Abstract
Study Design:
Retrospective study of prospectively collected data.
Objective:
To analyze the modified frailty index (mFI) as a predictor of adverse postoperative events following posterior lumbar fusion.
Methods:
The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database including all adult patients undergoing posterior lumbar interbody fusion or transforaminal lumbar interbody fusion between 2005 and 2012. Outcomes measured included mortality, postoperative complications, length of stay, reoperations, and readmissions. The previously described mFI was calculated, and univariate and multivariate logistic regression analysis were used to analyze risk factors associated with morbidity, mortality, and adverse postoperative events. This study was qualified as exempt by the Mount Sinai Hospital Institutional Review Board.
Results:
A total of 6094 patients met inclusion criteria. The mean mFI was 0.087(0-0.545). Increasing mFI score was associated with increased complications, reoperations, prolonged length of stay (LOS), and morbidity (P < .05). As the mFI score increased from 0.27 (3/11 variables present) to ≥0.36 (4/11), the rate of any complication increased from 26.8% to 35% (P < .0001), sepsis 2.4% to 5.2% (P < .0001), wound complications 4.4% to 6.5% (P < .0001), unplanned readmissions 4.7% to 20% (P = .02), and urinary tract infection 4.1% to 10.4% (P < .0001). An mFI of ≥0.36 was an independent predictor of any complication (odds ratio [OR]= 2.2, 95% confidence interval [CI] = 1.3-3.7), sepsis (OR = 6.3, 95%, CI = 1.8-21), wound complications (OR = 2.9, 95% CI = 1.1-8.2), prolonged LOS (OR = 2.3, 95% CI = 1.4-3.7), and readmission (OR = 4.3, 95% CI = 1.5-12.7).
Conclusion:
Patients with higher mFI scores (≥ 4/11 variables) are at a significantly higher risk of major complications, readmissions, and prolonged LOS following lumbar fusion.
Keywords: National Surgical Quality Improvement Program, frailty index, morbidity, mortality, posterior lumbar fusion
Introduction
Lumbar fusion is a common procedure for the treatment of elderly patients with degenerative conditions and spinal deformity using various types of instrumentation and bone graft. Posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) are the most common procedures with excellent outcomes supported by several well-designed studies, which has fueled an increase in utilization.1-5 The most dramatic increase in lumbar fusions has occurred in patients over 60 years old, and overall rates of these procedures has increased over 200% in the past 2 decades.5 Regardless, high complication rates are reported in the literature, and a growing elderly population raise concerns for the current preoperative risk models.3,5-7
The population over the age of 65 is expected to double over the next 3 decades, and comorbidities typically increase as individuals’ age, though considerable variability exists between patients.6-11 While older individuals are shown to be at higher risk than younger patients for surgical complications, biological age (physiologic reserve) is a better assessment than simply basing risk stratification on chronological age (measure of time).1,2,4,12 A greater understanding of the underlying physiologic state of a patient can determine whether they are in fact at a higher risk for postoperative complications.
Frailty is a term used to describe a decrease in physiologic reserve in elderly patients and is a process distinctly different than aging.7,8,10 Objectively measuring frailty and applying these models to patients undergoing surgery has shown to be a valid means of risk stratification across several surgical specialties.13-18 The Canadian Study of Health and Aging Frailty Index (CSHA-FI) has been defined as an index of 70 variables to measure the accumulative deficits with regard to physical, cognitive, functional, and social domains.11 This assessment tool along with modified versions (using 15 or less variables) have been studied and validated in patients undergoing surgery.13-18 Patel et al19 showed an association between frailty levels and mortality in patients with femoral neck fractures, while Obeid et al20 found a similar association in patients undergoing colectomy. In patients undergoing cardiac surgery, Afilalo et al showed frailty scores to be an independent predictor of postoperative morbidity.14
To the authors’ knowledge, frailty has not been studied with regard to patients undergoing lumbar spinal fusion. The modified frailty index (mFI) has been described in several studies and validated in the surgical literature.9,13,20,21 Our objective was to analyze the ability of the mFI to predict postoperative complications and mortality following surgery for PLIF and TLIF using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP). This database captures a large nationwide sample of patient factors, operative variables, and complications occurring within 30 days postoperatively.11,12 Our hypothesis was that a higher mFI score would be associated with a higher rate of postoperative complications and mortality in patients undergoing posterior lumbar fusion.
Materials and Methods
NSQIP Database
The ACS NSQIP database is a multicenter registry, which collects more than 200 variables on patients undergoing major surgical procedures. This database is maintained by on-site surgical clinical reviewers at participating institutions. In order to ensure data of the highest quality and reliability, NSQIP employs a series of rigorous training programs for the surgical clinical reviewers and conducts an ongoing internal auditing process. Per NSQIP, the variables collected include preoperative risk factors, intraoperative variables, and 30-day postoperative morbidity and mortality outcomes.11,12
All adult patients undergoing lumbar spinal fusion procedures and registered in the NSQIP database between 2005 and 2012 were included. The NSQIP collects data on more than 200 variables with regard to patient characteristics, comorbidities, operative variables, and 30-day postoperative events. The data was prospectively collected from more than 180 academic and private participating centers. A trained collector was staffed at each of the participating institutions to obtain this information. The NSQIP also utilized quality control processes to control interrater reliability and accuracy of data collection with the use of an auditing system.12
Study Cohort
In this study, we included patients ≥18 years old with the following Current Procedural Terminology (CPT) codes to identify patients who underwent lumbar spinal fusion, including posterior lumbar fusion (PLF), PLIF, TLIF, and PLF with PLIF or TLIF, between 2005 and 2012. The included codes were 22612, 22630, and 22633. Multilevel fusion was defined as having any of the following additional CPT codes: 22632, 22614, and 22632. Exclusion criteria were spinal deformity surgery, anterior lumbar fusion, nonelective, pregnant, ventilator dependent, underweight (body mass index [BMI], under 18.5 kg/m2), preoperative sepsis, emergencies, length of stay (LOS) longer than 365 days, central nervous system tumor, disseminated cancer, chemotherapy for malignancy within 30 days of operation, radiotherapy for malignancy within 90 days of operation, or acute renal failure.
Patient Factors
Demographic factors included age, race (Native Hawaiian or Pacific Islander, Asian, African American, Caucasian, other), gender, American Society of Anesthesiologists (ASA) score, alcohol use, smoking status, obesity, BMI score (<20 kg/m2, 20-30 kg/m2, or >30 kg/m2), diabetes (insulin dependent, insulin independent, oral medications), dyspnea, functional status prior to surgery (defined by NSQIP as independent: patient does not require assistance from another person for any activities of daily living [ADL]; partially dependent: the patient requires some assistance from another person for ADLs; or totally dependent: the patient requires total assistance for all ADLs), ventilator dependent, chronic obstructive pulmonary disease, congestive heart failure, prior myocardial infarction, prior percutaneous coronary intervention, prior cardiac surgery, history of angina, peripheral vascular disease, medication for hypertension, prior acute renal failure, dialysis, impaired sensorium, prior neuromuscular injury, history of stroke, steroid use within 30 days, recent weight loss, bleeding disorder, and preoperative transfusion. NSQIP has defined bleeding disorder as any patient at increased risk of bleeding (hemophilia, thrombocytopenia, chronic anticoagulation use, or vitamin K deficiency). Recent weight loss includes patients with greater than 10% decrease in body weight in the 6 months prior to surgery that was considered unintentional as reported by the patient. Neuromuscular injury includes a sustained acute or chronic neuromuscular condition resulting in paraplegia or quadriplegia. Steroid use was defined as the use of nontopical corticosteroid medications (for greater than 10 days) in the 30 days prior to surgery for a chronic medical condition.
Frailty Index
The mFI was previously described by Saxton and Velanovich by giving individuals a score based on 11 variables present in the CSHA-FI.16 Prior studies have validated using as few as 10 of the 70 variables defined in the CSHA-FI.17 The score is calculated by dividing the number of variables by the total number assessed (n/11). The 11 variables were a history of diabetes mellitus; functional status (independent or not independent); chronic obstructive pulmonary disease or pneumonia; congestive heart failure; prior myocardial infarction; percutaneous coronary intervention, stenting, or angina; hypertension requiring medication; peripheral vascular disease or ischemic rest pain; impaired sensorium; transient ischemic attack or cerebrovascular accident; or cerebrovascular accident with neurological deficits. Functional status was defined as 1 (patient is independent requiring no assistance for ADL), 2 (patient is partially dependent on another person for ADLs), or 3 (patient is totally dependent on another individual for ADLs; Table 1).
Table 1.
The 11 Variables of the Modified Frailty Index.
|
Outcomes
Our primary outcomes of interest were complications, reoperation, and mortality occurring within 30 days following posterior lumbar fusion. Postoperative complications included pneumonia, sepsis, deep vein thrombosis, pulmonary embolism, wound complication, deep infection, central nervous system complication, sepsis/septic shock, cardiac arrest, acute renal failure, or urinary tract infection. Additionally, reoperation, unplanned reoperation or readmission, and prolonged LOS of >5 days were recorded and analyzed.
Statistical Analysis
The statistical analysis was performed using the SAS software (Version 9.3, SAS Institute Inc, Cary, NC). The mFI was calculated by dividing the number of variables present from the mFI score by 11. We used χ2 test, t test, and Pearson correlation test. Multivariate analysis was performed to control for patient and operative variables and to assess which mFI scores were predictors of complications, mortality, and postoperative events. We also compared mFI score with age >60 years, ASA >3, and obese class III to assess the capacity to predict morbidity and mortality. To account for collinearity, the stepwise method included variables with P < .1 to enter into the model and P < .05 as independent predictors. Odds ratios (ORs) were calculated with 95% confidence intervals (CIs).
Results
Operative Variables
Overall, 6094 patients underwent lumbar spinal fusion surgery who were registered in the NSQIP database between 2005 and 2012. The mean age was 60 (SD 13.9, range 18-90), and 55% of the patients were female. The mean BMI was 30 (SD 6.3, range 18.5-98). PLF was documented in 70.6%, PLIF/TLIF in 24%, and PLF with PLIF/TLIF in 5% of cases based on CPT coding. Average operative time was 215 minutes (SD 103), and average LOS was 4.3 (SD 3.6; see Tables 2 and 3).
Table 2.
Operative Variables for Posterior Lumbar Fusion, N = 6094.
| Male | 45.0% |
| Female | 55.0% |
| PLF (CPT 22612) | 70.6% |
| PLIF/TLIF (CPT 22630) | 24.0% |
| PLF + PLIF/TLIF (CPT 22633) | 5.0% |
Abbreviations: CPT, current procedural terminology; PLF, posterior lumbar fusion; PLIF, posterior lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion.
Table 3.
Operative Variables for Posterior Lumbar Fusion, N = 6094.
| Variable | Mean | SD |
|---|---|---|
| Total relative value units | 52.7 | 26.5 |
| Operative time (minutes) | 214.6 | 103.4 |
| Length of stay (days) | 4.2 | 3.6 |
Frailty Index Distribution
The mean mFI for this study population was 0.087 (range 0-5.45), which corresponds to slightly less than 1 out of 11 variables present. The mFI score in 2266 patients was 0, 2309 was 0.09 (1/11 variables), 1147 was 0.18 (2/11 variables), 295 was 0.27 (3/11 variables), and 77 was ≥0.36 (4/11 variables; see Figure 1).
Figure 1.
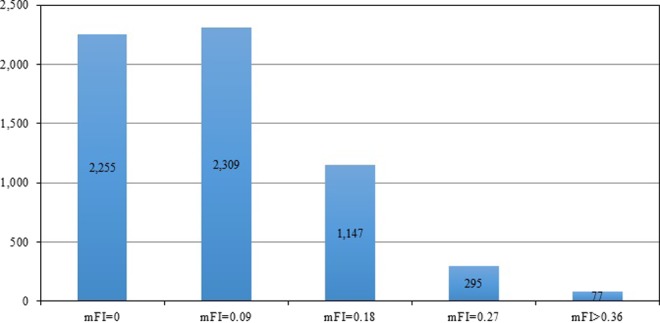
Histogram of patient distribution based on mFI scores.
Frailty Index as a Predictor of Postoperative Events
Patients with higher mFI scores had significantly higher rates of mortality, reoperation, longer LOS, unplanned readmission, and several postoperative complications (all P < .01). As the mFI increased from 0 to ≥0.36, rates of any complication increased from 14.7% to 35.1% (P < .0001), LOS >5 days increased from 13.4% to 35% (P < .0001), and unplanned readmission rates increased from 4.5% to 20% (P = .02; see Table 4). Rates of pulmonary, renal, pulmonary embolism/deep vein thrombosis, sepsis, urinary tract infection, blood transfusion, and wound complications all increased in a stepwise fashion with increasing mFI (all P < .02). Interestingly, mortality increased from 0.1% in patients with mFI scores of 0 to 1% in patients with mFI score of 0.27, and decreased to 0% in patients with mFI scores over 0.36.
Table 4.
Complications Associated With Increasing Modified Frailty Index.
| Modified Frailty Index, % | ||||||
|---|---|---|---|---|---|---|
| 0 | 0.09 | 0.18 | 0.27 | ≥0.36 | P | |
| N = 6094 | 2266 | 2309 | 1147 | 295 | 77 | |
| Any complication | 14.7% | 19.6% | 22.8% | 26.8% | 35.1% | <.0001 |
| Intra/postoperative blood transfusion | 11.8% | 15.9% | 17.1% | 20.3% | 23.4% | <.0001 |
| Death | 0.1% | 0.1% | 0.4% | 1.0% | 0.0% | .004 |
| Pulmonary complication | 0.4% | 0.9% | 2.1% | 2.0% | 2.6% | <.0001 |
| Renal complication | 0.0% | 0.1% | 0.7% | 0.7% | 1.3% | <.0001 |
| Central nervous system complication | 0.1% | 0.2% | 0.4% | 0.3% | 0.0% | .278 |
| Peripheral nerve injury | 0.4% | 0.2% | 0.3% | 0.0% | 0.0% | .236 |
| Cardiac complication | 0.4% | 0.3% | 0.9% | 1.0% | 0.0% | .053 |
| Venous thrombus emboli | 0.8% | 1.4% | 1.6% | 2.0% | 2.6% | .011 |
| Urinary tract infection | 1.2% | 2.4% | 2.7% | 4.1% | 10.4% | <.0001 |
| Sepsis | 0.6% | 0.8% | 1.7% | 2.4% | 5.2% | <.0001 |
| Wound complication | 1.8% | 2.0% | 3.4% | 4.4% | 6.5% | <.0001 |
| Graft failure | 0.2% | 0.0% | 0.1% | 0.0% | 0.0% | .235 |
| Other outcomes | ||||||
| Reoperation | 3.6% | 3.0% | 3.7% | 5.1% | 10.4% | .057 |
| Length of stay >5 days | 13.4% | 18.6% | 22.2% | 26.8% | 35.1% | <.0001 |
| Unplanned readmission (2011-2012) | 4.5% | 4.1% | 6.9% | 4.7% | 20.0% | .015 |
Multivariate Logistic Regression Analysis
Using multivariate analysis, a mFI score ≥0.36 (4/11 variables) was an independent predictor of any complication (OR = 2.2, 95% CI =1.3-3.7, P = .014), sepsis (OR = 6.3, 95% CI = 1.8-21, P = .01), wound complications (OR = 3.0, 95% CI = 1.1-8.2, P = .05), prolonged LOS (OR = 2.3, 95% CI = 1.4-3.7, P = .01), and unplanned readmission (OR = 4.3, 95% CI = 1.5-12.7, P = .005). Patients with mFI scores of >0.36 were compared with age over 60 years, obesity class of >III (BMI > 40), and ASA score ≥3, and mFI was a superior predictor of unplanned readmission and sepsis (Table 5).
Table 5.
Multivariate Logistic Regression to Assess Modified Frailty Index as an Independent Risk Factor.a
| Any Complication | Sepsis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||||
| Risk Factor | Adjusted OR | Lower | Upper | P | Risk Factor | Adjusted OR | Lower | Upper | P |
| Modified frailty index: 0.09 vs 0 | 1.113 | 0.938 | 1.321 | .023 | Modified frailty index: 0.09 vs 0 | 1.113 | 0.538 | 2.305 | .012 |
| 0.18 vs 0 | 1.21 | 0.98 | 1.494 | .294 | 0.18 vs 0 | 1.951 | 0.898 | 4.239 | .866 |
| 0.27 vs 0 | 1.367 | 0.994 | 1.879 | .791 | 0.27 vs 0 | 2.531 | 0.918 | 6.977 | .52 |
| 0.36 vs 0 | 2.196 | 1.303 | 3.702 | .014 | 0.36 vs 0 | 6.275 | 1.837 | 21.436 | .01 |
| Age: 65 to 79 vs 18 to 64 | 1.472 | 1.268 | 1.709 | .08 | Age: 65 to 79 vs 18 to 64 | 0.759 | 0.435 | 1.324 | .447 |
| ≥80 vs 18 to 64 | 1.604 | 1.219 | 2.11 | .036 | ≥80 vs 18 to 64 | 0.937 | 0.35 | 2.508 | .88 |
| BMI class: Obese I vs nonobese | 0.826 | 0.7 | 0.975 | .003 | BMI class: Obese I vs nonobese | 0.941 | 0.494 | 1.793 | .21 |
| Obese II vs nonobese | 0.924 | 0.748 | 1.142 | .315 | Obese II vs nonobese | 1.398 | 0.691 | 2.829 | .662 |
| Obese III vs nonobese | 1.315 | 1.024 | 1.687 | .003 | Obese III vs nonobese | 1.879 | 0.87 | 4.055 | .135 |
| ASA ≥3 | 1.281 | 1.098 | 1.495 | .002 | ASA ≥3 | 1.696 | 0.915 | 3.141 | .093 |
| Prolonged LOS (>5 Days) | 30=Day Unplanned Readmission (2011-2012) | ||||||||
| 95% CI | 95% CI | ||||||||
| Risk Factor | Adjusted OR | Lower | Upper | P | Risk Factor | Adjusted OR | Lower | Upper | P |
| Modified frailty index: 0.09 vs 0 | 1.186 | 0.999 | 1.407 | .0356 | Modified frailty index: 0.09 vs 0 | 0.836 | 0.527 | 1.326 | .0156 |
| 0.18 vs 0 | 1.277 | 1.037 | 1.573 | .3272 | 0.18 vs 0 | 1.345 | 0.792 | 2.284 | .9463 |
| 0.27 vs 0 | 1.489 | 1.093 | 2.029 | .5436 | 0.27 vs 0 | 0.851 | 0.336 | 2.152 | .2153 |
| 0.36 vs 0 | 2.252 | 1.362 | 3.723 | .0137 | 0.36 vs 0 | 4.3 | 1.458 | 12.68 | .0052 |
| Age: 65 to 79 vs 18 to 64 | 1.244 | 1.072 | 1.444 | .9312 | Age: 65 to 79 vs 18 to 64 | 0.906 | 0.609 | 1.35 | .634 |
| ≥80 vs 18 to 64 | 1.571 | 1.202 | 2.052 | .0084 | ≥80 vs 18 to 64 | 1.037 | 0.474 | 2.268 | .8252 |
| BMI class: Obese I vs nonobese | 0.895 | 0.76 | 1.054 | .0173 | BMI class: Obese I vs nonobese | 1.1 | 0.716 | 1.691 | .9339 |
| Obese II vs nonobese | 0.942 | 0.764 | 1.162 | .2048 | Obese II vs nonobese | 0.981 | 0.555 | 1.731 | .626 |
| Obese III vs nonobese | 1.388 | 1.089 | 1.768 | .0012 | Obese III vs nonobese | 1.287 | 0.687 | 2.413 | .4562 |
| ASA ≥3 | 1.543 | 1.323 | 1.798 | <.0001 | ASA ≥3 | 1.261 | 0.833 | 1.91 | .273 |
| Wound Complications | |||||||||
| 95% CI | |||||||||
| Risk Factor | Adjusted OR | Lower | Upper | P | |||||
| Modified frailty index: 0.09 vs 0 | 0.918 | 0.584 | 1.441 | .01 | |||||
| 0.18 vs 0 | 1.321 | 0.796 | 2.19 | .661 | |||||
| 0.27 vs 0 | 1.64 | 0.813 | 3.308 | .592 | |||||
| 0.36 vs 0 | 2.991 | 1.086 | 8.239 | .056 | |||||
| Age: 65 to 79 vs 18 to 64 | 0.727 | 0.494 | 1.071 | .115 | |||||
| ≥80 vs 18 to 64 | 1.088 | 0.543 | 2.177 | .477 | |||||
| BMI class: Obese I vs nonobese | 1.748 | 1.146 | 2.666 | .698 | |||||
| Obese II vs nonobese | 1.694 | 1.002 | 2.863 | .63 | |||||
| Obese III vs nonobese | 3.922 | 2.39 | 6.437 | <.0001 | |||||
| ASA ≥3 | 1.491 | 0.999 | 2.227 | .051 | |||||
Abbreviations: OR, odds ratio; CI, confidence interval; BMI, body mass index; ASA, American Society of Anesthesiologists.
aBoldface indicates P < .05.
Discussion
With the rapidly growing elderly population and increasing rates of surgery for lumbar spinal pathology, an accurate assessment of frailty and perioperative risk factors may help minimize complications and unnecessary costs. Elderly patients often have a considerable variability in physiologic reserve; therefore, spine surgeons should accurately assess which patients are at high risk for perioperative events and which patients may benefit from surgery. Frailty has been characterized by the mFI—a simple tool that has been validated as a predictor of postoperative events in several surgical cohorts.13,15,16,20,22-24 Our study is the first to analyze the utility of the mFI in patients undergoing common lumbar spine procedures, PLIF, and TLIF. Using a large nationwide database of more than 6000 patients, we found a low overall mean mFI of 0.087, though frailty was a powerful predictor of postoperative events.
As mFI scores increased, rates of any complication, several specific complications, unplanned readmission, and LOS beyond 5 days all increased significantly (P < .05). These variables increased in a stepwise manner as mFI scores increased from 0 to ≥0.36. Mortality rates also increased 10-fold (0.1% to 1.0%) as mFI increased from 0 to 0.27, though decreased to 0 in patients with mFI ≥0.36. It is unclear why this trend was observed, though the small sample size may suggest the analysis was slightly underpowered. Regardless, rates of pulmonary complications, renal complications, venous thrombus emboli, urinary tract infection, sepsis, and wound complications all increased significantly with increasing mFI (all P < .05). Lee et al found an association between frailty and prolonged institutional care and mortality in patients undergoing cardiac surgery.7 Similarly, Makary et al25 found an association between frailty and prolonged LOS, discharge to skilled care, and postoperative complications in more than 590 patients undergoing various elective surgeries. Interestingly, they utilized a frailty index that assessed phenotypic factors (weight loss, exhaustion, walking speed, and physical variables), which are not evaluated in the mFI and not possible to evaluate with the NSQIP database. Future studies may find improved predictability of complications in combining these variables with an index such as the mFI (which is based on comorbidities and systems deficits), as both have shown efficacy as preoperative screening tools. Similar to our findings, a study of patients undergoing head and neck surgery, the patients had similar baseline frailty (0.07 for inpatients and 0.04 for outpatients) and they also showed mFI to be associated with several postoperative complications.13
An mFI score of >0.36 was an independent predictor of any complication, sepsis, wound complications, and unplanned readmissions (P < .05). As mFI scores increased from 0 to >0.36, rates of wound complication increased from 1.8% to 6.5%, sepsis increased from 0.6% to 5.2%, any complication increased from 5% to 19.5%, and unplanned readmission increased from 4.5% to 20%. These were significant and concerning findings that could potentially be minimized by screening elderly patients planning for a lumbar fusion. In a study of more than 35 000 trauma patients over the age of 60 years, Farhat et al found a direct relationship between frailty index scores and rates of wound infections, any infection, and mortality.22 Adams et al found that an mFI score of >0.45 was an independent predictor of life-threatening complications, which included sepsis and other complications.13 Applying the mFI to patients undergoing PLIF or TLIF may help prevent these complications that have a tremendous impact on healthcare cost and patient outcomes.
Age, obesity, and ASA scores have also been associated with postoperative complications and poor outcomes, so we compared these variables with mFI scores. Frailty showed to be the superior predictor of any complication, sepsis, prolonged LOS, and unplanned readmission. Other studies have shown similar results with regard to postoperative complications in surgical cohorts.13,14,18,20,21 In a database analysis of more than 67 000 patients undergoing vascular surgery, frailty index was found to be a stronger predictor of mortality than age or ASA score.21 A possible explanation is that age and ASA scores are fairly nonspecific parameters and have been shown to be variable between individuals. For example, an 80-year-old patient may have normal physiologic capacity across all organ systems; however, a different 80-year-old patient may have extensive comorbidities, disability, and level of frailty. Older age may be associated with frailty, though prior studies have shown that 75% of patients over 85 are not frail.26 Similarly, ASA score is a nonspecific parameter that may be useful for guiding anesthesia requirements, though it was an inferior variable compared with frailty in predicting postoperative events.
Our study had several limitations, including the retrospective design, though the data was collected prospectively. Based on NSQIP criteria, there was a limit to the number of cases reported by each institution, which may lead to underreporting.16,27 The accuracy of data gathering was also difficult to evaluate with a large database collection, though the participating centers of NSQIP were trained prior to participation.27 As previously mentioned, the mFI criteria utilized in this study did not measure the presence of weight loss, slow walking speed, exhaustion, weakness, and low level of physical activity. These variables are considered part of the “phenotype” of frailty and have been shown to be predictive of postoperative complications. However, these measures cannot be evaluated using the NSQIP database as they are not recorded.17,23 The “deficit accumulation model” of frailty (as evaluated by the mFI) is based on current illness and function, and has shown to have clear advantages in evaluating elderly patients, though debate continues as to which model is superior.23,28 More comprehensive assessment tools such as the CSHA-FI and Comprehensive Geriatric Assessment have shown validity, though are more time consuming to utilize and would also not be possible to score using the NSQIP database. Pilotto et al compared 4 different frailty models in adults with femoral neck fractures and found that each was valid in associating frailty with short- and long-term mortality rates.28 The model we chose to apply to patients undergoing PLIF and TLIF has been validated by several other studies in using these 11 variables from the CSHA-FI, which allows for more widespread application.13,17,29 Future studies may combine the mFI with variables such as smoking status, laboratory values, or radiographic parameters to further improve the predictability of perioperative events, though our study did not assess these factors. For instance, Ronning et al showed that increased levels of interleukin-6 was an independent predictor of postoperative complications in patients undergoing colorectal surgery.28
Despite these limitations, our investigation showed that frailty was a strong predictor of several postoperative events following PLIF and TLIF. The mFI, which was utilized in our study, surpassed age, obesity, and ASA scores in ability to predict several postoperative events. Patients with mFI scores higher than 0.36 were at a significantly higher risk of any postoperative complication, prolonged LOS, and unplanned readmission. In this era of cost containment and high patient expectations, this screening tool can serve to improve our current risk stratification model. As indications for PLIF and TLIF are broadening and the elderly population is dramatically increasing, an augmented understanding of frailty can improve our ability to prevent postoperative complications and optimize long-term outcomes. Combining mFI with other variables in future studies may further improve our ability to risk stratify patients undergoing lumbar spinal fusion.
Footnotes
Authors’ Note: This study was qualified as exempt by the Mount Sinai Hospital Institutional Review Board.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SKC has received funding from Stryker and OREF outside the submitted work. All other authors declare no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, part II. 1-Year outcomes of surgical and nonsurgical management of sciatica. Spine (Phila Pa 1976). 1996;21:1777–1786. [DOI] [PubMed] [Google Scholar]
- 2. Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE. Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the Maine lumbar spine study. Spine (Phila Pa 1976). 2005;30:927–935. [DOI] [PubMed] [Google Scholar]
- 3. Davis H. Increasing rates of cervical and lumbar spine surgery in the United States, 1979-1990. Spine (Phila Pa 1976). 1994;19:1117–1123. [DOI] [PubMed] [Google Scholar]
- 4. Lee CS, Hwang CJ, Lee DH, Kim YT, Lee HS. Fusion rates of instrumented lumbar spinal arthrodesis according to surgical approach: a systematic review of randomized trials. Clin Orthop Surg. 2011;3:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. Four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009;91:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kulminski AM, Ukraintseva SV, Culminskaya IV, et al. Cumulative deficits and physiological indices as predictors of mortality and long life. J Gerontol A Biol Sci Med Sci. 2008;63:1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rockwood K. What would make a definition of frailty successful? Age Ageing. 2005;34:432–434. [DOI] [PubMed] [Google Scholar]
- 8. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karam JA, Shepard A, Peters D, Swartz A, Heath A, Rubinfeld IS. A simplified frailty index to predict postoperative adverse outcomes and mortality in vascular surgery patients. J Am Coll Surg. 2011;213:S156–S157. [Google Scholar]
- 10. Martin FC, Brighton P. Frailty: different tools for different purposes? Age Ageing. 2008;37:129–131. [DOI] [PubMed] [Google Scholar]
- 11. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhagat S, Vozar V, Lutchman L, Crawford RJ, Rai AS. Morbidity and mortality in adult spinal deformity surgery: Norwich Spinal Unit experience. Eur Spine J. 2013;22(suppl 1):S42–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams P, Ghanem T, Stachler R, Hall F, Velanovich V, Rubinfeld I. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg. 2013;139:783–789. [DOI] [PubMed] [Google Scholar]
- 14. Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222–228. [DOI] [PubMed] [Google Scholar]
- 15. Rowe R, Iqbal J, Murali-Krishnan R, et al. Role of frailty assessment in patients undergoing cardiac interventions. Open Heart. 2014;1:e000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011;253:1223–1229. [DOI] [PubMed] [Google Scholar]
- 17. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sundermann S, Dademasch A, Rastan A, et al. One-year follow-up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact Cardiovasc Thorac Surg. 2011;13:119–123. [DOI] [PubMed] [Google Scholar]
- 19. Patel KV, Brennan KL, Brennan ML, Jupiter DC, Shar A, Davis ML. Association of a modified frailty index with mortality after femoral neck fracture in patients aged 60 years and older. Clin Orthop Relat Res. 2014;472:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obeid NM, Azuh O, Reddy S, et al. Predictors of critical care-related complications in colectomy patients using the National Surgical Quality Improvement Program: exploring frailty and aggressive laparoscopic approaches. J Trauma Acute Care Surg. 2012;72:878–883. [DOI] [PubMed] [Google Scholar]
- 21. Karam J, Tsiouris A, Shepard A, Velanovich V, Rubinfeld I. Simplified frailty index to predict adverse outcomes and mortality in vascular surgery patients. Ann Vasc Surg. 2013;27:904–908. [DOI] [PubMed] [Google Scholar]
- 22. Farhat JS, Velanovich V, Falvo AJ, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72:1526–1530. [DOI] [PubMed] [Google Scholar]
- 23. Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodes-Cabau J, Webb JG, Cheung A, et al. Long-term outcomes after transcatheter aortic valve implantation: insights on prognostic factors and valve durability from the Canadian multicenter experience. J Am Coll Cardiol. 2012;60:1864–1875. [DOI] [PubMed] [Google Scholar]
- 25. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. [DOI] [PubMed] [Google Scholar]
- 26. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. [DOI] [PubMed] [Google Scholar]
- 27. American College of Surgeons. American College of Surgeons National Surgical Quality Improvement Program. http://site.acsnsqip.org/wp-content/uploads/2012/03/2010-User-Guide_FINAL.pdf. Accessed May 26, 2016.
- 28. Ronning B, Wyller TB, Seljeflot I, et al. Frailty measures, inflammatory biomarkers and post-operative complications in older surgical patients. Age Ageing. 2010;39:758–761. [DOI] [PubMed] [Google Scholar]
- 29. Pilotto A, Rengo F, Marchionni N, et al. Comparing the prognostic accuracy for all-cause mortality of frailty instruments: a multicentre 1-year follow-up in hospitalized older patients. PLoS One. 2012;7:e29090. [DOI] [PMC free article] [PubMed] [Google Scholar]


