Abstract
Restorative composites have evolved significantly since they were first introduced in the early 1960s, with most of the development concentrating on the filler technology. This has led to improved mechanical properties, notably wear resistance, and has expanded the use of composites to larger posterior restorations. On the organic matrix side, concerns over the polymerization stress and the potential damage to the bonded interface have dominated research in the past 20 y, with many “low-shrinkage” composites being launched commercially. The lack of clinical correlation between the use of these materials and improved restoration outcomes has shifted the focus more recently to improving materials’ resistance to degradation in the oral environment, caused by aqueous solvents and salivary enzymes, as well as biofilm development. Antimicrobial and ester-free monomers have been developed in the recent past, and evidence is mounting for their potential benefit. This article reviews literature on the newest materials currently on the market and provides an outlook for the future developments needed to improve restoration longevity past the average 10 y.
Keywords: permanent dental restorations, composite resins, polymerization, methacrylates, BisGMA, stress
Introduction
The significant evolution of composites for direct tooth restorations since their introduction on the market has allowed the expansion of their indications to larger posterior restorations, which were classically only restored with amalgams. Most of the developments have focused on the filler systems, leading to improvements mainly in mechanical properties and notably on wear resistance (Ferracane 2011). Irrespective of these improvements, the average life span of a composite restoration is still only around 10 y (Demarco et al. 2012). In the early 2000s, greater attention was focused on the further development of the organic matrix, which to date had been based exclusively on methacrylate chemistry, more specifically BisGMA (bisphenol A glycidyl dimethacrylate), TEGDMA (triethylene glycol dimethacrylate), BisEMA (ethoxylated bisphenol-A dimethacrylate), and UDMA (urethane dimethacrylate). Alternative monomers began to be developed with the common objective of reducing polymerization shrinkage and stress, as the possible association between stress development and gap formation at the bonded interface was being emphasized (Feng et al. 2010). The new monomers were either based on ring-opening polymerizable moieties (with the only commercial example being Filtek LS, based on silorane chemistry) or on higher molecular weight molecules (Fig. 1), with both strategies proving successful at reducing the molar shrinkage coefficient and ultimately the polymerization stress measured in vitro (Boaro et al. 2010). Clinical studies, however, have failed to show differences between the “low-shrinkage/low-stress” materials and their conventional counterparts in terms of restoration survival and incidence of secondary decay. This is likely due to the multifactorial nature of caries development and the overall technique sensitivity of the restorative procedure (Magno et al. 2016). More recently, the focus in resin development has shifted to improving the overall resistance of the restoration to degradation in the oral environment, including the actual hydrolysis of ester bonds present in methacrylates by salivary and bacterial enzymes, as well as by preventing biofilm formation on the surface and interface of composite restorations (Wu et al. 2015). In addition, much effort has been concentrated on developing materials that are simpler to use by virtue of requiring fewer application steps, such as bulk-fill and self-adhesive composites.
Figure 1.
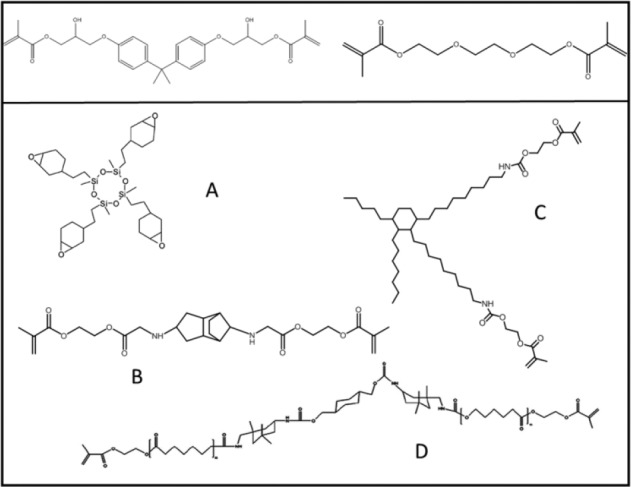
Top: Molecular structures of BisGMA (bisphenol A glycidyl dimethacrylate) (left) and TEGDMA (triethylene glycol dimethacrylate) (right). Bottom: Molecular structure of alternative monomers currently used in commercial products. (A) Oxirane (Filtek LS; 3M-ESPE), (B) TCD-urethane (Venus Diamond; Heraeus Kulzer), (C) dimeracid dimethacrylate (N’Durance; ConfiDental-Septodont), and (D) DuPont DX-511 (Kalore; GC America).
A 2011 review approached the inorganic phase of the resin composite (Ferracane 2011). Specifically on the organic matrix realm, another review has covered many aspects of the low-shrinkage/low-stress strategies, including multi-methacrylates, ultra-rapid methacrylates, thiol-ene polymerizations, and alternative polymerization routes, as well as aspects related to inclusion of nanoparticles and filler surface treatment (Cramer et al. 2011). The present review focuses solely on the organic phase of restorative composites, including novel monomer systems, polymeric additives, and other resin-related modifications. The text emphasizes literature published in the last 10 y, indexed in Scopus and/or in the patent literature. Developments in materials involving fewer application steps and self-adhesive, bioactive, and antibacterial materials with commercial examples are analyzed. Finally, we provide an outlook for the future of composites based on ongoing research efforts in the areas of network modification (i.e., through the use of covalent adaptable networks and the use of prepolymerized and tailorable nanoparticles) and the production of more degradation-resistant materials. It is evident from the amount of activity in the past 5 to 10 y that the topic of improvement of dental composites, specifically the organic matrix, remains relevant. The goal of researchers in this area is to produce a composite material with all the esthetic advantages of current materials but one that will last at least twice as long as current formulations, thus more capably preserving the patient’s natural tooth structure and avoiding costly retreatments.
Commercially Available Materials
One of the main drawbacks of resin composites compared with other direct placement materials, such as amalgam, is the technique sensitivity of the restorative procedure. This means the clinical outcomes of different types of restorations are strongly dependent on the operator (Scotti et al. 2016). For example, the adhesive application involves many steps, and there is ample opportunity for operator error. Also, the incremental layering technique is time-consuming and introduces additional variables to the treatment. With the aim of simplifying the procedure, bulk-fill and self-adhesive materials were developed, with the first few clinical trials being published.
Bulk-Fill Composites
Bulk-fill resin composites have been introduced to the market in both flowable and conventional/sculptable viscosities, with the premise of simplified application, while still ensuring adequate depth of cure. This has been achieved for different commercial materials via different routes, which include optimization of the initiator system (novel photoinitiators or greater concentration of conventional photoinitiators), modifications of the filler system (larger fillers or more translucent fillers), or inclusion of different chemistries in the composition (Miletic et al. 2017; Son et al. 2017). Flowable bulk-fill materials generally have lower filler loading than nonflowable, sculptable materials and require that the occlusal layer be filled with a “cap” of a more highly filled composite that is expected to be stronger and more wear resistant under occlusal loading. One example is SureFil SDR flow (Dentsply). According to the manufacturer, this product features, in addition to the lower filler content, a novel UDMA-based monomer with high molecular weight (849 g/mol), which helps to reduce shrinkage. The novel part of the monomer, to which the manufacturer refers as “polymerization modulator,” consists of photoactive groups embedded in the backbone of an oligomeric species (Xiaoming et al. 2015). The rationale is that as the material is exposed to light, the photoactive groups undergo photo-cleavage, at the same time breaking the oligomer chain to accommodate stress and generating radicals, which can further contribute to the overall conversion and crosslinking of the material. Indeed, these materials have been shown to reduce polymerization stress without reducing the polymerization rate or degree of conversion (Kim et al. 2015). In theory, this would eliminate the need for incremental filling on the basis of stress reduction.
Other nonflowable bulk-fill materials include Tetric EvoCeram Bulk-Fill (Ivoclar Vivadent) and Filtek Bulk-Fill (3M-ESPE). Tetric EvoCeram uses a photoinitiator system containing Ivocerin—a germanium-based light initiator, whose greater quantum yield conversion makes it more efficient in promoting polymerization in depth despite the shorter wavelengths needed for its optimal activation (λmax) (Moszner et al. 2009). For this material, shrinkage is reduced and stress is further relieved through the inclusion of prepolymerized resin filler particles. Studies have confirmed low shrinkage stress and efficient depth of cure (Jang et al. 2015). In the case of Filtek Bulk-Fill (3M-ESPE), among other compositional features, at least 1 monomer capable of undergoing addition fragmentation chain transfer is included in the formulation (3M technical profile). This mechanism has been widely described in the literature for applications where strain accommodation and stress reduction are desirable features, such as in the polymer coatings industry (Kloxin et al. 2010; Park et al. 2010). The main effect of this chemistry is that it renders the covalent network capable of adapting to stress generation via bond breakage and re-formation, without net loss of crosslinking via an allyl disulfide bond (Park et al. 2012). Results with experimental materials have demonstrated at least maintenance of mechanical properties and up to 30% reduction in polymerization stress with model molecules (Park et al. 2012).
In general, the utilization of bulk-fill resin composites in posterior restorations has been shown to reduce cusp deformation (Rosatto et al. 2015; Van Ende et al. 2017) and polymerization stress (Fronza et al. 2015), as well as increase the fracture resistance (Leprince et al. 2014; Rosatto et al. 2015) when tested in vitro. However, flowable bulk-fill materials produce lower mechanical properties in comparison with highly filled nano-hybrid composites and regular consistency bulk-fill materials, which can restrict their utilization under high occlusal load (Tomaszewska et al. 2015). Recent clinical studies indicate that flowable and regular-consistency bulk-fill materials present similar clinical performance compared with conventional materials (van Dijken et al. 2016).
Self-Adhesive Composites and Resin Cements
Self-adhesive resin composites, such as Vertise Flow and Dyad Flow (Kerr Corporation), have been developed with the goal of simplifying the composite restorative procedure by eliminating its most technique-sensitive step: the adhesive application. The resins in these composites contain glycerol phosphate dimethacrylate, a self-etching, dimethacrylate monomer capable of crosslinking and copolymerization with other methacrylates (Yuan et al. 2015), as well as the potential for chemical bonding with the tooth’s mineral content. There is also some evidence for micromechanical interlocking between polymerized monomers and partially demineralized collagen fibrils (Shafiei et al. 2016). All commercial self-adhesive resin-based materials commercialized to date are flowable (i.e., materials designed to enhance adaptation to the substrate by virtue of their low viscosity). Several studies published fairly recently have demonstrated, however, that the bond strength values of resin-based self-adhesive cements and restorative flowable composites are not as high as those achieved with separate adhesives and composite restoratives to tooth structure (Poitevin et al. 2013). This has been attributed to the fact that the acidity of the monomers in the self-adhesive materials is not low enough to promote extensive resin penetration through smear-covered surfaces or into aprismatic enamel, and to the fact that the viscosity presented by flowable materials is not low enough to ensure good adaptation to the cavity walls (Yuan et al. 2015). Their bond strength is improved only after traditional etch-and-rinse procedures with concentrated phosphoric acid are performed (Schuldt et al. 2015). In the case of bonding of fiber posts to root canals, which involves dentin in different depths and, therefore, with different structures, self-adhesive cements presented similar bond strengths compared with conventional controls (Vieira-Filho et al. 2017). In that case, the dentin structure seems to play a more important role than the cement composition. In addition, there is evidence for high water sorption and solubility, as well as increased microleakage for self-adhesive cements (Yuan et al. 2015), although hardness and elastic modulus appear to be comparable to other materials (Salerno et al. 2011).
Finally, even though this is not the focus of this review, it is important to note that soluble inorganic compounds, such as sodium silicates and biomimetic polyanions, can also be used to mediate adhesion (Sauro et al. 2016). Flowable resins containing these types of compounds have been demonstrated to stabilize the hybrid layer through apatite crystallite deposition in collagen (Sauro et al. 2013).
Bioactive Materials
In the past decade, in addition to lower stress generating/high-strength materials, researchers in academics and industry have also focused on developing materials with bioactive characteristics. The ideal restorative material of the future will not only be able to withstand occlusal loads and develop low polymerization stress but also be antifouling, antibacterial, and remineralizing, in addition to being biocompatible. Many efforts have been focused on each of these areas, as will be explored in the following sections as they relate to developments in resin-based systems.
Antimicrobial Materials
One of the main reasons for composite restoration failure is secondary caries development, presumably related to biofilm formation on and within gaps at the restoration margins (Lempel et al. 2015). Antimicrobial materials, therefore, have long been sought to either kill the bacteria on contact (bactericidal effect) or prevent bacterial adhesion (antifouling effect), and many strategies have been pursued. Quaternary ammonium methacrylates (QAMs) are being extensively studied and have been introduced in resin composites since they show bactericidal effects and are able to reduce bacterial adhesion (Cheng et al. 2015; K. Zhang et al. 2016). Dimethylaminododecyl methacrylate (DMAHDM), bis(2-methacryloyloxyethyl) dimethylammonium bromide (QADM), 12-methacryloyloxydodec-ylpyridinium bromide (MDPB), methacryloxylethylcetylammonium chloride (DMAE-CB), and quaternary ammonium polyethylenimine (QPEI) are some examples of the compounds that have been studied. Their demonstrated antibacterial effects come without compromise to the mechanical properties and short-term biocompatibility in highly filled composites (Ge et al. 2015). DMAHDM in particular seems to be able to inhibit the metabolic activity and lactic acid production of biofilm bacteria and to drastically reduce biofilm growth, since its positive charge interacts with the negatively charged bacterial wall, and the long side chain functions as a lancet to disrupt wall structure (Wu et al. 2015). Some examples of commercial materials relying on charged monomer technology include one adhesive system (Clearfil SE Protect, Kuraray Dental) that contains MDPB (12-methacryloyloxydodecyl pyridinium bromide) and Activa BioActive-Restorative resin composite (Pulpdent), which is formulated with an ionic-resin consisting of di-tri multifunctional monomers in an network crosslinked by polyacids (Activa technical profile). One aspect that cannot be ignored, despite the clear and encouraging evidence to support the use of antimicrobial materials, is the fact that for any agent to be effective in the long term, there needs to be substantial and prolonged activity at the surface, as well as in the bulk of the biofilm layer (Takenaka et al. 2008). Since the mechanism of action of QAMs necessarily involves contact with the bacterial cell surface, the reported effects in the bulk of the biofilm can be speculated to be due either to unreacted or degraded monomer leaching into the biofilm. If that is the case, toxicity concerns are raised.
One major point that relates to biofilm formation is that once the acquired pellicle is formed on the tooth or material surface, the contact kill mechanisms described so far are hampered, and bacterial adhesion is facilitated (S. Zhang et al. 2016). Oral microbes interact with surfaces coated with a glycoprotein-rich pellicle, to which early colonizers bind and then coadhere with other species (Paula et al. 2016). The growth and development of biofilms are regulated by the characteristics of oral surfaces, including energy, charge, chemical composition, mechanical properties, and hydrophilic character (Paula and Koo 2016). Negatively charged surfaces, highly hydrophobic or hydrophilic surfaces, and nanoscale surface roughness have all been investigated as a means to reduce bacterial attachment (Song et al. 2015). Since the bacterial cells are negatively charged, a normally negatively charged surface would repel bacterial and limit adhesion. However, some bacteria are remarkably resourceful and can adapt to better attach to negatively charged surfaces as well (Song et al. 2015). For this reason, it is advantageous to combine antibacterial and antifouling materials, with one example being 2-methacryloyloxyethyl phosphorylcholine, MPC (Zhang et al. 2015). Antifouling polymers are surface active and prevent bacterial attachment by disrupting the adsorption of proteins from the acquired pellicle (L. Zhang et al. 2016). The combination of MPCs with QAMs has shown a synergistic effect in inhibiting biofilm formation, without affecting materials’ mechanical properties (L. Zhang et al. 2016).
Remineralizing Materials
The rationale for the development of remineralizing dental materials is to replenish the lost mineral content from early disease to prevent cavitation of the lesion (L. Zhang et al. 2016). Calcium fluoride (nCaF2), amorphous calcium phosphate (nACP), and nanohydroxyapatite (nHA) and nanofluorohydroxyapatite (nFHA) nanoparticles have been widely studied as remineralizing agents, with studies predominantly focusing on repairing relatively small defects (Li et al. 2014). Nanoparticle characteristics such as surface charge, degree of hydrophobicity, ratio of surface area to biofilm mass, and the ability to adsorb or be collected on the surface of the biofilm can all be tailored (Melo et al. 2014). Importantly, it has been shown that the incorporation of acidic monomers, such as PMGDM (pyromellitic glycerol dimethacrylate), in a BisEMA resin positively affects the potential for calcium and phosphate ion recharge/release from the restoration to inhibit caries (L. Zhang et al. 2016). In addition, an enhancement in the remineralization effect provided by calcium phosphate (nACP) has been shown when it is used in combination with poly(amide amine) (PAMAM). PAMAM has been shown to be an excellent mineral nucleation template, and when combined with nACP, not only is remineralization favored, but demineralization is also hampered via the neutralization of biofilm acids by nACP (Liang et al. 2016).
Finally, attempts to combine QAMs with ACP mineral particles have also shown great promise in vitro and in vivo, as there seems to be a synergistic effect between the antibacterial and remineralizing agents in preventing caries initiation and progression (Cheng et al. 2015).
Future Developments
Stress-Reducing Materials
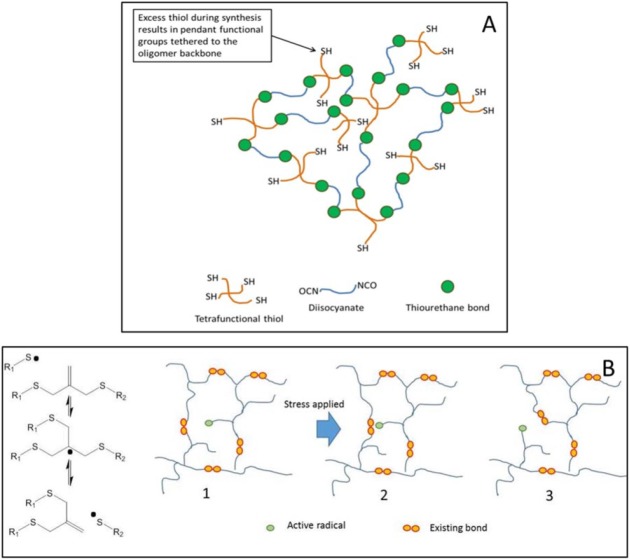
Stress-reducing materials continue to be the focus of investigations due to their utility in preventing gap formation at the tooth-restorative material interface. However, rather than concentrating on shrinkage reduction, most current strategies propose some type of modification to the polymer network that can simultaneously decrease stress and either maintain or enhance mechanical properties and monomer conversion. One example is the development of thiourethane oligomers (Bacchi and Pfeifer 2016). These molecules are synthesized under mild conditions through a click reaction and feature pendant thiol functionalities that are available to participate in chain transfer reactions with the secondary vinyl monomer matrix. In this sense, thiourethanes rely on the same stress reduction mechanism leading to delayed gelation/vitrification as seen in classic thiol-ene reactions (Boulden et al. 2011). At the same time, they provide network reinforcement stemming from the incorporation of tough thio-carbamate bonds into the network (Lowe et al. 2010). When used in highly filled composites, thiourethanes have been shown to reduce polymerization stress by up to 50%, while leading to a 2-fold increase in fracture toughness (Bacchi et al. 2016). Other nano-sized prepolymerized particles have also shown significant shrinkage/stress reduction and represent a highly tailorable platform for material modification (Moraes et al. 2011). Another example of a stress-reducing strategy is the use of monomers with addition-fragmentation chain-transfer capabilities, such as that used in the commercial bulk-fill material, Filtek Bulk Fill (3M ESPE). The multifunctional monomers in this product contain an allyl disulfide moiety (among other possible examples), which then becomes incorporated into the network as a crosslink (Fig. 2). That allyl bond can be broken and reformed as the radical propagates, while keeping the net crosslinking density essentially unchanged. As a result, polymerization stress can be accommodated and ultimately reduced, while maintaining mechanical properties comparable to traditional glassy networks (Park et al. 2012).
Figure 2.
Diagram for network modifications leading to stress reduction with the use of thiourethane oligomers (A) and covalent adaptable networks formed via addition-fragmentation chain transfer (B).
Degradation-Resistant Materials
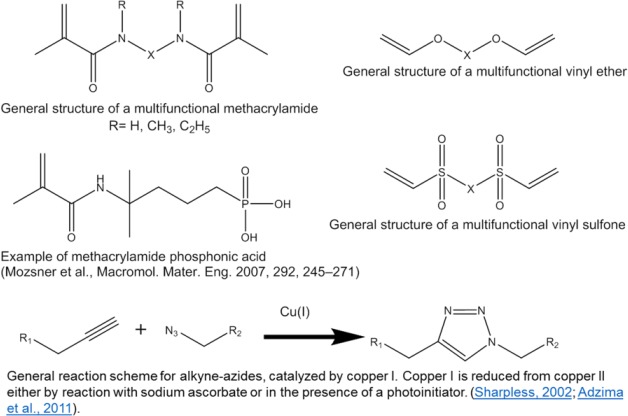
Despite the many changes to the organic matrix composition, dental composites are still polymerized via the vinyl bond of methacrylate monomers. This is due to many advantages associated with methacrylates, such as the ability to achieve degree of conversion values >60% to 70% in <1 min at room temperature using on-command photo-activated mechanisms, production of high Tg networks at room temperature, adequate mechanical properties, and highly esthetic restorations. One main disadvantage is that methacrylates are inherently hydrolyzable via their ester bonds, a process that can be accelerated at higher temperatures and low pH (Delaviz et al. 2014), which in turn are conditions consistent with those in the oral environment. In addition, incomplete conversion and water sorption/solubility decrease the stability of the polymer matrix and may, as some suggest, contribute to the less than optimal clinical lifetime (Delaviz et al. 2014). To address these shortcomings, materials departing from methacrylate chemistry have been proposed for use as restorative composites. These include methacrylamides, vinyl ethers, azide-alkyne, thiol-vinyl sulfone, and improved thiol-ene chemistry (Fig. 3). Methacrylamide monomers have been demonstrated to be more stable in aqueous environments, including acidic conditions (Moszner et al. 2001; Salz et al. 2010). Specifically for dental uses, they have been proposed as monofunctional monomers for adhesives (with 1 commercial example, Adhese One F [Ivoclar-Vivadent]) (Salz and Bock 2010) and as multifunctional monomers for composites. Several bisacrylamides have been evaluated as crosslinkers for dental composites with physical properties comparable to methacrylates and greater stability in an aqueous environment (Moszner et al. 2006). Vinyl ethers have also been shown to be very stable, even after enzymatic challenge, and novel multifunctional monomers have shown mechanical properties comparable to methacrylate controls (Gonzalez-Bonet et al. 2015). However, the homopolymerization of such monomers does not progress at room temperature, so they either require a postphotoactivation heat treatment or must be copolymerized with a methacrylate (Gonzalez-Bonet et al. 2015). Azide-alkyne click polymerizations are very efficient, copper-catalyzed reactions that produce polymer networks with high Tg and toughness at room temperature. These materials also present lower polymerization stress and can be photoactivated, albeit with reduced mechanical properties compared with methacrylate controls (Song et al. 2016). Potential pitfalls related to the copper catalyst (present in minute concentrations) include toxicity and discoloration. However, these materials are ester free and have the potential for very high stability in the oral environment. Finally, thiol vinyl sulfone polymerizations have been proposed. These additional materials can produce networks with degree of conversion approaching 80%, in part owing to the development of efficient photobase generator initiators and, despite containing thiols, Tg as high as 100°C (Podgórski et al. 2015). In addition, monomer structure can be tailored in these materials to eliminate degradable esters and produce networks that are highly solvent resistant (Podgórski et al. 2015).
Figure 3.
General molecular structure of materials being proposed as alternatives to methacrylates for dental applications.
Reinforcing and Self-Healing Materials
Self-healing materials are capable of restoring mechanical integrity after damage has occurred. The recovery is not always complete but allows for extension of the materials’ survival (Diesendruck et al. 2015). Self-healing materials are classified as either intrinsic or extrinsic, according to whether the reparative, highly reactive molecules are produced only when the damage occurs (intrinsic) or if they are somehow stored within the material (extrinsic) (Diesendruck et al. 2015). In the intrinsic self-healing materials, the healing occurs at the molecular level, and the bond-forming reactions begin when the reagents come within nanometer distances of each other, which makes it difficult for the healing of large gaps to occur. In addition, an external source of energy (light or heat) is required to impart the mobility needed to bring the reactants to within close proximity. Extrinsic self-healing materials usually rely on polymeric capsules loaded with the healing agent. In this situation, a polymerizable solution is encapsulated and the reactants are released when the capsules are physically damaged, for example, by the propagation of a crack within the matrix. An active catalyst, kept out of contact with the monomer, is also released by rupturing of a capsule and is responsible for crosslinking the polymer (Diesendruck et al. 2015) (Fig. 4). The first attempts to develop self-healing dental composites using the microcapsule approach resulted in materials showing significant fracture toughness recovery but equally significant biocompatibility concerns (Then et al. 2011). Several recent studies using microcapsules loaded with different healing agents have demonstrated at least 65% recovery of the virgin fracture toughness with minimal cytotoxicity. In at least 1 study, the self-healing efficacy was kept even after 6 mo of water storage, leading to the conclusion that the specific composition tested was promising for healing cracks, resisting fracture, and increasing the durability and longevity of dental restorations (Wu et al. 2016).
Figure 4.
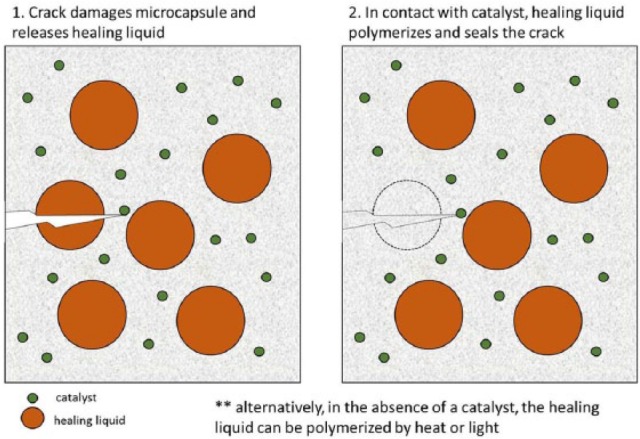
General scheme for the crack-healing mechanism using liquid-bearing microcapsules.
Conclusion
Ideally, directly placed esthetic dental restorations should 1) withstand occlusal loading, 2) be stable in the harsh oral environment, 3) minimize or prevent stress development and avoid gap formation, 4) prevent biofilm attachment/growth, 5) present remineralizing capabilities, 6) be able to self-repair, and 7) be easy to use. By any engineering measure, this is a very challenging list of prerequisites. To date, no material commercially available or under development is able to fulfill all of them. Resistance to wear and strength of composites in general have significantly increased over the years, and materials with low stress generation have been developed and commercialized. However, no currently available material has substantial antibacterial, remineralizing, or general bioactive/biomimetic capabilities. When all of these factors are considered in conjunction with the intrinsic susceptibility of methacrylate-based networks to undergo hydrolytically and enzymatically driven degradation, the relatively short life span of composite restorations is widely justified. Until regenerative therapies are not a reality for larger tooth defects, restorative materials will need to be improved. Most of the research in this field has shifted the focus to concentrate on biofilm and host interactions. The ultimate goal is to produce materials that are better able to integrate with the environment where they are applied and withstand the challenges imposed in the oral cavity. It is evident from the amount of research activity in the field of dental polymer chemistry that the organic phase of composite materials is likely to drastically change in the relatively near-term. Along with novel developments in filler systems and self-healing materials, this has the potential to shift the status quo of current materials used to directly restore diseased and damaged teeth.
Author Contributions
A.P.P. Fugolin, C.S. Pfeifer, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
C.S. Pfeifer acknowledges the National Institute of Dental and Craniofacial Research for funding (K02 DE025280, R01 DE026113, U01 DE023756, R15 DE023211).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bacchi A, Nelson M, Pfeifer CS. 2016. Characterization of methacrylate-based composites containing thio-urethane oligomers. Dent Mater. 32(2):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi A, Pfeifer CS. 2016. Rheological and mechanical properties and interfacial stress development of composite cements modified with thio-urethane oligomers. Dent Mater. 32(8):978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boaro LC, Gonçalves F, Guimarães TC, Ferracane JL, Versluis A, Braga RR. 2010. Polymerization stress, shrinkage and elastic modulus of current low-shrinkage restorative composites. Dent Mater. 26(12):1144–1150. [DOI] [PubMed] [Google Scholar]
- Boulden JE, Cramer NB, Schreck KM, Couch CL, Bracho-Troconis C, Stansbury JW, Bowman CN. 2011. Thiol-ene-methacrylate composites as dental restorative materials. Dent Mater. 27(3):267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Zhang K, Weir MD, Melo MA, Zhou X, Xu HH. 2015. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine (Lond). 10(4):627–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer NB, Stansbury JW, Bowman CN. 2011. Recent advances and developments in composite dental restorative materials. J Dent Res. 90(4):402–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaviz Y, Finer Y, Santerre JP. 2014. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 30(1):16–32. [DOI] [PubMed] [Google Scholar]
- Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJM. 2012. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 28(1):87–101. [DOI] [PubMed] [Google Scholar]
- Diesendruck CE, Sottos NR, Moore JS, White SR. 2015. Biomimetic self-healing. Angew Chem Int Ed Engl. 54(36):10428–10447. [DOI] [PubMed] [Google Scholar]
- Feng L, Suh BI, Shortall AC. 2010. Formation of gaps at the filler-resin interface induced by polymerization contraction stress: gaps at the interface. Dent Mater. 26(8):719–729. [DOI] [PubMed] [Google Scholar]
- Ferracane JL. 2011. Resin composite—state of the art. Dent Mater. 27(1):29–38. [DOI] [PubMed] [Google Scholar]
- Fronza BM, Rueggeberg FA, Braga RR, Mogilevych B, Soares LES, Martin AA, Ambrosano G, Giannini M. 2015. Monomer conversion, microhardness, internal marginal adaptation, and shrinkage stress of bulk-fill resin composites. Dent Mater. 31(12):1542–1551. [DOI] [PubMed] [Google Scholar]
- Ge Y, Wang S, Zhou X, Wang H, Xu HHK, Cheng L. 2015. The use of quaternary ammonium to combat dental caries. Materials (Basel). 8(6):3532–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bonet A, Kaufman G, Yang Y, Wong C, Jackson A, Huyang G, Bowen R, Sun J. 2015. Preparation of dental resins resistant to enzymatic and hydrolytic degradation in oral environments. Biomacromolecules. 16(10):3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Park SH, Hwang IN. 2015. Polymerization shrinkage and depth of cure of bulk-fill resin composites and highly filled flowable resin. Oper Dent. 40(2):172–180. [DOI] [PubMed] [Google Scholar]
- Kim RJ, Kim YJ, Choi NS, Lee IB. 2015. Polymerization shrinkage, modulus, and shrinkage stress related to tooth-restoration interfacial debonding in bulk-fill composites. J Dent. 43(4):430–439. [DOI] [PubMed] [Google Scholar]
- Kloxin CJ, Scott TF, Adzima BJ, Bowman CN. 2010. Covalent Adaptable Networks (CANs): a unique paradigm in crosslinked polymers. Macromolecules. 43(6):2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempel E, Tóth Á, Fábián T, Krajczár K, Szalma J. 2015. Retrospective evaluation of posterior direct composite restorations: 10-year findings. Dent Mater. 31(2):115–122. [DOI] [PubMed] [Google Scholar]
- Leprince JG, Palin WM, Vanacker J, Sabbagh J, Devaux J, Leloup G. 2014. Physico-mechanical characteristics of commercially available bulk-fill composites. J Dent. 42(8):993–1000. [DOI] [PubMed] [Google Scholar]
- Li F, Wang P, Weir MD, Fouad AF, Xu HHK. 2014. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater. 10(6):2804–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Weir MD, Xie X, Wang L, Reynolds MA, Li J, Xu HH. 2016. Dentin remineralization in acid challenge environment via PAMAM and calcium phosphate composite. Dent Mater. 32(11):1429–1440. [DOI] [PubMed] [Google Scholar]
- Lowe AB, Hoyle CE, Bowman CN. 2010. Thiol-yne click chemistry: a powerful and versatile methodology for materials synthesis. J Mater Chem. 20(23):4745–4750. [Google Scholar]
- Magno MB, Nascimento GCR, da Rocha YSP, d’Paula Gonçalves Ribeiro B, Loretto SC, Maia LC. 2016. Silorane-based composite resin restorations are not better than conventional composites—a meta-analysis of clinical studies. J Adhes Dent. 18(5):375–386. [DOI] [PubMed] [Google Scholar]
- Melo MA, Cheng L, Zhang K, Weir MD, Chow LC, Antonucci JM, Lin NJ, Lin-Gibson S, Xu HH. 2014. Combating dental caries via restorative materials containing antibacterial and remineralizing nanoparticles. Technical Proceedings of the 2014 NSTI Nanotechnology Conference and Expo, NSTI-Nanotech 2014 Nanotech 2014;2:289–292. [Google Scholar]
- Miletic V, Pongprueksa P, De Munck J, Brooks NR, Van Meerbeek B. 2017. Curing characteristics of flowable and sculptable bulk-fill composites. Clin Oral Investig. 21(4):1201–1212. [DOI] [PubMed] [Google Scholar]
- Moraes RR, Garcia JW, Barros MD, Lewis SH, Pfeifer CS, Liu J, Stansbury JW. 2011. Control of polymerization shrinkage and stress in nanogel-modified monomer and composite materials. Dent Mater. 27(6):509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moszner N, Fischer UK, Angermann J, Rheinberger V. 2006. Bis-(acrylamide)s as new cross-linkers for resin-based composite restoratives. Dent Mater. 22(12):1157–1162. [DOI] [PubMed] [Google Scholar]
- Moszner N, Salz U. 2001. New developments of polymeric dental composites. Prog Polym Sci (Oxford). 26(4):535–576. [Google Scholar]
- Moszner N, Zeuner F, Lamparth I, Fischer UK. 2009. Benzoylgermanium derivatives as novel visible-light photoinitiators for dental composites. Macromol Mater Eng. 294(12):877–886. [Google Scholar]
- Park HY, Kloxin CJ, Abuelyaman AS, Oxman JD, Bowman CN. 2012. Novel dental restorative materials having low polymerization shrinkage stress via stress relaxation by addition-fragmentation chain transfer. Dent Mater. 28(11):1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Kloxin CJ, Scott TF, Bowman CN. 2010. Stress relaxation by addition-fragmentation chain transfer in highly cross-linked thiol-yne networks. Macromolecules. 43(24):10188–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula AJ, Koo H. 2016. Nanosized building blocks for customizing novel antibiofilm approaches. J Dent Res. 96(2):128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgórski M, Becka E, Chatani S, Claudino M, Bowman CN. 2015. Ester-free thiol-X resins: new materials with enhanced mechanical behavior and solvent resistance. Polym Chem. 6(12):2234–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitevin A, De Munck J, Van Ende A, Suyama Y, Mine A, Peumans M, Van Meerbeek B. 2013. Bonding effectiveness of self-adhesive composites to dentin and enamel. Dent Mater. 29(2):221–230. [DOI] [PubMed] [Google Scholar]
- Rosatto CM, Bicalho AA, Verissimo C, Braganca GF, Rodrigues MP, Tantbirojn D, Versluis A, Soares CJ. 2015. Mechanical properties, shrinkage stress, cuspal strain and fracture resistance of molars restored with bulk-fill composites and incremental filling technique. J Dent. 43(12):1519–1528. [DOI] [PubMed] [Google Scholar]
- Salerno M, Derchi G, Thorat S, Ceseracciu L, Ruffilli R, Barone AC. 2011. Surface morphology and mechanical properties of new-generation flowable resin composites for dental restoration. Dent Mater. 27(12):1221–1228. [DOI] [PubMed] [Google Scholar]
- Salz U, Bock T. 2010. Adhesion performance of new hydrolytically stable one-component self-etching enamel/dentin adhesives. J Adhes Dent. 12(1):7–10. [DOI] [PubMed] [Google Scholar]
- Sauro S, Osorio R, Osorio E, Watson TF, Toledano M. 2013. Novel light-curable materials containing experimental bioactive micro-fillers remineralise mineral-depleted bonded-dentine interfaces. J Biomater Sci Polym Ed. 24(8):940–956. [DOI] [PubMed] [Google Scholar]
- Sauro S, Pashley DH. 2016. Strategies to stabilise dentine-bonded interfaces through remineralising operative approaches—state of the art. Int J Adhes Adhes. 69:39–57. [Google Scholar]
- Schuldt C, Birlbauer S, Pitchika V, Crispin A, Hickel R, Ilie N, Kuhnisch J. 2015. Shear bond strength and microleakage of a new self-etching/self-adhesive pit and fissure sealant. J Adhes Dent. 17(6):491–497. [DOI] [PubMed] [Google Scholar]
- Scotti N, Comba A, Gambino A, Manzon E, Breschi L, Paolino D, Pasqualini D, Berutti E. 2016. Influence of operator experience on non-carious cervical lesion restorations: clinical evaluation with different adhesive systems. Am J Dent. 29(1):33–38. [PubMed] [Google Scholar]
- Shafiei F, Saadat M. 2016. Micromorphology and bond strength evaluation of adhesive interface of a self-adhering flowable composite resin-dentin: effect of surface treatment. Microsc Res Tech. 79(5):403–407. [DOI] [PubMed] [Google Scholar]
- Son SA, Park JK, Seo DG, Ko CC, Kwon YH. 2017. How light attenuation and filler content affect the microhardness and polymerization shrinkage and translucency of bulk-fill composites? Clin Oral Investig. 21(2):559–565. [DOI] [PubMed] [Google Scholar]
- Song F, Koo H, Ren D. 2015. Effects of material properties on bacterial adhesion and biofilm formation. J Dent Res. 94(8):1027–1034. [DOI] [PubMed] [Google Scholar]
- Song HB, Sowan N, Shah PK, Baranek A, Flores A, Stansbury JW, Bowman CN. 2016. Reduced shrinkage stress via photo-initiated copper(I)-catalyzed cycloaddition polymerizations of azide-alkyne resins. Dent Mater. 32(11):1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka S, Trivedi HM, Corbin A, Pitts B, Stewart PS. 2008. Direct visualization of spatial and temporal patterns of antimicrobial action within model oral biofilms. Appl Environ Microbiol. 74(6):1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then S, Neon GS, Abu Kasim NH. 2011. Performance of melamine modified urea-formaldehyde microcapsules in a dental host material. J Appl Polym Sci. 122(4):2557–2562. [Google Scholar]
- Tomaszewska IM, Kearns JO, Ilie N, Fleming GJP. 2015. Bulk fill restoratives: to cap or not to cap—that is the question? J Dent. 43(3):309–316. [DOI] [PubMed] [Google Scholar]
- Van Dijken JW, Pallesen U. 2016. Posterior bulk-filled resin composite restorations: a 5-year randomized controlled clinical study. J Dent. 51:29–35. [DOI] [PubMed] [Google Scholar]
- Van Ende A, Lise DP, De Munck J, Vanhulst J, Wevers M, Van Meerbeek B. 2017. Strain development in bulk-filled cavities of different depths characterized using a non-destructive acoustic emission approach. Dent Mater. 33(4):e165–e177. [DOI] [PubMed] [Google Scholar]
- Vieira-Filho WS, Alonso RC, González AH, D’Alpino PH, Di Hipólito V. 2017. Bond strength and chemical interaction of self-adhesive resin cements according to the dentin region. Int J Adhes Adhes. 73:22–27. [Google Scholar]
- Wu J, Weir MD, Melo MA, Xu HH. 2015. Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. J Dent. 43(3):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Weir MD, Melo MAS, Strassler HE, Xu HHK. 2016. Effects of water-aging on self-healing dental composite containing microcapsules. J Dent. 47:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaoming J, Louis B, Qizhou D, O’Connor MT, Hammesfahr PD, Koltisko B. Inventors. 2015. DENTSPLY INTERNATIONAL INC., assignee. Low stress flowable dental composition. United States patent. [accessed 2017 Jun 21]. http://europepmc.org/patents/PAT/US2011315928
- Yuan H, Li M, Guo B, Gao Y, Liu H, Li J. 2015. Evaluation of microtensile bond strength and microleakage of a self-adhering flowable composite. J Adhes Dent. 17(6):535–543. [DOI] [PubMed] [Google Scholar]
- Zhang K, Cheng L, Weir MD, Bai YX, Xu HH. 2016. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int J Oral Sci. 8(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Weir MD, Chow LC, Antonucci JM, Chen J, Xu HH. 2016. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater. 32(2):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Chen C, Melo MA, Bai YX, Cheng L, Xu HH. 2015. A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int J Oral Sci. 7(2):103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, He L, Yang Y, Yang B, Liao Y, Xu X, Li J, Yang X, Li J. 2016. Effective: In situ repair and bacteriostatic material of tooth enamel based on salivary acquired pellicle inspired oligomeric procyanidins. Polym Chem. 7(44):6761–6769. [Google Scholar]