4 Cyclosporine A may interact with a number of different medications, and so care must be taken to avoid interactions.
Methotrexate, azathioprine and mycophenolate mofetil are antimetabolite medications that work as a folate antagonist, purine analog and purine synthesis inhibitor, respectively. These medications have been shown to be variably effective in treatment-resistant AD.48 As with cyclosporine, they are immunosuppressive and therefore can increase the risk of infection. They are also each associated with the potential for end-organ damage, including hepatotoxicity and cytopenias. Methotrexate should be used in conjunction with folic acid supplementation to reduce the risk of hematologic adverse events.52
Adjunctive treatment
First-generation oral antihistamines, such as hydroxyzine, may have clinical utility, primarily because of their sedating adverse effect, if a patient’s pruritus significantly impairs their ability to achieve restful sleep, leading to daytime dysfunction.9,53 It should be noted that there is no good evidence to suggest oral antihistamines effectively address the pruritic component of AD, and so the use of nonsedating oral antihistamines should be avoided.54
Patients with AD are at increased risk of bacterial and viral infections, most notably impetiginization of AD lesions and eczema herpeticum.55,56 In cases of active secondary infection, topical or systemic antibiotics or systemic antivirals are often needed. Topical antimicrobial agents, such as mupirocin, should be limited to the management of active bacterial skin infections and are not recommended for routine use in noninfected AD.8 Other means of normalizing the AD microbiome include dilute bleach baths, which have been shown to reduce AD severity.57
Targeted agents in development for AD
There are currently a large number of medications for the treatment of AD in various stages of development.58 This includes topical and systemic agents and both monoclonal antibodies and small molecules. The topical agent furthest along in development, not yet available in Canada but approved by the Food and Drug Administration in the United States, is crisaborole, a phosphodiesterase-4 inhibitor with good results in adults and children in phase 3 trials.59 With regard to systemic agents, dupilumab is the first biologic agent approved for use in AD.60 Dupilumab binds to the IL-4 receptor and interferes with the signaling of IL-4 and IL-13, 2 cytokines in the Th2 pathway that are important in AD pathogenesis. The results of phase 3 trials up to 16 weeks have been published with encouraging results, and longer trials are under way.60 While studies to date have been limited to adults, trials in children are planned as well (Clinicaltrials.gov: NCT02612454). While neither crisaborole nor dupilumab are currently approved in Canada, these and other new agents will hopefully increase the therapeutic armamentarium for AD in the coming years.
Addressing patient concerns regarding AD treatment adverse effects
The appropriate use of medications is essential in mitigating the likelihood of adverse effects.4,11 The distribution of accurate drug safety information is equally important in reducing patient fears.11 AD patients are often prescribed TCS as part of their treatment plan and the literature has reported public concerns of immune suppression and undesirable skin changes that have negatively impacted treatment compliance. Undertreatment in the context of “steroid phobia” can lead to poor disease control. Skin atrophy can be induced by any TCS,4,11 but there are various factors that can increase the risk of skin atrophy, including higher potency agents, combination products (i.e., high potency TCS with antibacterial or antifungal), concomitant occlusive dressing, use on thin skin and prolonged use.61 With respect to use of high-potency agents, the risk of hypothalamic-pituitary-adrenal axis suppression is low, but this risk can increase with prolonged use and use in children with a larger body surface area to weight ratio.62 Pharmacists have an opportunity to educate and reassure patients that appropriate use of TCS can safely relieve symptoms without adverse effects.
The public concern with TCI use arose with the previously mentioned FDA announcement of TCI’s lack of established long-term safety and subsequent “black box” warning designation.38,63-65 It was believed that there was potential for systemic absorption that could manifest as systemic adverse effects, including cancer. To date, there is no substantial evidence to suggest TCI are associated with increased cancer risk (including lymphomas) or systemic immunosuppression.38,63-65 Pharmacists can play an important role in mitigating patient concerns by communicating this evidence.
With respect to phototherapy in AD, it is generally considered safe and well tolerated. Commonly described adverse effects include photodamage, erythema, burn and tenderness.9 A large component of our understanding regarding the long-term safety of phototherapy and guidance with regard to phototherapy scheduling and dosing in AD arises from literature in psoriasis management.9 A review of 3867 patients on narrowband-UVB therapy, receiving a median of 29 treatments and as many as 352 patients receiving more than 100 treatments, found no significant association between narrowband-UVB and basal cell carcinoma, squamous cell carcinoma or melanoma after a median follow-up of 5.5 years.66 This review addresses the concern of the potential long-term risk of carcinogenesis associated with narrowband-UVB and suggests the judicious use of narrowband-UVB is a reasonable form of second-line therapy in patients failing topical therapy.
When used and monitored appropriately, systemic agents, including systemic corticosteroids, cyclosporine, methotrexate, azathioprine and mycophenolate mofetil, are usually tolerable and safe with the use of the lowest effective dose and for the shortest duration to allow for appropriate flare control.9 However, each of these medications is associated with immunosuppression as well as other potentially serious toxicities, as mentioned above. Prescribers and pharmacists must play an active role in ensuring these medications are being used and monitored appropriately to avoid potentially serious complications.
There is a lack of evidence evaluating the safety of oral antihistamines in AD management.67 This, however, does not preclude an adjunctive role for oral antihistamines in AD, as they have been used to provide relief of nocturnal pruritus.67 Patients should be aware of oral antihistamine side effects, including sedation and anticholinergic effects (blurry vision, dry mouth, tachycardia).
Patient education and adherence strategies
Pharmacists should
strive to improve adherence through patient education,
improve communication of instructions between the prescriber and the patient (the fingertip unit system),
advocate for community health literacy and
connect patients living with AD to patient-support organizations.
Pharmacists are inherently community medical educators, as they are easily accessible and trained to counsel in a patient-friendly manner. Pharmacists represent the interface between patients and prescribers and are ideally positioned to empower and encourage patients to consult with their primary care physician, pediatrician or dermatologist about any questions they have regarding their management plan (Box 1). Pharmacists can also take a proactive approach and advocate on behalf of their patients and communicate with the prescribing team to ensure that the plan is specific and well understood.
It is crucial for pharmacists to check on their patient’s understanding and concerns, clarifying any misconceptions related to their AD therapy plan to effectively address nonadherence in their counselling. In this way, fears and myths can be dispelled promptly. Furthermore, pharmacists can work with their patients to create SMART (Specific, Measurable, Attainable, Relevant and Time-based) goals as a means of providing patients with a structured framework to set treatment objectives for themselves in managing their AD.19,68 Equally important, following up with AD patients on their established SMART goals (Appendix 2, available in the online version of the article) is key to maintaining adherence and identifying whether more practical goals need to be established.
When TCS prescriptions use cautionary and imprecise phrasing in their instructions, such as “use sparingly,” it contributes to the fear and reluctance of patients about using TCS for their AD. A suggested method to improve the communication of instructions between the prescriber and the patient is the use of the fingertip unit system.4 A fingertip unit is defined as the amount of topical product expressed from a tube with a 5 mm diameter nozzle, applied from the distal skin crease to the tip of the index finger (Table 2).4,69 By incorporating this system of measurement with topical medications, health care prescribers can accurately convey how much product they would like their patients to use and ensure dosing consistency. This may help prevent clinical scenarios in which patients use either too little or too much topical product and subsequently experience either no clinical benefit or adverse effects. Pharmacists are ideal champions of the fingertip unit dosing system.
Table 2.
The fingertip unit (FTU) and number of FTUs to treat each anatomical area
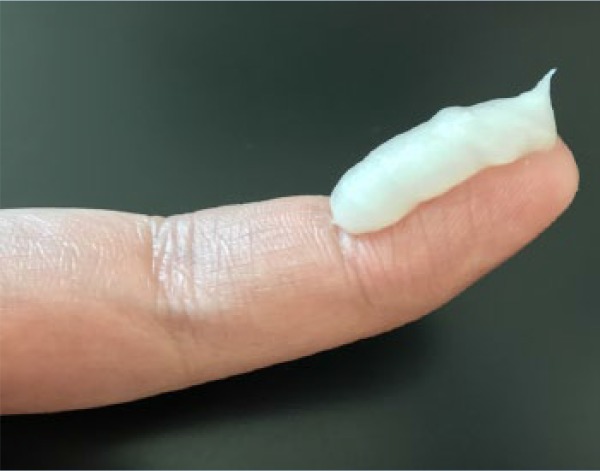
|
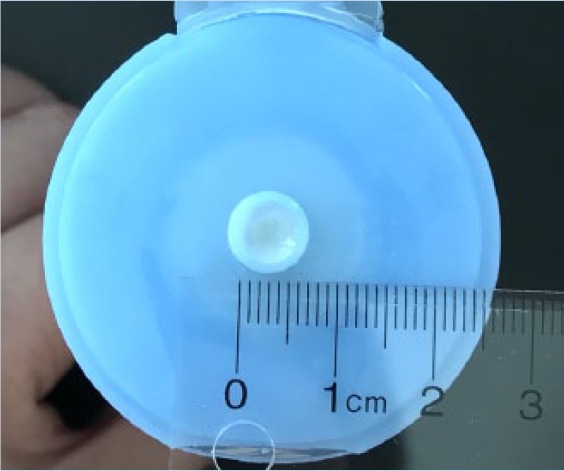
|
| (A) A fingertip unit (FTU) is defined as the amount of topical product expressed from a tube applied from the distal skin crease to the tip of the index finger. | (B) The tube in which the topical product is expressed from must have a 5 mm diameter nozzle. |
| Anatomical site | Number of FTUs to treat each anatomical area |
| Face and neck | 2.5 |
| Trunk | 7 (Front) 7 (Back) |
| 1 arm | 3 |
| 1 hand | 1 |
| 1 leg | 6 |
| 1 foot | 2 |
Adapted from Long and Finlay.69 The photos have been reproduced with permission from Ian T. Y. Wong, RPh.
Pharmacists can build on a trusting patient-pharmacist relationship and take the time to listen to their patients and learn how AD is affecting their quality of life. Showing appropriate empathy is essential in gaining trust from patients and destigmatizing AD. Inquiring about patient preferences in terms of cosmetic acceptability and cost consideration when selecting moisturizers for long-term use can often help patients take ownership of their AD management plan. Also, working with other members of the patient’s health care team (including dermatologists, allergists, respirologists, pediatricians and primary care physicians) can be helpful in ensuring patient continuity of care.
AD, much like other chronic diseases, has a complicated pathophysiology and can involve multiple treatment options and steps in one’s treatment plan. Thus, patient education is paramount to achieve and maintain adherence and good results. Highlighting the appropriate use of AD treatment, goals of therapy and AD triggers to avoid and reviewing myths and misunderstandings in AD management can encourage patient adherence. Time with physicians in the clinic is often limited, and pharmacist-led educational sessions can be beneficial for patients. Pharmacist-led community education can take on many different forms, from setting up a free educational talk for patients to attend in their pharmacy or community centre to contacting the local newspaper or radio station and offering to provide a short informative segment on AD and its management.
Pharmacists have the unique opportunity to oversee and optimize the ongoing care for skin health of patients living with AD in the community. In addition, pharmacists can continue to support patients living with AD by connecting them to community patient-support organizations, such as the Eczema Society of Canada, where they can gain access to further reputable, practical and patient-friendly information on AD. These resources are helpful not only for patients but also for pharmacists and other health care providers alike. Pharmacists can support AD patients by reinforcing basic skin care practices and aid in their personal selection of moisturizers with their knowledge of moisturizer characteristics and side effect profile and clinical understanding of AD. ■
The AD guidelines for pharmacists were developed in collaboration with the Eczema Society of Canada (ESC). The ESC is a registered Canadian charity, founded in 1997 and was established with the goal of improving the lives of Canadians living with AD. The society has contributed to communities across the nation through active education and awareness programming and the provision of support for patients and research endeavours in AD. The ESC aims to continue to raise awareness and destigmatize AD by helping the public gain insight into the tremendous burden AD can have for sufferers and their families. Health care professionals, patients and community members can access informative resources and learn about upcoming community education events at www.eczemahelp.ca/en/index.html.
Supplementary Material
Footnotes
Author Contributions:I. T. Y. Wong wrote the initial draft of the article. R. T. Tsuyuki, A. Cresswell-Melville, P. Doiron and A. M. Drucker reviewed and revised the article. All authors approved the final version of the article.
Declaration of Conflicting Interests:A. Cresswell-Melville is an employee of the Eczema Society of Canada (ESC), a registered Canadian charity, and has no conflict of interest or funding disclosure related to this publication. The Eczema Society of Canada receives funding from private citizen donations and foundations and funds, as well as from corporate partners, including Actelion Pharmaceuticals Ltd, Astellas Pharma, Beiersdorf Canada, Galderma Canada, Glaxo SmithKline Canada, Johnson & Johnson Inc., L’Oreal Canada Inc., Pediapharm Inc., Pierre Fabre Dermo-Cosmétique Canada Inc., Sanofi Canada, Skin Fix Inc., Valeant Canada and Wellspring Pharmaceuticals. A. M. Drucker is an investigator for Sanofi and Regeneron and a consultant for Sanofi. Dr. Drucker has received honoraria from Astellas Canada, Prime Inc. and Spire Learning.
Funding:The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1. Weidinger S, Novak N. Atopic dermatitis. Lancet Lond Engl 2016;387(10023):1109-22. [DOI] [PubMed] [Google Scholar]
- 2. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI; ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009;124(6):1251-8.e23. [DOI] [PubMed] [Google Scholar]
- 3. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol 2013;132(5):1132-8. [DOI] [PubMed] [Google Scholar]
- 4. Lee JH, Son SW, Cho SH. A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol Res 2016;8(3):181-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanifin JM, Reed ML; Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermat Contact Atopic Occup Drug 2007;18(2):82-91. [DOI] [PubMed] [Google Scholar]
- 6. Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol 2004;113(5):925-31. [DOI] [PubMed] [Google Scholar]
- 7. Garmhausen D, Hagemann T, Bieber T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy 2013;68(4):498-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014;71(1):116-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014;71(2):327-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis part 4: prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol 2014;71(6):1218-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saeki H, Nakahara T, Tanaka A, et al. Clinical practice guidelines for the management of atopic dermatitis 2016. J Dermatol 2016;43(10):1117-45. [DOI] [PubMed] [Google Scholar]
- 12. Lynde C, Barber K, Claveau J, et al. Canadian practical guide for the treatment and management of atopic dermatitis. J Cutan Med Surg 2005;8(suppl 5):1-9. [DOI] [PubMed] [Google Scholar]
- 13. Drucker AM, Wang AR, Li W-Q, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol 2017;137(1):26–30. [DOI] [PubMed] [Google Scholar]
- 14. Zuberbier T, Orlow SJ, Paller AS, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol 2006;118(1):226-32. [DOI] [PubMed] [Google Scholar]
- 15. Chamlin SL, Chren M-M. Quality-of-life outcomes and measurement in childhood atopic dermatitis. Immunol Allergy Clin North Am 2010;30(3):281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 2006;55(3):490-500. [DOI] [PubMed] [Google Scholar]
- 17. Filanovsky MG, Pootongkam S, Tamburro JE, Smith MC, Ganocy SJ, Nedorost ST. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr 2016;169:284-90.e5. [DOI] [PubMed] [Google Scholar]
- 18. Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol 2014;70(2):338-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Giam YC, Hebert AA, Dizon MV, et al. A review on the role of moisturizers for atopic dermatitis. Asia Pac Allergy 2016;6(2):120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol 2008;128(5):1067-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol 2008;121(6):1337-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Catherine Mack Correa M, Nebus J. Management of patients with atopic dermatitis: the role of emollient therapy. Dermatol Res Pract 2012;2012:e836931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol 2009;26(3):273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simpson E, Trookman NS, Rizer RL, et al. Safety and tolerability of a body wash and moisturizer when applied to infants and toddlers with a history of atopic dermatitis: results from an open-label study. Pediatr Dermatol 2012;29(5):590-7. [DOI] [PubMed] [Google Scholar]
- 25. Lucky AW, Leach AD, Laskarzewski P, Wenck H. Use of an emollient as a steroid-sparing agent in the treatment of mild to moderate atopic dermatitis in children. Pediatr Dermatol 1997;14(4):321-4. [DOI] [PubMed] [Google Scholar]
- 26. Nankervis H, Thomas KS, Delamere FM, Barbarot S, Rogers NK, Williams HC. Scoping systematic review of treatments for eczema [Internet]. Southampton (UK): NIHR Journals Library; 2016. (Programme Grants for Applied Research). Available: http://www.ncbi.nlm.nih.gov/books/NBK363127/ (accessed Dec. 10, 2016). [PubMed] [Google Scholar]
- 27. Wirén K, Nohlgård C, Nyberg F, et al. Treatment with a barrier-strengthening moisturizing cream delays relapse of atopic dermatitis: a prospective and randomized controlled clinical trial. J Eur Acad Dermatol Venereol 2009;23(11):1267-72. [DOI] [PubMed] [Google Scholar]
- 28. Cork M. Optimising treatment of atopic dermatitis: the emollient to topical steroid prescribing ratio. Sci Insights Rep 2003. [Google Scholar]
- 29. Zirwas MJ, Stechschulte SA. Moisturizer allergy. J Clin Aesthetic Dermatol 2008;1(4):38-44. [PMC free article] [PubMed] [Google Scholar]
- 30. Williams HC. Established corticosteroid creams should be applied only once daily in patients with atopic eczema. BMJ 2007;334(7606):1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Callen J, Chamlin S, Eichenfield LF, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol 2007;156(2):203-21. [DOI] [PubMed] [Google Scholar]
- 32. Nielsen NV, Sørensen PN. Glaucoma induced by application of corticosteroids to the periorbital region. Arch Dermatol 1978;114(6):953-4. [PubMed] [Google Scholar]
- 33. Aubert-Wastiaux H, Moret L, Le Rhun A, et al. Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol 2011;165(4):808-14. [DOI] [PubMed] [Google Scholar]
- 34. Ashcroft DM, Dimmock P, Garside R, Stein K, Williams HC. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials. BMJ 2005;330(7490):516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol 2015;26(4):306-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Papp KA, Werfel T, Fölster-Holst R, et al. Long-term control of atopic dermatitis with pimecrolimus cream 1% in infants and young children: a two-year study. J Am Acad Dermatol 2005;52(2):240-6. [DOI] [PubMed] [Google Scholar]
- 37. Hanifin JM, Paller AS, Eichenfield L, et al. Efficacy and safety of tacrolimus ointment treatment for up to 4 years in patients with atopic dermatitis. J Am Acad Dermatol 2005;53(2 suppl 2):S186-94. [DOI] [PubMed] [Google Scholar]
- 38. Hanifin JM. A reassuring rejoinder against malignant influences of topical calcineurin use in children. JAMA Dermatol 2015;151(6):587-8. [DOI] [PubMed] [Google Scholar]
- 39. Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol 2002;147(3):528-37. [DOI] [PubMed] [Google Scholar]
- 40. Berth-Jones J, Damstra RJ, Golsch S, et al. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: randomised, double blind, parallel group study. BMJ 2003;326(7403):1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glazenburg EJ, Wolkerstorfer A, Gerretsen AL, Mulder PGH, Oranje AP. Efficacy and safety of fluticasone propionate 0.005% ointment in the long-term maintenance treatment of children with atopic dermatitis: differences between boys and girls? Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol 2009;20(1):59-66. [DOI] [PubMed] [Google Scholar]
- 42. Peserico A, Städtler G, Sebastian M, Fernandez RS, Vick K, Bieber T. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled study. Br J Dermatol 2008;158(4):801-7. [DOI] [PubMed] [Google Scholar]
- 43. Van Der Meer JB, Glazenburg EJ, Mulder PG, Eggink HF, Coenraads PJ. The management of moderate to severe atopic dermatitis in adults with topical fluticasone propionate. The Netherlands Adult Atopic Dermatitis Study Group. Br J Dermatol 1999;140(6):1114-21. [DOI] [PubMed] [Google Scholar]
- 44. Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol 2011;164(2):415-28. [DOI] [PubMed] [Google Scholar]
- 45. Breneman D, Fleischer AB, Abramovits W, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol 2008;58(6):990-9. [DOI] [PubMed] [Google Scholar]
- 46. Wollenberg A, Reitamo S, Girolomoni G, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy 2008;63(7):742-50. [PubMed] [Google Scholar]
- 47. Suárez-Fariñas M, Tintle SJ, Shemer A, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 2011;127(4):954-64.e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol 2014;133(2):429-38. [DOI] [PubMed] [Google Scholar]
- 49. Schmitt J, Schäkel K, Fölster-Holst R., et al. Prednisolone vs. ciclosporin for severe adult eczema: an investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol 2010;162(3):661-8. [DOI] [PubMed] [Google Scholar]
- 50. Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum 2006;55(3):420-6. [DOI] [PubMed] [Google Scholar]
- 51. Bolanos SH, Khan DA, Hanczyc M. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol 2004;92(5):500-5. [DOI] [PubMed] [Google Scholar]
- 52. Shea B, Swinden MV, Ghogomu ET, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. J Rheumatol 2014;41(6):1049-60. [DOI] [PubMed] [Google Scholar]
- 53. Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol 2015;135(1):56-66. [DOI] [PubMed] [Google Scholar]
- 54. Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol 1999;135(12):1522-5. [DOI] [PubMed] [Google Scholar]
- 55. Langan SM, Abuabara K, Henrickson SE, Hoffstad O, Margolis DJ. Increased risk of cutaneous and systemic infections in atopic dermatitis: a cohort study. J Invest Dermatol [Internet]. February 12, 2017. Available: http://www.jidonline.org/article/S0022-202X(17)30175-6/abstract (accessed Apr. 5, 2017). [DOI] [PMC free article] [PubMed]
- 56. Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol 2003;49(2):198-205. [DOI] [PubMed] [Google Scholar]
- 57. Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009;123(5):e808-14. [DOI] [PubMed] [Google Scholar]
- 58. Eichenfield LF, Friedlander SF, Simpson EL, Irvine AD. Assessing the new and emerging treatments for atopic dermatitis. Semin Cutan Med Surg 2016;35(5 suppl):S92-6. [DOI] [PubMed] [Google Scholar]
- 59. Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol 2016;75(3):494-503.e4. [DOI] [PubMed] [Google Scholar]
- 60. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2017;376(1):1090. [DOI] [PubMed] [Google Scholar]
- 61. Simpson EL. Atopic dermatitis: a review of topical treatment options. Curr Med Res Opin 2010;26(3):633-40. [DOI] [PubMed] [Google Scholar]
- 62. Ellison JA, Patel L, Ray DW, David TJ, Clayton PE. Hypothalamic-pituitary-adrenal function and glucocorticoid sensitivity in atopic dermatitis. Pediatrics 2000;105(4 pt 1):794-9. [DOI] [PubMed] [Google Scholar]
- 63. Margolis DJ, Hoffstad O, Bilker W. Lack of association between exposure to topical calcineurin inhibitors and skin cancer in adults. Dermatol Basel Switz 2007;214(4):289-95. [DOI] [PubMed] [Google Scholar]
- 64. Arellano FM, Wentworth CE, Arana A, Fernández C, Paul CF. Risk of lymphoma following exposure to calcineurin inhibitors and topical steroids in patients with atopic dermatitis. J Invest Dermatol 2007;127(4):808-16. [DOI] [PubMed] [Google Scholar]
- 65. Tennis P, Gelfand JM, Rothman KJ. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol 2011;165(3):465-73. [DOI] [PubMed] [Google Scholar]
- 66. Hearn RM, Kerr AC, Rahim KF, Ferguson J, Dawe RS. Incidence of skin cancers in 3867 patients treated with narrow-band ultraviolet B phototherapy. Br J Dermatol 2008;159(4):931-5. [DOI] [PubMed] [Google Scholar]
- 67. Apfelbacher CJ, van Zuuren EJ, Fedorowicz Z, Jupiter A, Matterne U, Weisshaar E. Oral H1 antihistamines as monotherapy for eczema. Cochrane Database Syst Rev 2013;(2):CD007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Substance Abuse and Mental Health Services Administration. S.M.A.R.T. treatment planning. Available: http://www.samhsa.gov/samhsa_news/volumexiv_5/article2.htm (accessed Dec. 11, 2016).
- 69. Long CC, Finlay AY. The finger-tip unit—a new practical measure. Clin Exp Dermatol 1991;16(6):444-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.