Abstract
Study Design:
Review.
Objectives:
Cervical spondylotic myelopathy (CSM) is a major cause of disability, particular in elderly patients. Awareness and understanding of CSM is imperative to facilitate early diagnosis and management. This review article addresses CSM with regard to its epidemiology, anatomical considerations, pathophysiology, clinical manifestations, imaging characteristics, treatment approaches and outcomes, and the cost-effectiveness of surgical options.
Methods:
The authors performed an extensive review of the peer-reviewed literature addressing the aforementioned objectives.
Results:
The clinical presentation and natural history of CSM is variable, alternating between quiescent and insidious to stepwise decline or rapid neurological deterioration. For mild CSM, conservative options could be employed with careful observation. However, surgical intervention has shown to be superior for moderate to severe CSM. The success of operative or conservative management of CSM is multifactorial and high-quality studies are lacking. The optimal surgical approach is still under debate, and can vary depending on the number of levels involved, location of the pathology and baseline cervical sagittal alignment.
Conclusions:
Early recognition and treatment of CSM, before the onset of spinal cord damage, is essential for optimal outcomes. The goal of surgery is to decompress the cord with expansion of the spinal canal, while restoring cervical lordosis, and stabilizing when the risk of cervical kyphosis is high. Further high-quality randomized clinical studies with long-term follow up are still needed to further define the natural history and help predict the ideal surgical strategy.
Keywords: degenerative disc disease, cervical spondylosis, cervical spondylotic myelopathy, cervical spine stenosis, anterior cervical discectomy and fusion, cervical laminoplasty, cervical disk replacement
Introduction
Cervical spondylotic myelopathy (CSM) is a progressive degenerative disease and is the most common cause of cervical spinal cord dysfunction.1,2 CSM can be due to direct compression of the spinal cord, or surrounding blood vessels, resulting in varied clinical symptoms. Spondylosis has been shown as the most common etiology for cervical myelopathy in people aged 55 years or older.3 The incidence of hospitalizations related to CSM has been estimated at 4.04 per 100 000 person-years, and the number of patients undergoing surgical treatment each year has increased up to 7-fold.4,5 The rate and degree of neurologic deterioration is variable and optimal management strategies are complex. Early recognition and treatment of CSM, before the onset of spinal cord damage, is essential for optimal outcomes. As such, a better understanding of this pathology is warranted. This review article addresses CSM with regard to its epidemiology, anatomical considerations, pathophysiology, clinical manifestations, imaging characteristics, treatment approaches and outcomes, and the cost-effectiveness of surgical options.
Epidemiology
The reported prevalence and incidence of CSM is varied due to the diverse classification of degenerative processes defined as CSM. Current data is limited to population-based studies on CSM-related hospitalization rates. Boogaarts and Bartels6 estimated the prevalence of CSM to be 1.60 per 100 000 inhabitants based on CSM cases that were treated surgically at a hospital in the Netherlands.6 Evaluating a 12-year nationwide database in Taiwan, Wu et al5 retrospectively estimated that the overall incidence of CSM-related hospitalization was 4.04 per 100 000 person-years. Wu et al5 observed that older age and male gender were associated with a higher incidence of CSM.
The incidence of CSM is steadily increasing and carries a high risk for disability. Examining nontraumatic rates of spinal cord injury (SCI), Nouri et al7 estimated that the incidence and prevalence of CSM-related SCI in the North American region is 4.10 and 6.05 per 100 000, respectively. A prospective study found CSM to be the most common diagnosis (23.6%) in 585 patients admitted to a UK hospital with tetraparesis or paraparesis.8 In the United States, the number of CSM patients admitted from the emergency department has increased 2-fold from 1993 to 2002 (3.73 to 7.88 per 100 000).4 The number of these patients that underwent surgical reconstruction of the cervical spine demonstrated a 7-fold increase. The incidence of CSM and subsequent number of cervical spine surgery may continue to increase as the elderly population in the United States increases.
Anatomical Considerations
In addition to acquired degenerative processes, congenital cervical spine stenosis is also widely recognized as a significant predisposing factor for developing CSM. Congenital cervical spine stenosis commonly occurs with short pedicles. Using measurements proposed by Pavlov et al,9 congenital stenosis can be defined with radiographs when the canal diameter divided by the vertebral body diameter is less than 0.82. Bajwa et al10 analyzed 1066 skeletal specimens with digital calipers and suggested that sagittal canal diameter <13 mm and interpedicular distance <23 mm is associated with the presence of cervical spine stenosis at all cervical spine levels.10 Individual variability in the area of the cord avoids firm conclusions with these measurements; rather clinically relevant stenosis should be referenced to the size of the spinal cord. Further degenerative processes at the level of the intervertebral disc, facet joints, and capsules, ligamentum flavum can also increase canal encroachment.
Pathophysiology
The pathophysiology of CSM is multifactorial and its chronic nature likely induces compensatory mechanisms within the cord. From an anatomical standpoint, the cord is at risk of compression from protruding vertebral discs, deformed vertebral bodies, facet joint hypertrophy, osteophytic lesions, hypertrophic ligamentum flavum, and ossified posterior longitudinal ligament. These degenerative processes can result in static compression, and they can exacerbate compression of the spinal cord under dynamic movements. Ultimately, the static and dynamic compression may result in axonal stretch-associated injuries, spinal cord ischemia from vascular compression and venous congestion.7 Experimental validation of cord ischemia in the setting of CSM is lacking. The pathophysiology of CSM is not fully explained with the aforementioned static and dynamic model. From a molecular standpoint, ischemia would result in cellular death via necrosis; however, neural cells have been observed to undergo apoptosis with CSM.11 Emerging evidence from basic science studies has demonstrated an association between myelopathy and disruption of the blood–spinal cord barrier, acute and chronic neuroinflammation.
Clinical Presentation
CSM is the most common cause of nontraumatic spinal cord dysfunction and it often presents with a variety of subtle neurologic findings. Characteristic symptoms and signs can present insidiously and include the loss of manual dexterity in the hands, weakness, stiffness, increased urinary urgency, frequency or hesitancy, spasticity in extremities, and gait dysfunction including stiff or spastic gait (Table 1).12–14 While gait and balance disturbances from proximal lower extremity weakness are common early manifestations they are usually attributed to old age and has been reported to delay the diagnosis by 6 years.15 Sensory findings often include proprioceptive loss and glove sensation loss in the hands, which could be confounded by diabetes mellitus or a concurrent peripheral neuropathy.
Table 1.
Common Clinical Presentation and Examination Tools.
Motor signs
|
Upper motor neuron signs such as Hoffman’s sign, inverted radial reflex, pathological clonus and Babinski’s sign may also be present. The Hoffman’s sign was described as quick flexion of both the thumb and index finger when the middle finger nail is snapped. Lhermitte’s sign is an electric shock-like sensation that runs down the center of the patient’s back and enters the limbs during flexion of the neck, which may be present in CSM or multiple sclerosis patients. Additional clinical tests can help delineate CSM-like symptoms, including Romberg’s test, number of handgrip and releases, 9-hole peg test, timed gait, tandem gait, and the triangle step test. Rarely do severely affected individuals present with paralysis affecting bowel and bladder control.
Differential Diagnosis
The aforementioned presenting clinical symptoms and signs are not specific to CSM. Therefore, it is important to exclude other diagnoses that could present in a similar fashion. Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder that can be difficult to recognize due to overlapping demographics and clinical symptoms with CSM. There is a high incidence of spondylosis in patients at the mean age of onset (55.7 years) of ALS.16 ALS can present with upper and lower motor neuron deficits, as well as cranial nerve deficits. The possibility of ALS should be considered in the evaluation of patients with weakness even in the presence of radiological findings. The presence of fasciculation on clinical examination and or the absence of sensory findings make the diagnosis ALS much more likely. Continued neurologic deterioration following surgical decompression should raise suspicion for ALS. An electromyography demonstrating a denervation pattern can serve as diagnostic evidence for ALS.
Other pathologies to include in the differential are a demyelinating process, tumor, trauma and normal pressure hydrocephalus. Guillain-Barré syndrome can present with a subacute onset of progressive weakness. Furthermore, absent reflexes and cranial nerve deficits makes Guillain-Barré syndrome more likely. Gait and bladder dysfunction can be found in patients with normal pressure hydrocephalus. Additional cranial nerve abnormalities and/or a hyperactive jaw jerk reflex would suggest the presence of a brainstem or intracranial lesion.2 Cognitive dysfunction can help differentiate normal pressure hydrocephalus from CSM. Imaging studies can be utilized to further differentiate CSM from most other pathologies.
Imaging
Computed tomography (CT) can give accurate assessment due to its superiority in evaluating bone. Myelography or intrathecal injection of a contrast agent can be used with CT imaging. However, this is not commonly used with the advent of magnetic resonance imaging (MRI), unless there is a contraindication to acquiring an MRI. An MRI of the cervical spine can serve as the initial test for patients with suspected CSM. High signal changes seen in the spinal cord with T2-weighted imaging can indicate permanent spinal cord damage or myelomalacia. MRI alone, has low sensitivity for detecting subtle spinal cord damage, especially in patients with chronic symptoms.17 A meta-analysis of diffuse tensor imaging (DTI) studies of CSM patients demonstrated a significant reduction in fractional anisotropy and increase in apparent diffusion coefficient when compared with healthy subjects.17 In the future, there may be an increasing role for DTI in differentiating CSM patients from healthy subjects.
Functional Scales
A variety of scales have been developed to assess and quantify the functional disability of patients with CSM. The two most commonly utilized scales in the U.S. are the Nurick grade (Table 2)18 and the modified Japanese Orthopaedic Association (mJOA) scale (Table 3).19 The Nurick classification is a 6-grade ordinal scale that is primarily based on employment and gait impairment. The Nurick scale has been shown to have good correlation with the Japanese Orthopaedic Association (JOA) scale with high inter- and intrarater reliability; however, it has low sensitivity and responsiveness to change.20 The original JOA was modified from grading the use of chopsticks (widely used in East Asian countries) to grading the use of a spoon (widely used in Western countries).19 The JOA scales are multidimensional, and assess upper extremity dysfunction, lower extremity dysfunction, and bladder dysfunction in patients with CSM. The mJOA has been shown to strongly correlate with the JOA,21 and the use of mJOA has been advocated as the standard scale for CSM grading in the Western population.20
Table 2.
Nurick Grades.15
| 0 | Signs or symptoms of root involvement but without evidence of spinal cord disease |
| 1 | Signs of spinal cord disease but no difficulty in walking |
| 2 | Slight difficulty in walking which did not prevent full-time employment |
| 3 | Difficulty in walking which prevented full-time employment or the ability to do all housework, but which was not so severe as to require someone else’s help to walk |
| 4 | Able to walk only with someone else’s help or with the aid of a frame |
| 5 | Chair bound or bedridden |
Table 3.
Modified Japanese Orthopaedic Association Scoring System.16
| Motor dysfunction | |
| Upper extremities | |
| 0 | Unable to move hands |
| 1 | Unable to eat with a spoon but able to move hands |
| 2 | Unable to button shirt but able to eat with a spoon |
| 3 | Able to button shirt with great difficulty |
| 4 | Able to button shirt with slight difficulty |
| Lower extremities | |
| 0 | Complete loss of motor & sensory function |
| 1 | Sensory preservation without ability to move legs |
| 2 | Able to move legs but unable to walk |
| 3 | Able to walk on flat floor with a walking aid (cane or crutch) |
| 4 | Able to walk up- &/or downstairs w/aid of a handrail |
| 5 | Moderate-to-significant lack of stability but able to walk up &/or downstairs without handrail |
| 6 | Mild lack of stability but able to walk unaided with smooth reciprocation |
| 7 | No dysfunction |
| Sensory dysfunction | |
| Upper extremities | |
| 0 | Complete loss of hand sensation |
| 1 | Severe sensory loss or pain |
| 2 | Mild sensory loss |
| 3 | No sensory loss |
| Sphincter dysfunction | |
| 0 | Unable to micturate voluntarily |
| 1 | Marked difficulty in micturition |
| 2 | Mild-to-moderate difficulty in micturition |
| 3 | Normal micturition |
Natural History
The natural history of CSM can widely vary and is often unpredictable. In 1956, Clark and Robinson22 were first to investigate the natural history of CSM in 26 patients. The authors found the course of the disease to follow 1 of 3 patterns: (1) 75% deteriorated in a stepwise fashion, (2) 20% slow, steady progression of disease, and (3) 5% developed rapid onset of symptoms and signs, then remained stable for years. In 1963, Lees and Turner23 examined the natural history of 22 patients over 10 years and also found that the initial decrease in function may level off and stable for years. These results were corroborated by Nurick24 in 1972 after examining 37 conservatively treated CSM patients. Both groups also observed a percentage of patients that continued to worsen.23,24 Cusick25 and Cooper26 noted that a number of comparative clinical studies observed up to 50% of CSM patients can continue to decline in function over time. In 1998, Nakamura et al27 retrospectively analyzed 64 patients treated conservatively with a follow-up range of 3 to 10 years and approximately 26% to 27% of patients reported no disability in the upper or lower extremity. In a 12-year nationwide database from Taiwan, Wu et al5 observed that SCI (12 in 1000 person-years) was more likely to develop in conservative management than the surgical group. In sum, these results suggest that mild CSM could be successfully treated with conservative measures for a significant number of patients. However, in select patients, deterioration can occur over time and these patients need to be closely followed for signs of neurologic decline.
Risk factors for poor prognosis in patients with CSM have included the severity of disability on presentation, age and the duration of symptoms. Radiological imaging has also been investigated as a prognostic tool. Two studies investigated intensity signals on MRI and while using the JOA as a functional grading scale.28 Matsumoto et al29 retrospectively examined increased signal intensity on T2-weighted images in 52 patients treated conservatively. The average follow-up was 3-years and the increased signal intensity was not found to be correlated to a poor outcome.29 Shimomura et al30 prospectively examined prognostic factors that exacerbate clinical symptoms of CSM in 56 patients after conservative treatment. Shimomura et al30 found that 11/56 (19.6%) deteriorated to moderate or severe, but no statistical differences in JOA scores were observed after conservative treatment. The presence of a high T2 signal on sagittal MRI did not significantly affect the clinical condition; rather the only factor that was found to significantly exacerbate clinical symptoms was circumferential spinal cord compression at the maximum compression segment on axial MRI.
Conservative Versus Surgical Approach
Conservative treatments for CSM often include neck immobilization, pharmacologic treatments, lifestyle modifications, and physical modalities. There is a lack of high-level studies comparing these modalities to surgical intervention. Therefore, conservative therapies are often initiated based on a clinician’s preference or specialty. Kadanka et al31 conducted a randomized controlled trial to compare conservative and operative treatments of mild and moderate, nonprogressive, and slowly progressive forms of CSM (mJOA score >12) in 68 patients over 3 years. Randomization occurred by a coin toss and outcomes assessed included JOA score, 10-minute walk, score for daily activities recorded by video, at 6 to 36-month follow-up time points. The 3-year follow-up study did not show that surgery is superior to conservative treatment. Perhaps a longer follow-up period was needed to assess the differences. However, these results suggest that treatment of mild CSM may involve conservative therapy for the first 3 years after diagnosis.
Two comparative studies concluded that surgery was more favorable. Sampath et al32 conducted a prospective multicenter questionnaire for 43 patients with mild CSM treated surgically and conservatively. The average follow-up was 11.2 months and the surgical cohort had more severe myelopathy at baseline. No direct comparisons were made between the surgical and conservative groups. Surgical patients improved, whereas the conservative group declined. Sampath et al32 concluded that surgery was associated with improved functional status, improved neurologic status and improved patient satisfaction. Yoshimatsu et al33 retrospectively compared patients with CSM elected to undergo either immediate surgical treatment (32 patients, JOA <13) or conservative care (69 patients, JOA >13). The authors found that surgically treated patients demonstrated greater improvements than the conservative group. Rhee et al34 systematically reviewed the literature and concluded that there was low evidence that conservative treatment yield different outcomes than surgery in those with mild myelopathy. However, for moderate to severe myelopathy, conservative treatment had inferior outcomes versus surgery in retrospective studies, even though patients treated surgically were worse at baseline.
In a review of surgical indications for cervical myelopathy, Law et al35 identified several poor prognostic factors with conservative treatment, which included the progression of symptoms, presence of myelopathy for more than 6 months, compression ratio approaching 0.4 (indicating flattening of the cord), and transverse area of the cord <40 mm. The presence of any of these factors can be an indication for surgical intervention. Many factors play a role in the decision-making process for surgical intervention, which include duration of symptoms, degree of spinal cord dysfunction, general health of the patient, degree of functional deterioration, and radiographic findings.
Surgical Prognostic Factors
The impact of preoperative radiological findings and age on the clinical outcome of CSM patients from surgical intervention has been investigated. Alafifi et al36 retrospectively analyzed 76 patients with CSM that underwent cervical decompressive surgery with pre- and postoperative (2-4 months) MRI studies available for review. The authors examined pre- and postoperative MRI with outcome scores (Nurick scores; Odom’s criteria). Alafifi et al36 observed a less favorable surgical outcome with the presence of a low intramedullary signal on T1-weighted imaging, clonus and spasticity. The results suggest that surgical timing is important for optimal surgical outcomes in patients with CSM. Holly et al37 retrospectively analyzed a cohort of 36 elderly patients (>75 years) and 34 younger patients (<65 years) that underwent decompressive surgery for CSM. The mean follow-up was 24 months. Both groups demonstrated significant improvements and the differences among the groups were not significant. Of note, postoperative complication rates were much higher in the elderly group (38% vs 6%). However, the authors reported that the complications were self-limiting and did not adversely affect neurological outcomes.
Anterior Versus Posterior or Combined
The optimal surgical approach is not always clear and has been under investigation over the past 30 years (Table 4). An anterior approach offers the following advantages: direct decompression of pathologies in the anterior cervical spine (ie, osteophytes, ossification of the posterior longitudinal ligament, disc herniations), a muscle sparing dissection to minimize postoperative pain, lower infection rates, the ability to decompress and correct cervical kyphosis, and for those with prominent radiculopathy. Most spine surgeons prefer an anterior approach when 1 to 2 levels are involved (Figure 1 and Table 5).3 When 3 or more levels are involved, the complication rates with an anterior approach rise and a posterior approach should be considered. If there is focal kyphosis and the compressive pathology is posterior, then a combined approach can also be considered. The posterior approach allows for a wider decompression and is dependent on the ability of the cord to drift away from anterior lesions. It is therefore important to take cervical sagittal alignment into consideration, as the cord may not drift posteriorly with significant cervical kyphosis.
Table 4.
Common Anterior and Posterior Approaches.
| Surgical Technique | Main Indications | Pros | Cons | Common Complications | Contraindications |
|---|---|---|---|---|---|
| Anterior cervical discectomy and fusion |
|
|
|
|
|
| Anterior corpectomy |
|
|
|
|
|
| Arthroplasty |
|
|
|
|
|
| Cervical laminectomy only |
|
|
|
|
|
| Cervical laminectomy and fusion |
|
|
|
|
|
| Cervical laminoplasty |
|
|
|
|
|
| Combined ACDF and laminectomy and fusion |
|
|
|
|
|
Abbreviations: ACDF, anterior cervical discectomy and fusion; CSF, cerebrospinal fluid; CSM, cervical spondylotic myelopathy.
Figure 1.
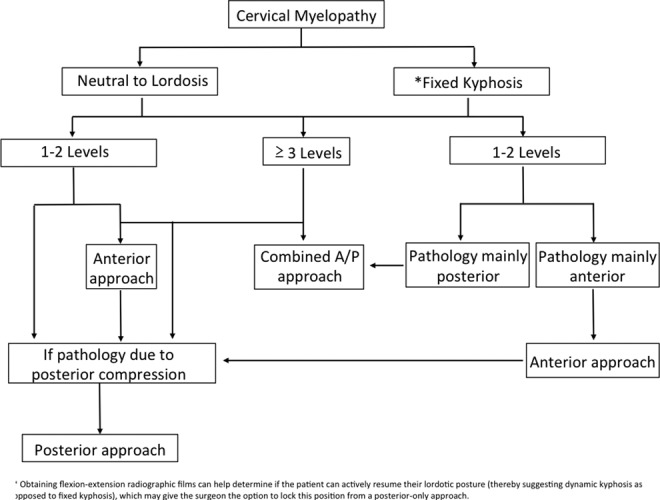
A general algorithm in the surgical approach of treating cervical spondylotic myelopathy.
Table 5.
Factors That Would Promote One Approach Over Another.
| Sagittal alignment | Kyphosis | Fixed →Anterior Flexible → Anterior or posterior with fusion |
| Neutral or lordotic | → Posterior (laminoplasty) > Anterior | |
| Number of levels | ≥3 | → Posterior (laminoplasty) > Anterior |
| ≤2 | → Anterior > Posterior | |
| Age and comorbidities | Elderly, greater comorbidities | → Posterior > Anterior |
| Healthier | → Anterior > Posterior | |
| Preoperative Pain Levels | Moderate—High | → Anterior or posterior with fusion |
| None—Low | →Posterior (laminoplasty) or anterior | |
| Instability | Yes | → Anterior or posterior with fusion |
| No | → Posterior (laminoplasty) or anterior |
The degree of kyphosis or lordosis can be quantitatively calculated by the sagittal cervical Cobb angle (in degrees). C2-C7 lordosis is measured as the angle of intersection between vertical lines drawn from lines parallel to the inferior end plates of C2 and C7. Shamji et al38 prospectively analyzed the neurologic outcomes (mJOA, Nurick, Neck Disability Index) in 124 patients with CSM based on cervical sagittal Cobb angles. The authors found that lordotic patients exhibited similar improvement when approached anteriorly or posteriorly, whereas kyphotic patients exhibited greater improvement when approached by an anterior or combined approach. More recently, the cervical sagittal alignment, as assessed by the C2-C7 sagittal vertical axis (SVA, in mm displacement) has also been shown to play a major role in clinical outcomes (Figure 2). The cervical (C2-C7) SVA is measured as the deviation of the C2 plumb line from the posterior superior end plate of C7, and it has previously been shown to correlate with postoperative disability scores.39 Tang et al39 retrospectively analyzed 113 patients that underwent multilevel posterior cervical fusion for cervical stenosis, myelopathy, and kyphosis, and found a threshold of ≥40 mm where disability correlated the strongest with cervical SVA. Hardacker et al40 previously demonstrated that cervical SVA from radiographic imaging of 100 adult volunteers with no neck or radicular arm symptoms had a cervical SVA of 16.8 ± 11.2 mm. These measurements can help the spine surgeon to choose the approach for the optimal postoperative cervical sagittal alignment.
Figure 2.
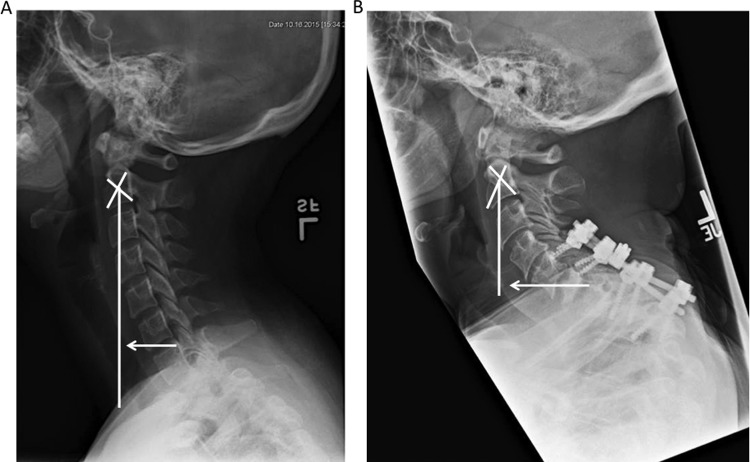
Cervical sagittal alignment parameters can be associated with clinical symptoms. A. Cervical spine lateral radiograph in an asymptomatic patient. The C2-C7 sagittal vertical axis (SVA) is measured as the deviation of the C2 plumb line from the posterior superior end plate of C7 (white arrow) B. Patient presented with severe myelopathy and radiograph demonstrated bony destruction at C6-C7 and T1 vertebral bodies with an angulated kyphosis at the C7-T1 region and compression fracture T1-T2. There was evidence of failure in the posterior lateral mass screws at C5-6 and pedicle screws at C7-T1. There was also an obvious kyphotic deformity along that region and evidence of pedicle screw failure in the lower levels. Cobb angle measured approximately 80° going from the endplate of C5 to the endplate of T2. C2-C7 SVA is also more pronounced in this patient (white arrow).
The current evidence is not clear on whether an anterior or posterior approach is superior; rather the sagittal alignment, number of pathological levels and degree of anterior or posterior compression dictates what would be the most direct approach for the decompression. Luo et al41 systematically reviewed controlled trials that compared anterior and posterior approaches for patients with multilevel (≥3) CSM. The outcomes investigated included the recovery rate, JOA score, complication rate, reoperation rate, blood loss, operation time and length of stay. The authors included 10 comparative studies, which were rated high quality by the Newcastle-Ottawa scale. The subgroup meta-analysis demonstrated the following results: 24-month postoperative JOA score was significantly higher in the anterior surgery group, recovery rate between the groups were similar at 24 months, postoperative complication rate was significantly higher in the anterior surgery group, the reoperation rate was significantly higher in the anterior surgery group, the intraoperative blood loss and operation time was significantly higher in the anterior surgery group and the length of stay was significantly lower in the anterior surgery group. There was no apparent difference in the neurologic recovery and a definitive conclusion regarding which surgical approach is more effective for the treatment of multilevel CSM could not be made. Pooling the results from low-level studies with various surgical indications and technologies may have contributed to the clinical heterogeneity observed in their analysis. These findings are in line with previous investigations3,42 and may be clarified when the results from an ongoing multi-institutional randomized controlled trial (NCT02076113) are available.43
Methods of Anterior Decompression
Methods of anterior decompression include anterior cervical discectomy and fusion (ACDF), anterior cervical corpectomy and fusion (ACCF), hybrid procedures, and cervical arthroplasty (Tables 4 and 5). Anterior approaches generally demonstrate lower perioperative complications and morbidity and tend to be performed on younger patients when compared to other approaches.44 Clinical series have demonstrated successful arthrodesis in the majority of patients (92%-96%) after single-level ACDF with satisfactory clinical outcomes.2 For compression at multiple levels, numerous options exist, including multilevel ACDF, corpectomy and hybrid techniques. While the rate of neurologic improvement remains high for multilevel ACDFs, the incidence of nonunion can increase with the number of levels being fused. Anterior cervical plating has increased the fusion rate, as Wang et al45 demonstrated that the fusion rate for three-level ACDF was 82% with plating and 63% without plating. An alternative method to improve fusion rates and provide a more a more extensive decompression would be to employ a corpectomy, which can also be combined with anterior discectomy procedures (see case 3). Combined anterior circumferential decompression and fusion techniques have been advocated for cases involving 3 or more segments, and the addition of a posterior segmental fusion can increase the rate of fusion and decrease the incidence of graft and implant-related problems.2
Complications of anterior procedures can include postoperative dysphagia (2%-48%), hoarseness (temporary in 3%-11%, permanent in 0.33%), injury to the vertebral artery (0.03%) and carry an incidence of adjacent segment disease of 3% per year.2 A potential lethal complication can include postoperative airway obstruction due to edema or hematoma formation; therefore patients with severe myelopathy are often kept intubated until appropriate weaning parameters are met.
The literature on arthroplasty for patients with myelopathy is limited. Hu et al46 reported pooled the data of eight prospective randomized controlled trials investigating the outcome of ACDF and cervical disc arthroplasty for the treatment of 1- to 2-level CSM. With an overall follow-up of 2 to 7 years, the meta-analysis demonstrated that cervical arthroplasty group achieved significantly higher rates of overall success, long-term functional outcomes and a lower incidence of adjacent segment degeneration. However, patients with significant degenerative changes in the cervical spine may be better suited for surgical spinal stabilization with fusion to prevent further degenerative changes at the effected regions. These patients may not be ideal candidates for cervical arthroplasty, as the increased motion can exacerbate the degenerative changes. Instead, cervical arthroplasty could be reserved for patients with acute neurologic deficits due to a herniated disc. Higher quality clinical studies with longer follow-up are still needed to confirm the therapeutic value of arthroplasty in CSM.
Methods of Posterior Decompression
Posterior approaches offer the opportunity to avoid technical problems encountered with anterior approaches that result from obesity, a short neck, barrel chest, or previous anterior cervical surgery. Options for a posterior approach includes a laminoplasty or a laminectomy with or without fusion (Tables 4 and 5). The laminoplasty technique is often ideal for the patient with spinal stability, good cervical lordosis and minimal neck pain. In a retrospective analysis of outcomes of 11 patients who underwent laminoplasty for CSM, Suda et al47 showed that as long as there is no local kyphosis of >13° and signal intensity change on MRI, laminoplasty can still produce good clinical outcomes.
The laminoplasty technique offers the opportunity to preserve some of the natural cervical biomechanical motion without necessitating fusion, but it comes at the expense of less extensive cord decompression.48 Variations in the laminoplasty technique include the open door laminoplasty, the double door laminoplasty, and various muscle-sparing laminoplasty alterations. The open-door laminoplasty involves a thinned hinge on one side of the lamina, and a complete cut through the lamina on the opposite side.48 The laminae on the open side can then be reconstructed with miniplates, anchored with a stitch between the spinous process and the hinged lamina, or plated open by fixing the open lamina with subsequent levels. The double-door laminoplasty expands the canal symmetrically as the opening is created in the midline.49 This is accomplished by splitting the spinous processes in the midline with the left and right hemilaminae hinging on the laminaspinous process and ligamentum flavum complex bilaterally. Various muscle-sparing techniques (sparing semispinalis cervicis, multifidus, and C7 musculoligamentous attachments) have also been described with the aim to limit postoperative axial neck pain, kyphosis, and segmental instability.50
Complications from the laminoplasty procedure can include delayed C5 nerve root injury (2%-13.3%), neck pain (40%-60%), loss of range of motion, (20%-50%) or new-onset kyphosis (2%-15%).51,52 Suk et al53 prospectively investigated 85 patients that underwent a C3-C7 open door laminoplasty. At 2 years’ follow-up, the authors found a 30.5% decrease in range of motion and 10.6% of patients developed kyphosis (average 12.2°).53 A recent meta-analysis pooled 2470 patients from studies that reported on various laminoplasty techniques during 2003-2013.54 Duetzmann et al54 found no significant difference in the preoperative 14.17° (±0.19°) to postoperative 13.98° (±0.19°) C2-C7 lordosis at a mean follow-up of 39 months. Of the various laminoplasty methods, the authors found decreased postoperative kyphosis in specific studies that employed posterior element sparing techniques. Duetzmann et al54 also observed an overall mean of 47.3% loss of range of motion in 2390 patients. Compared with the laminectomy technique, the laminoplasty preserved the posterior tension band for greater stability. Matsunaga et al55 retrospectively analyzed the cervical alignment of 64 patients who underwent laminoplasty and 37 patients who underwent laminectomy for CSM or ossification of the posterior longitudinal ligament. The authors reported that postoperative kyphosis or swan neck deformity is more common after laminectomy alone (34%) versus laminoplasty (7%) with a follow-up greater than 5 years.
In the past, a laminectomy alone was regarded as the standard treatment for multilevel CSM. Many surgeons still perform laminectomy only, but the incidence of postoperative kyphosis (6%-46%) and segmental instability (∼18%) requiring additional stabilization has led many to believe that it is not a routinely advisable approach.2,3,56–58 The differences in the range of postoperative kyphosis can be due to preoperative sagittal alignment and directly related to the extent of the laminectomy procedure (amount of lateral dissection, facet capsule disruption, etc). Segmental hypermobility of the cervical spine occurs when a foraminotomy involves resection of >50% of the facet.59 Cervical laminectomy and fusion offers the advantage to stabilize the decompressed segment in a lordotic posture while preventing segmental instability, thereby allowing for a more expansive decompression. There is insufficient evidence to indicate whether the addition of cervical fixation improves functional outcome.60 The addition of fixation also carries the risks of complications related to misplaced screws, long-term hardware failure, and the alteration of the natural cervical biomechanics distributed to adjacent levels.
Lee at al58 systematically analyzed the outcomes from a variety of laminoplasty techniques to laminectomy and fusion on patients with multilevel CSM. The authors performed a meta-analysis that included 1 low-powered randomized controlled trial and 6 observational studies comprising of 302 patients treated with a laminoplasty technique, and 290 patients treated with laminectomy and fusion. The groups were matched by cervical lordosis and neck disability. Both groups similarly improved in their JOA score and VAS for neck pain. Both groups evenly lost cervical lordosis after treatment. The overall sagittal alignment progressed to kyphosis, regardless of the surgical method, and there was no substantial difference between the groups. In the subgroup analysis of 3 observational studies with follow-up periods of 18 months or more, the laminectomy and fusion group may have had superior results in preserving lordosis in the long term.
When the clinical scenario allows the opportunity, these general techniques can be further tailored to be less invasive. For example, skip laminectomy can be utilized to limit the disruption of the posterior cervical tension band.61 By employing this method, decompression between C3-C7 can be accomplished by a C4 and C6 skip laminectomy to preserve the C3, C5, and C7 posterior arches as well as all the muscular attachments to those spinous processes. More recently, minimally invasive endoscopic approaches for posterior decompression have also been utilized for CSM. Minamide et al62 retrospectively analyzed 51 patients that underwent endoscopic posterior decompression. With a mean follow-up of 20.3 months, the JOA score improved from 10.1 (±2.7) to 13.6 (±2.3) and the postoperative Cobb angle remained the same (6.7°). Among 51 patients, 4 complications occurred (1 compressive epidural hematoma that required operative evacuation), and the length of hospital stay was 8.6 days. Dahdaleh et al63 retrospectively analyzed a case series of 10 patients that underwent endoscopic posterior decompression. The authors found an improvement in Nurick score from 1.6 ± 0.7 to 0.3 ± 0.7 with an average follow-up of 18.9 months. Albeit the power of the study was small, no postoperative worsening of the Cobb angle occurred, no complications occurred, and all patients were discharged within 2 days.
Case Illustrations of Cervical Decompression Techniques
The complexities in the decision making of the surgical approach can be illustrated with the following 3 case examples.
Case 1
A 60-year-old man who presented with progressive bilateral radiculopathy and left bicep weakness. His examination was significant for weakness in his biceps and with wrist extension. Imaging demonstrated multilevel advanced degenerative disc disease and loss of his cervical lordosis (Figure 3A). Recent MRI demonstrated worsening central and foraminal stenosis at C4-C7. The patient underwent an anterior cervical discectomy C4-C7 for decompression of central and bilateral foraminal stenosis (Figure 3B). The potential need for further surgery, including a posterior approach was discussed with the patient. No complications occurred during the operation, and the patient was back to his neurologic baseline.
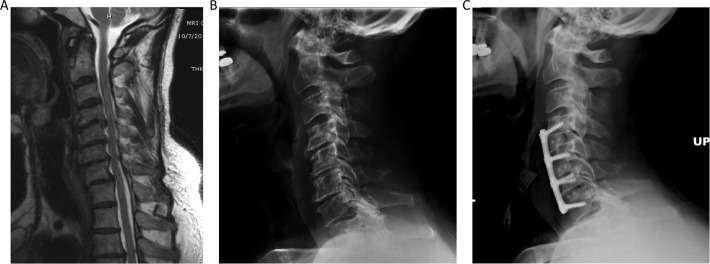
Figure 3.
Illustrative case demonstrating a 3-level anterior cervical discectomy and fusion (ACDF). (A) Magnetic resonance imaging (MRI) of the cervical spine demonstrating multilevel cervical stenosis due to disc herniation from C4-C6. (B) Lateral cervical radiograph demonstrated multilevel advanced degenerative disc disease and straightening of the normal cervical lordosis. (B) ACDF extending from the C4-C7 levels with interbody graft seen at the C4-5, C5-6, and C6-7 levels.
Case 2
The right surgical approach is not always straightforward, and it is important to include patients in the decision-making process. A 44-year-old man presented with complaints of several months of worsening upper and lower extremity weakness, difficulty with hand function as well as balance difficulties. On examination, the patient had significant difficulty with tandem walking, 4+/5 strength of bilateral upper and lower extremities, with Hoffman’s bilaterally as well as several beats of clonus bilaterally. On MRI, diffuse cervical spondylosis with multilevel cervical stenosis due to a combination of disc and ligamentous hypertrophy, worse at C4-5 and C5-6 with moderate to severe stenosis at these levels with cord signal change (Figure 4A). The patient had good lordosis and denied significant neck pain; therefore, the decision was made to perform a posterior decompression with the laminoplasty technique. This was employed by creating a hinge on the left side to expose the ventral cortical bone and the open-door side of the laminoplasty was performed on the right side at the junction again of the lamina and lateral mass (Figure 4B and 4C). No complications occurred during the operation, and on postoperative follow-up the patient had significant improvement in his balance and hand function.
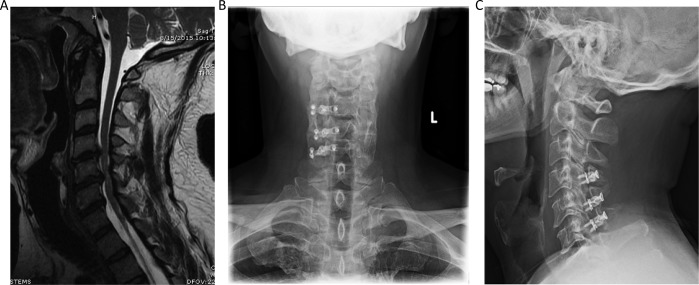
Figure 4.
Case example of a 3-level laminoplasty. (A) Magnetic resonance imaging (MRI) cervical spine shows diffuse cervical spondylosis with multilevel cervical stenosis due to a combination of disc and ligamentous hypertrophy, worse at C4-5 and C5-6 with moderate to severe stenosis at these levels and some suggestion of cord signal change. (B) Anterior-posterior and (C) lateral radiographic views of the laminoplasty technique, with the open door side on the right side with plates, and the hinged side was on the left.
Alternative methods of posterior decompression with fixation are also available.
Case 3
A 70-year-old man with a history of chronic renal failure presented with progressive neck pain and upper extremity weakness. On examination, the patient demonstrated significant bilateral weakness in his biceps, triceps, and handgrip. On imaging, the patient was noted to have severe stenosis at multiple levels, and vertebral body fractures and collapse of C4, C5, C6 vertebral bodies with corresponding kyphosis (Figure 5A). Given his comorbidities and progressive myelopathic symptoms, the patient opted for surgery. A combined anterior-posterior approach was employed in 2 stages, with the intent for the anterior approach to correct the overall kyphotic posture followed by posterior stabilization and further decompression (Figure 5B and C). Posterior stabilization of C2-T2 was warranted due to the requirement of a 3-level corpectomy and the patient being at high risk for pseudarthroses, given his osteoporosis. No complications occurred during the operation, and the patient was back to his neurologic baseline.
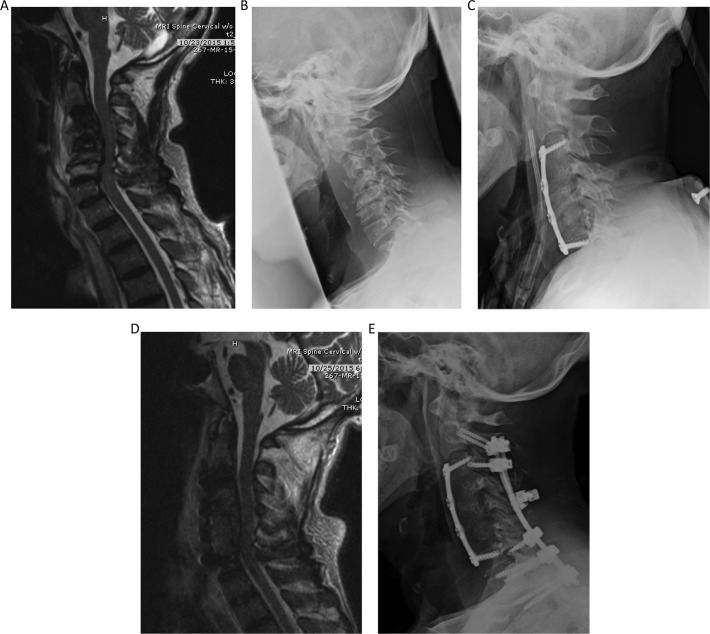
Figure 5.
Illustrative case demonstrating a combined anterior-posterior approach completed in 2 stages. (A) Magnetic resonance imaging (MRI) demonstrating moderate cord compression C3-C6. With normal signal within the C3-C6 vertebral bodies with large heterogeneous prevertebral fluid collection at these levels. possibly reflecting severe spondyloarthropathy of dialysis. (B) Lateral views of the cervical spine demonstrate vertebral body deformity, height loss, near complete loss of the disc spaces and endplate irregularity from C3-C6. Anterior osteophytes were also seen at all levels in the cervical spine. (C) Demonstrating cervical corpectomy at C4-C6 with graft placement at C3-C7 levels, and anterior plate fusion extending from C3 to C7. (D) MRI demonstrating postsurgical changes related to anterior cervical corpectomy at C4- C6 and anterior plate fusion from C3-C7. Subsequent decompression of the cervical spine at the operated levels was appreciated. (E) C2-T2 posterolateral fusion with rod and lateral mass and pedicle screw fixation, with lateral mass screws sparing the C4-C6 levels and pedicle screws in the thoracic levels.
It should be noted that there is a significantly higher rate of perioperative complications with anterior-posterior combined procedures when compared with when compared with anterior or posterior approaches alone.44 In part this may be because combined procedures are generally indicated for patients with severe kyphosis, severe spinal instability, fixed kyphotic deformity, or complex pathology and associated comorbidities that require a more rigid construct. Combined procedures are more technically demanding, and can result in longer surgery time.
These case examples highlight the methods in choosing the approach by factoring in the location of spinal cord compression, number of levels involved, sagittal alignment, instability, associated axial neck pain, risk factors for pseudarthrosis, and patient comorbidities. However, these variables are frequently equivocal, and surgeon training and patient preference are often the deciding factor for treatment rendered.
Economic Analyses
Analysis of the cost-effectiveness of treatment approaches is also an important consideration. The outcome measure of quality-adjusted life year (QALY) is a product of both the quantity and quality of life gained by the intervention, and its reporting is recommended by the Panel of Cost-Effectiveness in Health and Medicine.64 QALY is calculated as the change in HRQOL between 2 time points multiplied by the amount of time. Fehlings et al65 analyzed the cost-utility measurement per QALY of treatment for 70 patients undergoing surgery for CSM at a single institution in Canada to represent a reasonable global median costing model. The authors found that the cost-utility ratio for CSM surgeries is $32 916/QALY, which is below the national benchmark to be considered highly cost-effective. For reference to other medical treatments, US$/QALY for other procedure include: cataract surgery 2020, hip replacement 6668, knee replacement 28 100 to 30 695, and gastric bypass surgery 35 600.
Conclusion
CSM is a major cause of disability, particular in elderly patients. Awareness and understanding of CSM is imperative to facilitate early diagnosis and management. Current static and dynamic models do not fully explain the mechanisms underlying CSM, and emerging research at the molecular level may help elucidate this. The clinical presentation and natural history of CSM is variable, alternating between quiescent and insidious to stepwise decline or rapid neurological deterioration. The use of diffusion MRI may hold some potential in diagnosing and treating spinal cord dysfunction earlier. For mild CSM, conservative options could be employed with careful observation. However, surgical intervention has shown to be superior for moderate to severe CSM. The success of operative or conservative management of CSM is multifactorial and high-quality studies are lacking. The optimal surgical approach is still under debate, and can vary depending on the location of the spinal cord compression, number of levels involved, sagittal alignment, instability, associated axial neck pain, risk factors for pseudarthrosis, and patient comorbidities. The goal of surgery is to decompress the cord with expansion of the spinal canal while restoring cervical lordosis and stabilizing when the risk of cervical kyphosis is high. Further high-quality randomized clinical studies with long-term follow up are still needed to further define the natural history and help predict the most ideal surgical strategy.
Abbreviations
- Cervical spondylotic myelopathy
(CSM)
- spinal cord injury
(SCI)
- Amyotrophic lateral sclerosis
(ALS)
- Diffuse Tensor Imaging
(DTI)
- Japanese Orthopedic Association
(JOA)
- modified Japanese Orthopedic Association
(mJOA)
- visual analog scale
(VAS)
- Quality-adjusted life year
(QALY)
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Singh A, Tetreault L, Casey A, Laing R, Statham P, Fehlings MG. A summary of assessment tools for patients suffering from cervical spondylotic myelopathy: a systematic review on validity, reliability and responsiveness. Eur Spine J. 2015;24(suppl 2):209–228. doi:10.1007/s00586-013-2935-x. [DOI] [PubMed] [Google Scholar]
- 2. Edwards CC, 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. current diagnostic and treatment strategies. Spine J. 2003;3:68–81. [DOI] [PubMed] [Google Scholar]
- 3. Klineberg E. Cervical spondylotic myelopathy: a review of the evidence. Orthop Clin North Am. 2010;41:193–202. doi:10.1016/j.ocl.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 4. Lad SP, Patil CG, Berta S, Santarelli JG, Ho C, Boakye M. National trends in spinal fusion for cervical spondylotic myelopathy. Surg Neurol. 2009;71:66–69. doi:10.1016/j.surneu.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 5. Wu JC, Ko CC, Yen YS, et al. Epidemiology of cervical spondylotic myelopathy and its risk of causing spinal cord injury: a national cohort study. Neurosurg Focus. 2013;35:E10 doi:10.3171/2013.4.FOCUS13122. [DOI] [PubMed] [Google Scholar]
- 6. Boogaarts HD, Bartels RH. Prevalence of cervical spondylotic myelopathy. Eur Spine J. 2015;24(suppl 2):139–141. doi:10.1007/s00586-013-2781-x. [DOI] [PubMed] [Google Scholar]
- 7. Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics and pathogenesis. Spine (Phila Pa 1976). 2015;40:E675–E693. doi:10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 8. Moore AP, Blumhardt LD. A prospective survey of the causes of non-traumatic spastic paraparesis and tetraparesis in 585 patients. Spinal Cord. 1997;35:361–367. [DOI] [PubMed] [Google Scholar]
- 9. Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology. 1987;164:771–775. doi:10.1148/radiology.164.3.3615879. [DOI] [PubMed] [Google Scholar]
- 10. Bajwa NS, Toy JO, Young EY, Ahn NU. Establishment of parameters for congenital stenosis of the cervical spine: an anatomic descriptive analysis of 1,066 cadaveric specimens. Eur Spine J. 2012;21:2467–2474. doi:10.1007/s00586-012-2437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karadimas SK, Gatzounis G, Fehlings MG. Pathobiology of cervical spondylotic myelopathy. Eur Spine J. 2015;24(suppl 2):132–138. doi:10.1007/s00586-014-3264-4. [DOI] [PubMed] [Google Scholar]
- 12. Crandall PH, Batzdorf U. Cervical spondylotic myelopathy. J Neurosurg. 1966;25:57–66. doi:10.3171/jns.1966.25.1.0057. [DOI] [PubMed] [Google Scholar]
- 13. Echols DH. The Hoffmann sign. J Nerv Ment Dis. 1936;84:427–431. [Google Scholar]
- 14. Denno JJ, Meadows GR. Early diagnosis of cervical spondylotic myelopathy. A useful clinical sign. Spine (Phila Pa 1976). 1991;16:1353–1355. [DOI] [PubMed] [Google Scholar]
- 15. Sadasivan KK, Reddy RP, Albright JA. The natural history of cervical spondylotic myelopathy. Yale J Biol Med. 1993;66:235–242. [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshor D, Klugh A, 3rd, Appel SH, Haverkamp LJ. Incidence and characteristics of spinal decompression surgery after the onset of symptoms of amyotrophic lateral sclerosis. Neurosurgery. 2005;57:984–989. [DOI] [PubMed] [Google Scholar]
- 17. Guan X, Fan G, Wu X, et al. Diffusion tensor imaging studies of cervical spondylotic myelopathy: a systemic review and meta-analysis. PLoS One. 2015;10:e0117707 doi:10.1371/journal.pone.0117707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100. [DOI] [PubMed] [Google Scholar]
- 19. Benzel EC, Lancon J, Kesterson L, et al. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4:286–295. [DOI] [PubMed] [Google Scholar]
- 20. Kopjar B, Tetreault L, Kalsi-Ryan S, Fehlings M. Psychometric properties of the modified Japanese Orthopaedic Association scale in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2015;40:E23–E28. doi:10.1097/BRS.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 21. Kato S, Oshima Y, Oka H, et al. Comparison of the Japanese Orthopaedic Association (JOA) score and modified JOA (mJOA) score for the assessment of cervical myelopathy: a multicenter observational study. PLoS One. 2015;10:e0123022 doi:10.1371/journal.pone.0123022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clarke E, Robinson PK. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79:483–510. [DOI] [PubMed] [Google Scholar]
- 23. Lees F, Turner JW. Natural history and prognosis of cervical spondylosis. Br Med J. 1963;2:1607–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101–108. [DOI] [PubMed] [Google Scholar]
- 25. Cusick JF. Pathophysiology and treatment of cervical spondylotic myelopathy. Clin Neurosurg. 1991;37:661–681. [PubMed] [Google Scholar]
- 26. Cooper PR. Cervical spondylotic myelopathy. Contemp Neurosurg. 1997;19(25):1–7. [Google Scholar]
- 27. Nakamura K, Kurokawa T, Hoshino Y, Saita K, Takeshita K, Kawaguchi H. Conservative treatment for cervical spondylotic myelopathy: achievement and sustainability of a level of “no disability”. J Spinal Disord. 1998;11:175–179. [PubMed] [Google Scholar]
- 28. Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976). 2001;26:1890–1894. [DOI] [PubMed] [Google Scholar]
- 29. Matsumoto M, Toyama Y, Ishikawa M, Chiba K, Suzuki N, Fujimura Y. Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy. Does it predict the outcome of conservative treatment? Spine (Phila Pa 1976). 2000;25:677–682. [DOI] [PubMed] [Google Scholar]
- 30. Shimomura T, Sumi M, Nishida K, et al. Prognostic factors for deterioration of patients with cervical spondylotic myelopathy after nonsurgical treatment. Spine (Phila Pa 1976). 2007;32:2474–2479. doi:10.1097/BRS.0b013e3181573aee. [DOI] [PubMed] [Google Scholar]
- 31. Kadanka Z, Mares M, Bednanik J, et al. Approaches to spondylotic cervical myelopathy: conservative versus surgical results in a 3-year follow-up study. Spine (Phila Pa 1976). 2002;27:2205–2210. doi:10.1097/01.BRS.0000029255.77224.BB. [DOI] [PubMed] [Google Scholar]
- 32. Sampath P, Bendebba M, Davis JD, Ducker TB. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine (Phila Pa 1976). 2000;25:670–676. [DOI] [PubMed] [Google Scholar]
- 33. Yoshimatsu H, Nagata K, Goto H, et al. Conservative treatment for cervical spondylotic myelopathy. prediction of treatment effects by multivariate analysis. Spine J. 2001;1:269–273. [DOI] [PubMed] [Google Scholar]
- 34. Rhee JM, Shamji MF, Erwin WM, et al. Nonoperative management of cervical myelopathy: a systematic review. Spine (Phila Pa 1976). 2013;38(22 suppl):S55–S67. doi:10.1097/BRS.0b013e3182a7f41d. [DOI] [PubMed] [Google Scholar]
- 35. Law MD, Jr, Bernhardt M, White AA., 3rd Cervical spondylotic myelopathy: a review of surgical indications and decision making. Yale J Biol Med. 1993;66:165–177. [PMC free article] [PubMed] [Google Scholar]
- 36. Alafifi T, Kern R, Fehlings M. Clinical and MRI predictors of outcome after surgical intervention for cervical spondylotic myelopathy. J Neuroimaging. 2007;17:315–322. doi:10.1111/j.1552-6569.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 37. Holly LT, Moftakhar P, Khoo LT, Shamie AN, Wang JC. Surgical outcomes of elderly patients with cervical spondylotic myelopathy. Surg Neurol. 2008;69:233–240. doi:10.1016/j.surneu.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 38. Shamji MF, Mohanty C, Massicotte EM, et al. The association of cervical spine alignment with neurologic recovery in a prospective cohort of patients with surgical myelopathy: analysis of a series of 124 cases. World Neurosurg. 2016;86:112–119. doi:10.1016/j.wneu.2015.09.044. [DOI] [PubMed] [Google Scholar]
- 39. Tang JA, Scheer JK, Smith JS, et al. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery. 2015;76(suppl 1):S14–S21. doi:10.1227/01.neu.0000462074.66077.2b. [DOI] [PubMed] [Google Scholar]
- 40. Hardacker JW, Shuford RF, Capicotto PN, Pryor PW. Radiographic standing cervical segmental alignment in adult volunteers without neck symptoms. Spine (Phila Pa 1976). 1997;22:1472–1480. [DOI] [PubMed] [Google Scholar]
- 41. Luo J, Cao K, Huang S, et al. Comparison of anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy. Eur Spine J. 2015;24:1621–1630. doi:10.1007/s00586-015-3911-4. [DOI] [PubMed] [Google Scholar]
- 42. Zhu B, Xu Y, Liu X, Liu Z, Dang G. Anterior approach versus posterior approach for the treatment of multilevel cervical spondylotic myelopathy: a systemic review and meta-analysis. Eur Spine J. 2013;22:1583–1593. doi:10.1007/s00586-013-2817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghogawala Z, Benzel EC, Heary RF, et al. Cervical spondylotic myelopathy surgical trial: randomized, controlled trial design and rationale. Neurosurgery. 2014;75:334–346. doi:10.1227/NEU.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Macagno A, Liu S, Marascalchi BJ, et al. Perioperative risks associated with cervical spondylotic myelopathy based on surgical treatment strategies. Int J Spine Surg. 2015;9:24 doi: 10.14444/2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang JC, McDonough PW, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2000;25:41–45. [DOI] [PubMed] [Google Scholar]
- 46. Hu Y, Lv G, Ren S, Johansen D. Mid- to long-term outcomes of cervical disc arthroplasty versus anterior cervical discectomy and fusion for treatment of symptomatic cervical disc disease: a systematic review and meta-analysis of eight prospective randomized controlled trials. PLoS One. 2016;11:e0149312 doi:10.1371/journal.pone.0149312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suda K, Abumi K, Ito M, Shono Y, Kaneda K, Fujiya M. Local kyphosis reduces surgical outcomes of expansive open-door laminoplasty for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2003;28:1258–1262. doi:10.1097/01.BRS.0000065487.82469.D9. [DOI] [PubMed] [Google Scholar]
- 48. Abdullah KG, Yamashita T, Steinmetz MP, et al. Open-door cervical laminoplasty with preservation of posterior structures. Global Spine J. 2012;2:15–20. doi:10.1055/s-0032-1307258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kurokawa T, Tsuyama N, Tanaka H. Enlargement of spinal canal by the sagittal splitting of the spinous process. Bessatsu Seikeigeka. 1982;2:234–240. [Google Scholar]
- 50. Mitsunaga LK, Klineberg EO, Gupta MC. Laminoplasty techniques for the treatment of multilevel cervical stenosis. Adv Orthop. 2012;2012:307916 doi:10.1155/2012/307916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang MY, Shah S, Green BA. Clinical outcomes following cervical laminoplasty for 204 patients with cervical spondylotic myelopathy. Surg Neurol. 2004;62:487–492. doi:10.1016/j.surneu.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 52. Ratliff JK, Cooper PR. Cervical laminoplasty: a critical review. J Neurosurg. 2003;98:230–238. [DOI] [PubMed] [Google Scholar]
- 53. Suk KS, Kim KT, Lee JH, Lee SH, Lim YJ, Kim JS. Sagittal alignment of the cervical spine after the laminoplasty. Spine (Phila Pa 1976). 2007;32:E656–E660. doi:10.1097/BRS.0b013e318158c573. [DOI] [PubMed] [Google Scholar]
- 54. Duetzmann S, Cole T, Ratliff JK. Cervical laminoplasty developments and trends, 2003-2013: a systematic review. J Neurosurg Spine. 2015;23:24–34. doi:10.3171/2014.11.SPINE14427. [DOI] [PubMed] [Google Scholar]
- 55. Matsunaga S, Sakou T, Nakanisi K. Analysis of the cervical spine alignment following laminoplasty and laminectomy. Spinal Cord. 1999;37:20–24. [DOI] [PubMed] [Google Scholar]
- 56. van Geest S, de Vormer AM, Arts MP, Peul WC, Vleggeert-Lankamp CL. Long-term follow-up of clinical and radiological outcome after cervical laminectomy. Eur Spine J. 2015;24(suppl 2):229–235. doi:10.1007/s00586-013-3089-6. [DOI] [PubMed] [Google Scholar]
- 57. McAllister BD, Rebholz BJ, Wang JC. Is posterior fusion necessary with laminectomy in the cervical spine? Surg Neurol Int. 2012;3(suppl 3):S225–S231. doi:10.4103/2152-7806.98581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lee CH, Lee J, Kang JD, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical myelopathy: a meta-analysis of clinical and radiological outcomes. J Neurosurg Spine. 2015;22:589–595. doi:10.3171/2014.10.SPINE1498. [DOI] [PubMed] [Google Scholar]
- 59. Zdeblick TA, Ducker TB. The use of freeze-dried allograft bone for anterior cervical fusions. Spine (Phila Pa 1976). 1991;16:726–729. [DOI] [PubMed] [Google Scholar]
- 60. Anderson PA, Matz PG, Groff MW, et al. Laminectomy and fusion for the treatment of cervical degenerative myelopathy. J Neurosurg Spine. 2009;11:150–156. doi:10.3171/2009.2.SPINE08727. [DOI] [PubMed] [Google Scholar]
- 61. Shiraishi T, Fukuda K, Yato Y, Nakamura M, Ikegami T. Results of skip laminectomy-minimum 2-year follow-up study compared with open-door laminoplasty. Spine (Phila Pa 1976). 2003;28:2667–2672. doi:10.1097/01.BRS.0000103340.78418.B2. [DOI] [PubMed] [Google Scholar]
- 62. Minamide A, Yoshida M, Yamada H, et al. Clinical outcomes of microendoscopic decompression surgery for cervical myelopathy. Eur Spine J. 2010;19:487–493. doi:10.1007/s00586-009-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dahdaleh NS, Wong AP, Smith ZA, et al. Microendoscopic decompression for cervical spondylotic myelopathy. Neurosurg Focus. 2013;35:E8 doi:10.3171/2013.3.FOCUS135. [DOI] [PubMed] [Google Scholar]
- 64. Whitmore RG, Schwartz JS, Simmons S, Stein SC, Ghogawala Z. Performing a cost analysis in spine outcomes research: comparing ventral and dorsal approaches for cervical spondylotic myelopathy. Neurosurgery. 2012;70:860–867. doi:10.1227/NEU.0b013e3182367272. [DOI] [PubMed] [Google Scholar]
- 65. Fehlings MG, Jha NK, Hewson SM, Massicotte EM, Kopjar B, Kalsi-Ryan S. Is surgery for cervical spondylotic myelopathy cost-effective? A cost-utility analysis based on data from the AOSpine North America prospective CSM study. J Neurosurg Spine. 2012;17:89–93. doi:10.3171/2012.6.AOSPINE111069. [DOI] [PubMed] [Google Scholar]