Abstract
Study Design:
Retrospective consecutive case series.
Objective:
The objective of this case series was to demonstrate the safety of a modified transfacet pedicle–sparing decompression and instrumented fusion in patients with thoracic disc herniations (TDHs).
Methods:
Consecutive patients undergoing operative management of TDH from July 2007 to December 2011 using a posterior unilateral modified transfacet pedicle–sparing approach were identified. All patients underwent open or minimally invasive modified transfacet pedicle–sparing discectomy and segmental instrumentation with interbody fusion, performed by four different surgeons. Pre- and postoperative visual analog scale (VAS) pain scores, Nurick grade, and American Spinal Injury Association Impairment Scale (AIS) were analyzed from a retrospective chart review. Estimated blood loss and complications were also obtained.
Results:
Fifty-one patients were included that had operations for TDH. Thirty-nine patients had single level decompression and 12 had multilevel decompression. The total number of levels operated on was 64. Five patients were treated with minimally invasive surgery. A herniated disc level of T11-12 (n = 17) was treated most often. One major complication of epidural hematoma occurred. Minor complications such as malpositioned hardware, postoperative hematoma, wound infection, pseudoarthrosis, and pulmonary complications occurred in a few patients. Follow-up ranged from 1 to 46 months with 1 patient lost to follow-up. From preoperative to final postoperative: mean VAS scores improved from 8.31 to 4.05, AIS in all patients remained stable or improved, and Nurick scores improved from 3 to 2.6 on average. No intraoperative or permanent neurological deficit occurred.
Conclusion:
In our surgical series, 51 consecutive patients underwent modified transfacet pedicle–sparing approach to TDHs and experienced improvement of functional status as well as improvement of objective pain scales with no neurological complications. The posterior unilateral modified transfacet pedicle–sparing decompression and instrumented fusion approach to the thoracic spine is a safe and reproducible procedure for the treatment of TDHs.
Keywords: thoracic disc herniation, transfacet approach, thoracic discectomy, thoracic myelopathy, thoracic radiculopathy
Introduction
Symptomatic thoracic disc herniations (TDHs) are a relatively uncommon yet challenging condition to treat. The annual incidence of TDH is reported at approximately 1 per million patients.1 TDH tends to affect men more than women, with a peak age for both sexes at 40 to 50 years.2,3 The majority of TDHs occur in the lower thoracic spine, with the most common level reported to be T11-12.2 While the mechanism of TDH is not completely understood, several researchers postulate that the relative weakness of the posterior longitudinal ligament and the higher mobility of the lower thoracic spine are involved.4,5
Treatment of TDH can be technically challenging due to the unique anatomy of the thoracic spine. The thoracic spinal cord occupies a greater portion of the spinal canal creating an increased risk for damage from disc herniations or intraoperative manipulation.4,6,7 Historically, a posterior approach utilizing conventional laminectomy was the surgical treatment of choice. This approach yielded poor success rates with high morbidity and mortality, resulting in postoperative neurological deficits.2,4,8 In subsequent years, several alternative approaches have been reported with varying degrees of success. Among these techniques, the transthoracic approach has the highest rate of success, especially for treatment of central and paracentral TDH.5,9–13 The transthoracic approach is not appropriate for patients with significant comorbidities, such as pulmonary or cardiac disease due to the invasiveness and consequent difficult recovery. Recovery from the transthoracic approach can include chest tube placement, intensive monitoring, and subsequent increased risk for deep venous thrombosis, atelectasis, and pneumonia. As a result of the anterior technique, the lungs, heart, great vessels, and the artery of Adamkiewicz are at risk.14 In addition, the procedure can be technically challenging as the surgical corridor is generally limited in size.
Several other surgical approaches have been described, such as posterolateral variants, lateral extracavitary, costotransversectomy, thorascopic and microendoscopic keyhole discectomy. In 1995, Stillerman et al15 proposed a cadaveric morphometric analysis and preliminary clinical experience of a transfacet pedicle–sparing approach for TDHs in 15 patients. This posterior approach, the transfacet pedicle–sparing technique, was then reported with good results in the same year. However, it provided suboptimal exposure and its efficacy on central TDH remained limited.16 In 2010, a modified transfacet pedicle–sparing decompression and instrumented fusion technique was described, which exhibited improved exposure allowing for better removal of central TDHs. Results were inconclusive as the sample size was relatively small, with only 18 patients in the treated group.17 In the present outcome study of 51 patients, a modified unilateral transfacet pedicle–sparing approach using open or minimally invasive techniques was utilized for discectomy and fusion. This modified technique allows unilateral circumferential decompression of the spinal cord and instrumented stabilization of the segment(s) during surgery.
The fundamental questions to answer for any surgical technique include: Is it reproducible? Is it safe?, and Does it achieve the surgical goal of improving patient outcomes and quality of life? With our retrospective look at this series of cases, we add to the literature on the safety, reproducibility, and outcomes of the modified transfacet pedicle–sparing decompression and instrumented fusion as an approach to thoracic discectomy and fusion.
Materials and Methods
Patient Population
After approval by the institutional review board, retrospective chart reviews of the Michigan Spine and Brain Surgeons clinic database and the St John Providence Hospital medical records search were performed to identify patients with TDHs, who underwent surgical treatment, in the form of discectomy and fusion, between July 2007 and December 2011. Fifty-one consecutive patients were identified from these chart reviews. Details of patient clinic visits, hospital records, and operative records from St John Providence in Southfield, Michigan, and St John Providence Park in Novi, Michigan were then reviewed.
Imaging
Diagnosis of TDH was made by means of MRI or computed tomographic (CT) myelography. Herniated discs were classified as either central or paracentral as previously described in the literature.6 Calcified discs were noted on preoperative CT scans or radiographs. Furthermore, MRIs and/or CT myelograms were used to identify the precise level of the herniated disc.
Postoperative radiographs were obtained within 24 hours of surgery. CT scans were obtained during hospitalization only if needed to assess the accuracy of instrumentation and the graft position. Upright radiographs were obtained 1 month postoperatively in clinic follow up, to assess for baseline alignment followed by routine flexion/extension radiographs at subsequent clinic visits to confirm maintenance of alignment, healing, and evolution of fusion at the surgical level.
Implant Materials
Segmental fixation was performed using K2 M Denali (K2 M, Inc, Leesburg, VA), Stryker Xia (Stryker, Kalamazoo, MI), or Medtronic Legacy (Medtronic, Minneapolis, MN). If surgery was carried out with percutaneous technique, Synthes Viper (Depuy-Synthes, West Chester, PA), Medtronic Longitude (Medtronic, Minneapolis, MN) or Medtronic Sextant (Medtronic, Minneapolis, MN) was used depending on the location of desired fixation. Interbody arthrodesis was accomplished with machined freeze-dried allograft interbody fusion cage by Synthes (Depuy-Synthes, West Chester, PA) or machined freeze-dried polyetheretherketone (PEEK) cage by K2 M (K2 M, Inc, Leesburg, VA). Cages were packed with local morselized bone graft, allograft cancellous chips and/or Medtronic Infuse bone morphogenic protein (BMP; Medtronic, Minneapolis, MN). In general, at least half of the material was composed of morselized local bone graft.
Surgical Technique
Surgery was performed by 4 different surgeons from Michigan Spine and Brain Surgeons (Southfield, MI) between July 2007 and December 2011. Open techniques were utilized for thoracic discectomy and fusion in 46 patients. Minimally invasive techniques were utilized in 5 cases. A summary description of the surgical technique is presented below. A detailed description of the surgical technique has been previously described by Bransford et al.17 In contrast to Bransford et al, however, we chose to perform unilateral discectomy as opposed to bilateral discectomy.
Surgery was performed with patients in a prone position on a Jackson table (Mizuho/OSI, Union City, CA) with monitoring of somatosensory-evoked potentials (SSEPs) and motor-evoked potentials (MEPs). Prepositioning SSEPs were established as baseline and were rechecked once patients were positioned on the Jackson table. Standard dissection and exposure was performed for the indicated thoracic levels with fluoroscopic guidance.
Pedicle screw instrumentation was performed at the designated vertebral levels bilaterally in a standard fashion. Autologous bone graft was harvested throughout the case as a central laminectomy was performed using the Medtronic Midas Rex high-speed drill (Medtronic, Minneapolis, MN) with any remaining ligament and bone at the medial aspect of the facets was removed in a piecemeal fashion with Kerrison rongeurs and curettes. (Figure 1A and B). This allowed for a wide central decompression at the designated level.
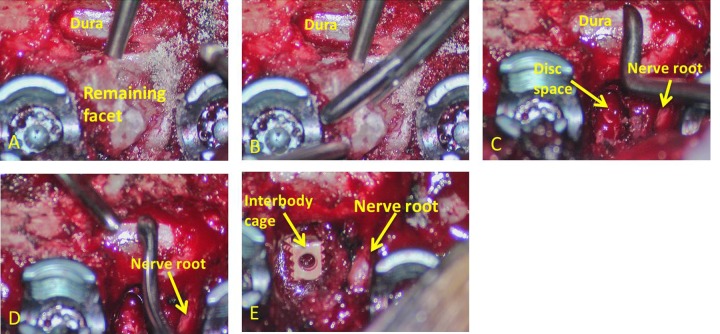
Figure 1.
Intraoperative photos illustrating the extent of osteotomies, facetectomies, and discectomy with interbody cage placement. (A) After placement of pedicles screws and the laminectomy is complete, dura of the spinal cord is visualized. Subsequently, with use of the Midas Rex drill, Kerrison rongeurs, and curettes, a complete unilateral facetectomy is performed. (B) Decompressive boundaries are denoted by the inferior edge of the pedicle above and the superior edge of the pedicle below. (C) The disc space and exiting nerve root are identified, and a discectomy is performed with use of down-going, angled curettes and shavers. (D) The length of the curette and its ability to reach underneath to the thecal sac allows for circumferential decompression. (E) Once complete removal of the disc is achieved, the cavity is packed with autologous bone, demineralized bone matrix or bone morphogenic protein, followed by insertion of a cage, packed with local bone graft.
After a wide central decompression, unilateral facet osteotomies were performed in a piecemeal fashion with a high-speed Midas Rex M8 drill, Kerrison rongeur and Codman curettes. This allowed for exposure and visualization of the exiting nerve root at that level (Figure 1C). Posterior foraminal decompression was declared complete with exposure of the superior pedicle wall of the caudal level and the inferior pedicle of the rostral level denoted by pedicle screws above and below (Figure 1A and B). From an oblique angle the exiting nerve root and thecal sac were identified and the designated disk was visualized. Using a series of endplate shavers, curettes and rongeurs a unilateral discectomy was carried out (Figure 1C and D). Next, a series of custom made, progressively longer, downgoing curettes (K2 M, Inc, Leesburg, VA) were used to achieve removal of the herniated portion of the disc (Figure 2). Use of the angled downgoing curettes allows the herniated portion of the disc to be pushed down into the cavity created during initial discectomy. With this technique and the use of custom curettes we are able to achieve a circumferential decompression and removal of paracentral and central disc fragments from a unilateral approach (Figure 1C and D and Figure 2).
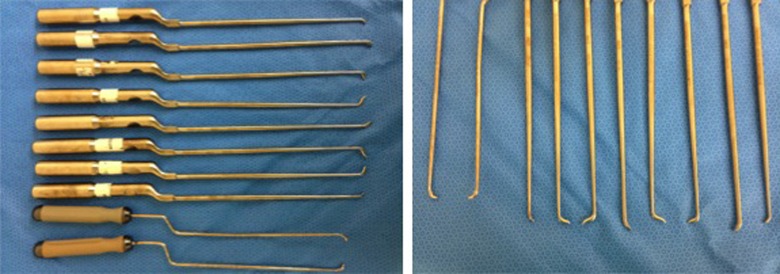
Figure 2.
Intraoperative photographs illustrating customized long, downgoing, and various angled curettes to achieve a unilateral decompression. With the use of progressively longer down-going angled curettes one can work across the entire anterior aspect of the thecal sac, removing the remainder of the designated disc.
Because of the amount of bony decompression and discectomy performed, a segmental fusion was performed to prevent instability and postoperative back pain. The disc space was prepared with intervertebral shavers. Cartilage was removed from the superior and inferior endplates until bleeding from the subchondral bone was noted. Next the bone obtained from the posterior laminectomy and ipsilateral facetectomy along with demineralized bone matrix was packed into the anterior disc space. The interbody cage was then packed with morselized local bone graft and at times with BMP and inserted under fluoroscopic guidance with distraction across the disc space (Figure 1E). Final construct was then secured using 6-mm titanium rods bilaterally.
Postoperative Care
All patients were mobilized early, beginning on the first postoperative day, and received postoperative antibiotic therapy for 24 hours postoperative, unless the patient had a drain, in which case intravenous antibiotics were continued for 48 hours or until the drain was removed. Each patient underwent an anteroposterior and lateral radiography within 24 hours of the procedure to evaluate instrumentation. Immediately postoperatively and during subsequent follow-up visits, visual analog scale (VAS) was recorded in hospital and office chart records. During subsequent visits to the clinic, patients obtained flexion-extension radiographs to evaluate for fusion across the surgical segment(s). If there was radiolucency noted surrounding the screws, or motion across the instrumented level, a CT scan was obtained to further evaluate for pseudoarthrosis. Nurick grades and American Spinal Injury Association Impairment Scale (AIS) scores were recorded during clinic visits with the surgeons. VAS and Nurick grades were analyzed with paired 2-tailed T tests (Microsoft Excel).
Results
Fifty-one patients with symptomatic TDHs were treated via an open or minimally invasive, modified transfacet pedicle–sparing decompression and fusion technique. The average patient age was 60 years (range 39-85 years) and 31 of 51 patients were female. The average body mass index was 35.8 and 31.9 kg/m2 for males and females, respectively. Sixteen patients had thoracic myelopathy with signal cord changes present on MRI imaging. Eighteen patients complained of radicular symptoms. Eight patients had myelopathy as well as radiculopathy. Ten patients had intractable back pain referable to the thoracic disc based on clinical evaluation. Twenty-one patients had TDH at the apex of the thoracic curve.
Thirty-nine patients had 1 TDH. Of the 12 remaining patients, 10 had 2 TDHs, 1 patient had 3 TDHs, and 1 patient had 4 TDHs. Herniated discs were found at the following levels: T1-2 (n = 1), T2-3 (n = 0), T3-4 (n = 1), T4-5 (n = 1), T5-6 (n = 1), T6-7 (n = 10), T7-8 (n = 8), T8-9 (n = 5), T9-10 (n = 5), T10-11 (n = 12), T11-12 (n = 17), T12-L1 (n = 5). Twenty-five patients had paracentral herniations, 2 of which were calcified; 21 patients had central herniations, 4 of which were calcified, and 5 patients were classified as having evidence of both types of herniation, with no evidence of calcification (Figure 3).
Figure 3.
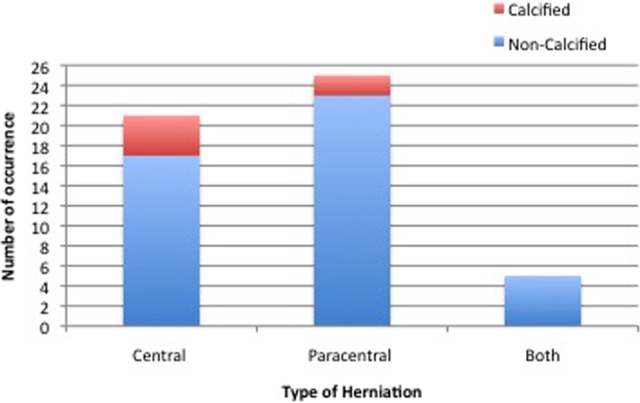
Disc herniation types classified as central, paracentral, or both and whether the disc was calcified.
Estimated blood loss was available from operative notes in 32 of 51 operations. Average blood loss was 770 mL (range 25-2000 mL). Neurophysiological status was monitored via SSEP and MEP testing intraoperatively with no deterioration in signals noted. The average length of stay was 6 days (range 3-15 days), with 4 patients staying longer than 10 days.
Complications
There was no permanent postoperative nerve injury, paralysis, or pneumothorax. The most significant complication occurred in a 62-year-old male who had a postoperative epidural hematoma with significant cord compression. He underwent an immediate exploration and evacuation of hematoma and had a successful recovery with no neurological deficit.
Forty-seven patients showed no signs of instrument migration or failure and went on to achieve radiographic fusion. Three patients were noted to have suboptimal hardware or graft placement with no complications. One patient had a migrated bone fragment into the T10-T11 foramen. This was treated conservatively without neurological injury. Another patient was noted to have anterior migration of a T6-T7 interbody cage into the mediastinum. The patient had relief of preoperative symptoms and went on to successful fusion without reoperation. The third patient had a long screw placed at T9 during fusion. One year after surgery, the patient underwent hardware removal due to painful hardware. During attempts to remove the T9 screw, pulsatile bleeding was encountered and the screw was quickly replaced to tamponade the bleeding. Postoperatively, the patient was placed in the intensive care unit, and vascular surgery was consulted. Vascular surgery recommended observation for several days. During his hospitalization, there was no vascular complication including no arterial thrombus and no decrease in hemoglobin. The patient was discharged and on follow-up had no vascular complications. One patient was noted to have pseudoarthrosis on follow-up imaging in the office and underwent revision surgery.
Postoperative wound infections occurred in 2 patients. These required simple incision and drainage. Two patients suffered from acute respiratory failure due to aspiration pneumonia and underwent a prolonged stay in the intensive care unit with a normal recovery. One patient developed a pulmonary embolism.
Overall, no neurological deficits occurred during this approach. One major complication of postoperative hematoma occurred (1.9%). Other minor complications included 1 screw malposition (1.9%), 1 cage malposition (1.9%), 1 allograft malposition (1.9%), 1 pseudoarthrosis (1.9%), 2 superficial wound infections requiring incision and drainage (3.9%), and 3 pulmonary complications (5.8%).
Clinical Outcomes
Fifty of 51 patients were available for follow-up. The average length of follow-up was 14 months (range 1-46 months). One patient was lost to follow-up for unknown reasons despite a concerted effort to contact the patient.
Forty-three patients reported improvement of pain by VAS score, 5 patients reported the same VAS score, and 3 patients reported worse pain via VAS. When averaged, VAS scores improved from 8.31 to 4.05 (P < .01), preoperative to final follow-up.
Functional status was evaluated with the Nurick grade. Twenty-six patients had stable Nurick grade from pre- to postoperative. With the exception of 1 patient, the rest of the patients experienced improvement of 1, 2, or 3 Nurick grade. One patient had a decline of 1 Nurick grade. On average, Nurick grade improved from 3 to 2.6 (P < .01) (Table 1).
Table 1.
Nurick Grade: Individual Patients.a
| Postoperative | |||||||
|---|---|---|---|---|---|---|---|
| 5 | 4 | 3 | 2 | 1 | 0 | ||
| Preoperative | 5 | 2 | 4 | 0 | 1 | 0 | 0 |
| 4 | 0 | 6 | 10 | 2 | 1 | 0 | |
| 3 | 0 | 1 | 6 | 4 | 1 | 2 | |
| 2 | 0 | 0 | 0 | 4 | 1 | 0 | |
| 1 | 0 | 0 | 0 | 0 | 5 | 0 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
aNurick grade is a 6-point system (0-5) assessing the “difficulty in walking“ with worsening in ascending order. Patients in blue remained stable in their Nurick grade. Patients in green improved 1 or more grades. Only 1 patient had an increase in grade.
No deterioration in neurological status as measured by the AIS at the time of the surgery and following completion of the surgical procedure was observed. AIS scores improved 1 level in 14 patients, and improved 2 levels in 5 patients. All other patients had stable AIS scores from pre- to postoperative (Table 2).
Table 2.
ASIA Impairment Scale: Individual Patients.a
| Postoperative | ||||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| Preoperative | A | 0 | 0 | 0 | 0 | 0 |
| B | 0 | 0 | 1 | 0 | 0 | |
| C | 0 | 0 | 2 | 8 | 4 | |
| D | 0 | 0 | 0 | 22 | 6 | |
| E | 0 | 0 | 0 | 0 | 8 | |
aPre- and postoperative evaluation of given score results, where the American Spinal Injury Association (ASIA) Impairment Scale pertains to assessment of at least 10 muscle groups on each side of body, proprioception and position sense graded A-E. Blue shading indicates stability of AIS scale pre- and postoperation. Green shading indicates improvement of AIS scale from pre- to postoperation.
Discussion
While TDHs are rare in comparison with cervical or lumbar, consensus opinion on surgical approaches to thoracic discectomy is elusive among spine surgeons. The patients in our case series represent a similar pattern of disc herniation as previously described in the literature with respect to demographics, level of disc herniation, and contiguity of disc herniations.1,17–19 Historically, posterior approaches for the decompression of thoracic disc herniations via laminectomy yielded less than ideal results, with reports of increased paresis or paralysis in 24% of patients by Love and Keifer,10 and 27% by Logue.8 Subsequently these results led to the development of transthoracic approaches as the mainstay in the management of central and paracentral TDHs.17 The transthoracic approach inherently puts the patient at increased risk of certain cardiothoracic complications including atelectasis, lung contusion, intercostal neuralgia, pneumothorax, and hemothorax.8,10,11,17,20 The anterior approach is not a viable option in many patients with significant co-morbidities due to the increased surgical risk. In recent years, the literature has described several posterior and minimally invasive techniques to perform a thoracic discectomy. Some approaches include thorascopic anterior approach, mini-open thoracotomy,21 transfacet pedicle–sparing,22 transpedicular,23,24,25 modified costotransversectomy,26 and microendoscopic keyhole discectomy. Many of these techniques are still being explored. Our series of 51 patients demonstrates the technical feasibility and safety of performing a posterior unilateral modified transfacet pedicle–sparing discectomy and instrumented fusion in the thoracic spine to achieve circumferential decompression and fusion.
In this series of consecutive patients, there were no instances of postoperative neurological deficit. The complications noted during in this series are potential complications of any spinal surgery, including postoperative hematoma, infection, postoperative respiratory failure, and implant or allograft malposition. One major epidural bleeding event was successfully evacuated with a return to neurological baseline. The initial report on TDHs through a modified pedicle–sparing approach by Bransford et al17 listed complications in 6 patients with 7 total complications, 5 postoperative wound infections, 1 misplaced hardware, and 1 transient neurological deficit related to a fracture 20 days after surgery, with all patients experiencing a normal recovery.17 The most important finding in our review is that no patient experienced an intraoperative neurological deficit as a result of the surgical approach or fusion. With experience, this approach provides a significant size operative corridor to allow circumferential discectomy and safe insertion of interbody grafts, while limiting the morbidity and neurological injury.
Describing each disc herniation within a series of 51, patients can be difficult to put into words. This series provides a range of paracentral and central disc herniations, both calcified and not calcified to show the range of this technique (Figure 3). An illustrative case involves a 48-year old woman with thoracic myelopathy and a TDH at T6-7 (Figures 4 -6). Postoperative CT imaging shows decompression of large central calcified disc hernation through the posterior unilateral modified transfacet pedicle–sparing decompression and instrumented fusion (Figures 7 and 8). Postoperative MRI was obscured by metal artifact. More important, this patient’s AIS score improved from C to D and Nurick score from 3 to 1.
Figure 4.
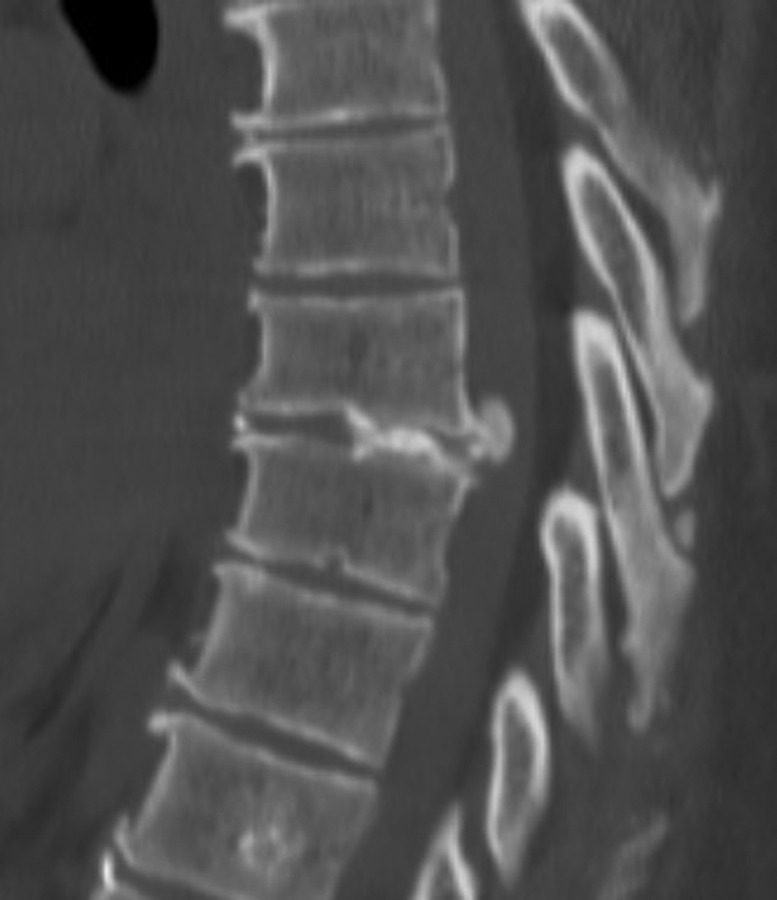
Sagittal preoperative computed tomography scane of T6-7 large central calcified disc herniation causing thoracic myelopathy.
Figure 5.
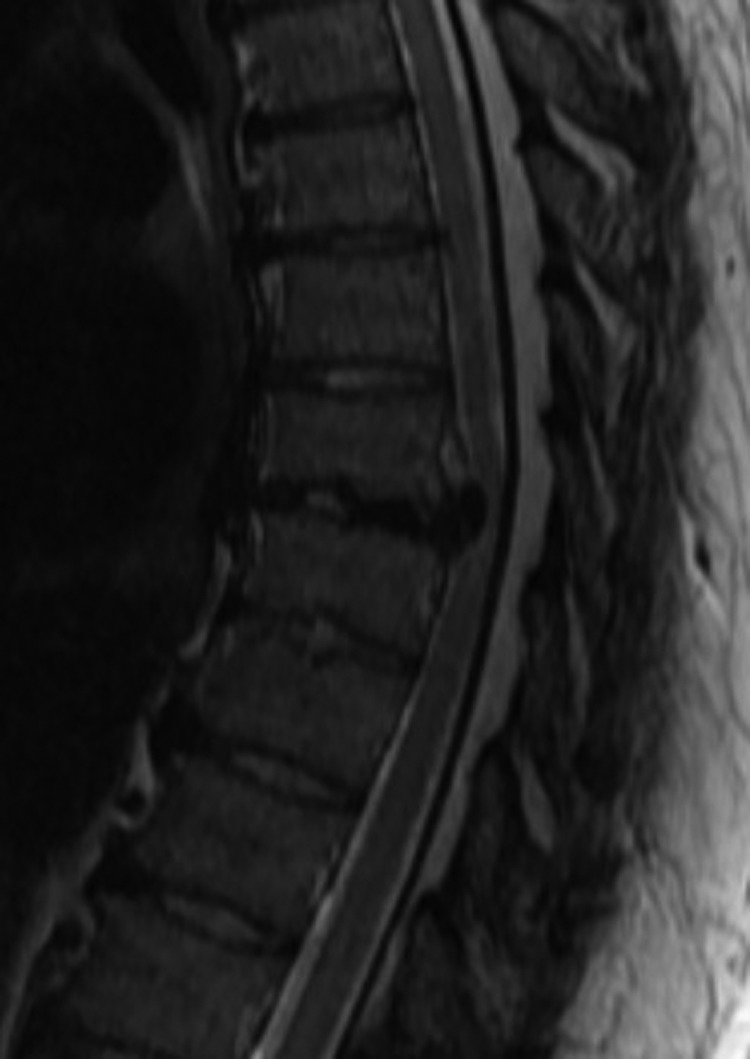
Sagittal preoperative magnetic resonance imaging of T6-7 herniation with cord compression.
Figure 6.
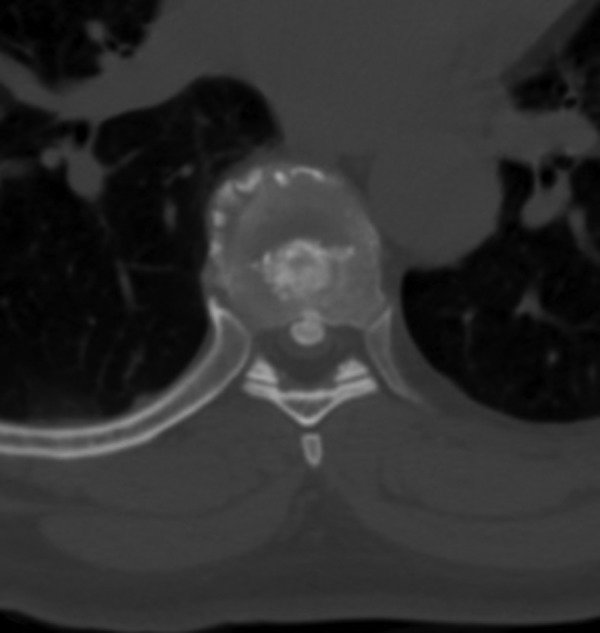
Axial imaging of T6-7 central calcified disc.
Figure 7.
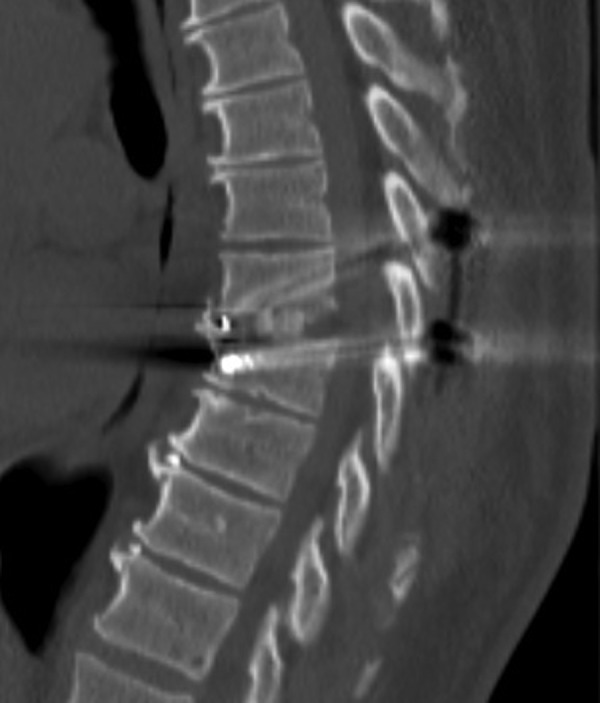
Sagittal postoperative imaging of calcified disc removal and placement of interbody cage and segmental instrumentation.
Figure 8.
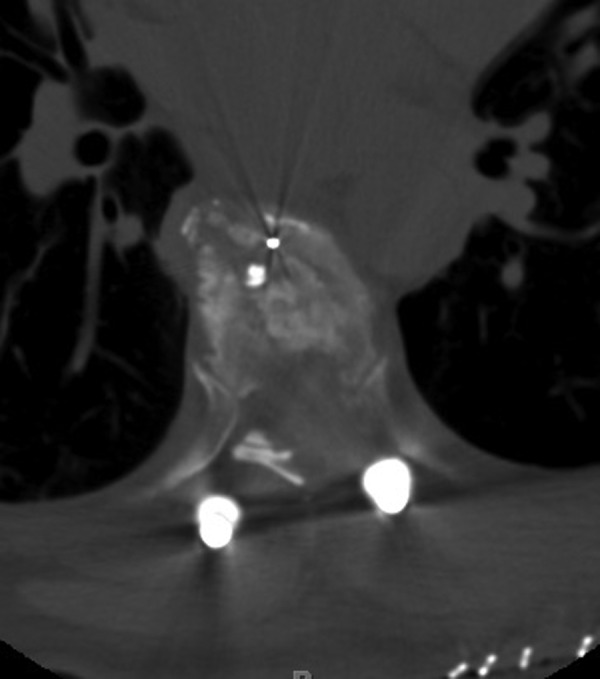
Axial postoperative imaging of illustrative case.
As time passes, the minimally invasive approach has become more utilized in spinal surgery. The modified posterior unilateral transfacet pedicle–sparing discectomy approach can be performed in a minimally invasive fashion using tubular retractors. In this series of patients, minimally invasive techniques were used in 5 cases to perform the discectomy. Benefits of the minimally invasive route specific to TDH include ability to leave posterior tension band intact, limited muscle dissection, and limited blood loss. A larger series can provide a more robust representation of the safety and feasibility of the minimally invasive technique. Data collection for a minimally invasive cohort is underway.
This study is limited by several factors. This study analyzes information in a retrospective rather than a prospective fashion. However given that the pathology of TDH requiring operation is rare, it was difficult to amass many patients in a prospective fashion. Going forward, collection of data in a prospective fashion and the pursuit of minimally invasive modified transfacet pedicle–sparing decompression and instrumented fusion approach will lead to a more robust and complete analysis of this technique. Despite the retrospective nature of this report, the goal of describing feasibility and safety is confirmed with this series. Another limitation of this study is inherent in the nature of a case series. Collecting patients in a consecutive series allows for significant variance in data. It is possible that the rate of complications such as infection may be higher or lower in the overall population than the consecutive patients selected in this series.
The patients in our study who underwent unilateral modified transfacet pedicle–sparing approach had improved pain control as well as remained stable or improved functionally based on data provided by VAS, AIS, and Nurick scores. This data shows that a unilateral modified transfacet pedicle–sparing approach can be performed safely and is reproducible, with surgery being conducted by 4 different surgeons in the same practice and institution. To date, this is one of the largest case series and overview of patient outcomes after being treated for TDHs, via the modified transfacet pedicle–sparing approach.17,18 A modified unilateral transfacet pedicle–sparing decompression and instrumented fusion is a reasonable surgical technique that should be considered when treating thoracic disc pathology due to the safety described by this retrospective study.
Conclusion
TDHs, while a rare disease process, present a unique challenge to the spine surgeon. It is a pathology that often requires treatment to prevent progression of myelopathy, intractable pain, and disability. Several surgical corridors have been proposed to address this pathology. Based on the results presented here, we believe that a posterior unilateral modified transfacet pedicle–sparing approach to TDHs with segmental instrumentation and interbody fusion is a safe and reproducible technique in achieving circumferential decompression, segmental stabilization, and fusion.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Carson J, Gumpert J, Jefferson A. Diagnosis and treatment of thoracic intervertebral disc protrusions. J Neurol Neurosurg Psychiatry. 1971;34:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angevine PD, McCormick PC. Thoracic disc. J Neurosurg Spine. 2012;16:261–262; discussion 262-263. [DOI] [PubMed] [Google Scholar]
- 3. Mulier S, Debois V. Thoracic disc herniations: transthoracic, lateral, or posterolateral approach? A review. Surg Neurol. 1998;49:599–606; discussion 606-598. [DOI] [PubMed] [Google Scholar]
- 4. Dickman CA, Rosenthal D, Regan JJ. Reoperation for herniated thoracic discs. J Neurosurg. 1999;91:157–162. [DOI] [PubMed] [Google Scholar]
- 5. Melissano G, Bertoglio L, Civelli V, et al. Demonstration of the Adamkiewicz artery by multidetector computed tomography angiography analysed with the open-source software OsiriX. Eur J Vasc Endovasc Surg. 2009;37:395–400. [DOI] [PubMed] [Google Scholar]
- 6. el-Kalliny M, Tew JM, Jr, van Loveren H, Dunsker S. Surgical approaches to thoracic disc herniations. Acta Neurochir (Wien). 1991;111:22–32. [DOI] [PubMed] [Google Scholar]
- 7. Murthy NS, Maus TP, Behrns CL. Intraforaminal location of the great anterior radiculomedullary artery (artery of Adamkiewicz): a retrospective review. Pain Med. 2010;11:1756–1764. [DOI] [PubMed] [Google Scholar]
- 8. Logue V. Thoracic intervertebral disc prolapse with spinal cord compression. J Neurol Neurosurg Psychiatry. 1952;15:227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bohlman HH, Zdeblick TA. Anterior excision of herniated thoracic discs. J Bone Joint Surg Am. 1988;70:1038–1047. [PubMed] [Google Scholar]
- 10. Love JG, Kiefer EJ. Root pain and paraplegia due to protrusions of thoracic intervertebral disks. J Neurosurg. 1950;7:62–69. [DOI] [PubMed] [Google Scholar]
- 11. McCormick WE, Will SF, Benzel EC. Surgery for thoracic disc disease. Complication avoidance: overview and management. Neurosurg Focus. 2000;9:e13. [DOI] [PubMed] [Google Scholar]
- 12. Patterson RH, Jr, Arbit E. A surgical approach through the pedicle to protruded thoracic discs. J Neurosurg. 1978;48:768–772. [DOI] [PubMed] [Google Scholar]
- 13. Vollmer DG, Simmons NE. Transthoracic approaches to thoracic disc herniations. Neurosurg Focus. 2000;9:e8. [DOI] [PubMed] [Google Scholar]
- 14. Charles YP, Barbe B, Beaujeux R, Boujan F, Steib JP. Relevance of the anatomical location of the Adamkiewicz artery in spine surgery. Surg Radiol Anat. 2011;33:3–9. [DOI] [PubMed] [Google Scholar]
- 15. Stillerman CB, Chen TC, Day JD, Couldwell WT, Weiss MH. The transfacet pedicle-sparing approach for thoracic disc removal: cadaveric morphometric analysis and preliminary clinical experience. J Neurosurg. 1995;83:971–976. [DOI] [PubMed] [Google Scholar]
- 16. Stillerman CB, Chen TC, Couldwell WT, Zhang W, Weiss MH. Experience in the surgical management of 82 symptomatic herniated thoracic discs and review of the literature. J Neurosurg. 1998;88:623–633. [DOI] [PubMed] [Google Scholar]
- 17. Bransford R, Zhang F, Bellabarba C, Konodi M, Chapman JR. Early experience treating thoracic disc herniations using a modified transfacet pedicle-sparing decompression and fusion. J Neurosurg Spine. 2010;12:221–231. [DOI] [PubMed] [Google Scholar]
- 18. Arce CA, Dohrmann GJ. Herniated thoracic disks. Neurol Clin. 1985;3:383–392. [PubMed] [Google Scholar]
- 19. Brown CW, Deffer PA, Jr, Akmakjian J, Donaldson DH, Brugman JL. The natural history of thoracic disc herniation. Spine (Phila Pa 1976). 1992;17(6 suppl):S97–S102. [DOI] [PubMed] [Google Scholar]
- 20. Okada Y, Shimizu K, Ido K, Kotani S. Multiple thoracic disc herniations: case report and review of the literature. Spinal Cord. 1997;35:183–186. [DOI] [PubMed] [Google Scholar]
- 21. Russo A, Balamurali G, Nowicki R, Boszczyk BM. Anterior thoracic foraminotomy through mini-thoracotomy for the treatment of giant thoracic disc herniations. Eur Spine J. 2012;21(suppl 2): S212–S220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arnold PM, Johnson PL, Anderson KK. Surgical management of multiple thoracic disc herniations via a transfacet approach: a report of 15 cases. J Neurosurg Spine. 2011;15:76–81. [DOI] [PubMed] [Google Scholar]
- 23. Bilsky MH. Transpedicular approach for thoracic disc herniations. Neurosurg Focus. 2000;9:e3. [DOI] [PubMed] [Google Scholar]
- 24. Le Roux PD, Haglund MM, Harris AB. Thoracic disc disease: experience with the transpedicular approach in twenty consecutive patients. Neurosurgery. 1993;33:58–66. [DOI] [PubMed] [Google Scholar]
- 25. Uribe JS, Smith WD, Pimenta L, et al. Minimally invasive lateral approach for symptomatic thoracic disc herniation: initial multicenter clinical experience. J Neurosurg Spine. 2012;16:264–279. [DOI] [PubMed] [Google Scholar]
- 26. Simpson JM, Silveri CP, Simeone FA, Balderston RA, An HS. Thoracic disc herniation. Re-evaluation of the posterior approach using a modified costotransversectomy. Spine (Phila Pa 1976). 1993;18:1872–1877. [PubMed] [Google Scholar]