Abstract
The ratio between proteases and their inhibitors is unbalanced in cancer. The cysteine protease inhibitor cystatin C is internalized by some cancer cells, which affects cellular properties. Here we aimed to investigate if uptake of cystatin C and the related inhibitor cystatin E/M occur in melanoma cell lines and to evaluate to what extent the uptake affects the legumain activity that is typically increased in melanoma. First we studied the basic expression, secretion, and intracellular content of all type 2 cystatins as well as expression and activity of their possible target enzymes legumain and cathepsin B in MDA-MB-435S, A375, and C8161 melanoma cells. Legumain activity was measureable in all cell lines, and of the potential legumain inhibitors, cystatin C, E/M, and F, cystatin C was the one mainly produced. All cells internalized cystatin C added to culture media, leading to increased intracellular cystatin C levels by 120–200%. Cystatin E/M was internalized as well but at a modest rate. The effects on intracellular legumain activity were nevertheless pronounced, probably because the cells lacked this inhibitor, and its affinity for legumain is 100-fold higher than that of cystatin C. Likewise, the low-degree uptake resulted in reduced migration and invasion of A375 cells in Matrigel to an extent comparable with the W106F variant of cystatin C with optimal uptake properties and resulting in much higher intracellular levels. Thus, cystatin E/M appears to be a good candidate to efficiently down-regulate the increased legumain activity, possibly important for the malignant phenotype of melanoma cells.
Keywords: cancer, cathepsin B (CTSB), cysteine protease, enzyme inhibitor, melanoma, cystatin, legumain
Introduction
Lysosomal proteases, like cathepsin B and legumain, are fundamental in the cellular metabolism of proteins. Some of these enzymes are, moreover, involved in the processes of cancer invasion (1), growth, and metastasis (2–4). Cathepsin B is one of 11 human “cysteine cathepsins,” members of protease family C1 according to the MEROPS classification (5). Legumain or asparaginyl endopeptidase is another type of cysteine protease belonging to protease family C13 (5). In human cells it is mainly found in endolysosomal compartments but has also been detected on the cellular surface of tumor cells or as a secreted enzyme (6, 7).
We have in the present study aimed to address a cancer-associated mis-regulation of legumain and cathepsin B activity appearing to be at hand in melanoma cells by investigating the natural inhibitors of these two enzymes, the cystatins. Of these, cystatin C and E/M are two-headed inhibitors capable of binding one cysteine cathepsin and one legumain molecule concurrently by opposing parts of the molecule (8, 9). The cystatin family consists of three subgroups of protease inhibitors; type 1 cystatins, which are intracellular cysteine cathepsin inhibitors, type 2 cystatins, which are synthesized with a signal peptide and thus secreted, and type 3 cystatins, which are intravascular and also act as kinin precursors, the kininogens. Both cystatin C and cystatin E/M are type 2 cystatins and, together with cystatin F are the only cystatins capable of legumain inhibition (8).
Typical type 2 cystatins are small molecules with a size of 13–14 kDa. They consist of ∼120-amino acid-residue-long polypeptides, which generally show a high degree of sequence similarity, but cystatin C and cystatin E/M are quite distantly related (<35% sequence identity when aligned). The genes coding for type 2 cystatins are generally located on chromosome 20, but the gene coding for cystatin E/M is located on chromosome 11. Cystatin E/M is different from most other type 2 cystatins, as it is found in either a glycosylated or unglycosylated form (10, 11). Cystatins C and E/M also show marked differences in tissue distribution. Cystatin C is expressed in virtually all tissues and cells and secreted into all body fluids in biologically significant quantities (12). Therefore, it is potentially the most important cysteine protease inhibitor in humans, generally speaking. In contrast, cystatin E/M expression is lower in level, much more specific, and seems to be restricted to epithelial cells (10), especially those of the skin (13). Thus, both cystatin C and cystatin E/M should be highly relevant for our study aim to address the apparent mis-regulation of legumain and cathepsin B activity at hand in melanoma.
CST6, the gene encoding cystatin E/M, has been proposed to be a tumor suppressor gene. It was first recognized as a down-regulated gene in breast cancer metastases (11), but later studies have shown that expression of cystatin E/M is epigenetically silenced in many cancers including those of the breast and prostate as well as in gastric carcinoma and glioma cells (14–18). Of relevance for the skin, where the cystatin E/M expression normally is high relatively speaking (13), and hence for our study aim, one study has reported undetectable cystatin E/M expression in both established melanoma cell lines and primary melanoma cell cultures from patients, whereas the legumain activity in the cells was high (19). The legumain activity was reduced when these melanoma cells were transfected to overproduce cystatin E/M as were the cells' proliferation, migration, invasion, and adhesion to endothelial cells (19). In another study cystatin E/M was detected within HEK293 cells when incubated with conditioned medium from cells overproducing the inhibitor (20), indicating that a cellular uptake of cystatin E/M could occur. This could well coincide with the cellular uptake system we recently delineated, leading to high-level internalization of the more abundant inhibitor cystatin C into model cancer cells in biologically relevant amounts sufficient to down-regulate activities of intracellular target enzymes such as cysteine cathepsins and legumain (21, 22).
Taken together, available data indicate that a parallel investigation of cystatin C and cystatin E/M with respect to potential for cellular internalization should be relevant for our study aim to elucidate the means to affect a cancer-associated mis-regulation of protease activity in melanoma. Specifically, we aimed to analyze the intracellular effects of such uptake with a focus on legumain inhibition, as cystatin E/M seems to have a net effect as a tumor suppressor in melanoma and also is the tightest-binding legumain inhibitor known (8). A basic evaluation of the expression, intracellular content, and secretion of all type 2 cystatins in melanoma cell lines was furthermore pursued in order to put the results into perspective.
Results
Expression of enzymes and inhibitors in melanoma cell lines
Total RNA was isolated from the melanoma cell lines A375, C8161, and MDA-MB-435S as well as the breast adenocarcinoma cell line MCF-7. The relative mRNA expression of the type 2 cystatins (cystatins C, D, E/M, F, S, SA, and SN) and the enzymes legumain and cathepsin B was analyzed by quantitative real-time (qRT)2-PCR and correlated to the expression of 18S rRNA (Fig. 1). Expression of legumain and cathepsin B mRNA was found in all cell lines, and the highest levels were seen in C8161 cells. The mRNA levels for all of the cystatins were close to the detection limit with the exception of cystatin C mRNA, which had a high expression level in all cell lines. The highest degree of cystatin C expression was detected in MCF-7 cells, which expressed twice as much compared with the other cell lines. Interestingly, expression of the salivary cystatin SN was detected in MDA-MB-435S and MCF-7 cells. In addition, a low level of mRNA for cystatin F, which normally is expressed in blood cells, was detected in the MDA-MB-435S cells.
Figure 1.
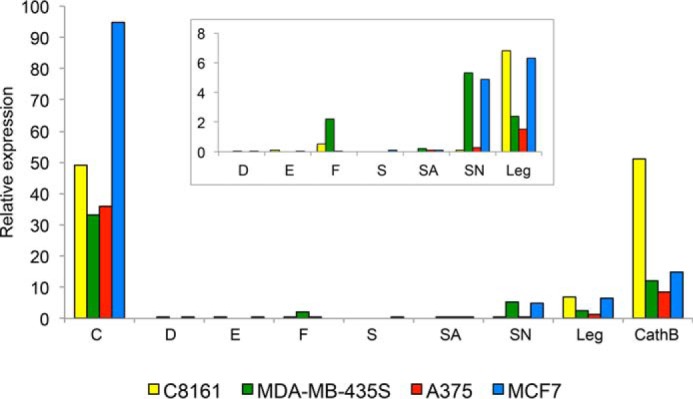
Relative expression of type 2 cystatins, legumain, and cathepsin B. Levels of mRNA encoding type 2 cystatins, legumain (Leg), and cathepsin B (CathB) in three melanoma cell lines and the breast cancer cell line MCF-7 were measured by qRT-PCR and related to 18S rRNA levels. Each bar represents the mean of triplicate measurements, and the value is multiplied by a factor of 106. For each cell line cDNA from the same culture was used. Inset, blow-up to visualize the relative levels of the lowly expressed cystatins and legumain. The CV was below 3% in all analyses.
Secretion and intracellular content of cystatins in melanoma cell lines
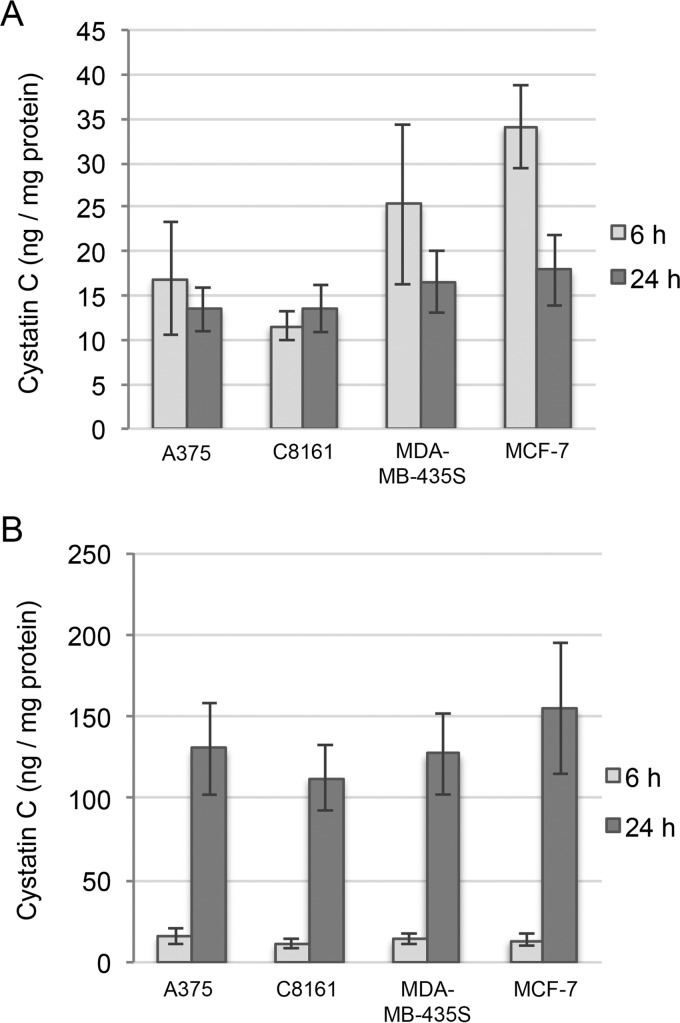
The amount of cystatins C, D, E/M, and F in conditioned media and cell lysates was analyzed by ELISA. The levels of cystatins E/M, D, and F were below detection limits in all samples (data not shown). Cystatin C could be measured in both media and lysates from all cell lines, in agreement with the high levels of mRNA shown by qRT-PCR. The secreted cystatin C accumulated in the medium with time and was found in analogous amounts in all samples (24 h: 112–155 ng/mg of protein). The cystatin C content in the lysates from A375 and C8161 cells did not change between 6 and 24 h but decreased in MCF-7 and MDA-MB-435S cells (Fig. 2, A and B).
Figure 2.
Secretion and intracellular content of cystatins produced by melanoma cell lines. Cells were cultured for 6 or 24 h in standard growth medium. A and B, cystatin C was quantified in the cell homogenates (A) and media (B) by ELISA, and the values were correlated to total protein content of the homogenates. Bars represent mean values of duplicate wells from three experiments. The samples were run in duplicate wells in the ELISA measurements. Error bars represent standard deviation (S.D.) of results.
Immunoblotting was performed with the aim to detect cystatin S, SA, and SN because no ELISA methods were available. As these cystatins share 90% identical amino acid residues, it is impossible to detect them individually with the available antibodies, which will cross-react (12). Because the expression was low, the cystatins were first captured on carboxymethylated (CM)-papain-Sepharose beads. No immunoreactive bands were detected in the lysates (data not shown). In the conditioned media from the melanoma cell lines, weak 14-kDa immunoreactive bands were seen corresponding to a band in the positive control sample included as a reference, which consisted of saliva (data not shown). Cystatin SN is the most expressed of the salivary cystatins, cystatin S, SA, and SN. The strongest band was detected in the MDA-MB-435S medium, in line with the expression pattern analyzed by qRT-PCR, showing the highest cystatin SN mRNA level in these cells.
Cystatin uptake in melanoma cells
It has been shown that legumain activity is suppressed in melanoma cells that overexpress cystatin E/M and that these cells are less invasive in Matrigel (19). Another study reported on detection of intracellular cystatin E/M when cells were incubated in conditioned medium from cells expressing cystatin E/M. This led us to examine if cystatin E/M was internalized into the melanoma cells, as cystatin C is taken up by other cancer cells (21–23).
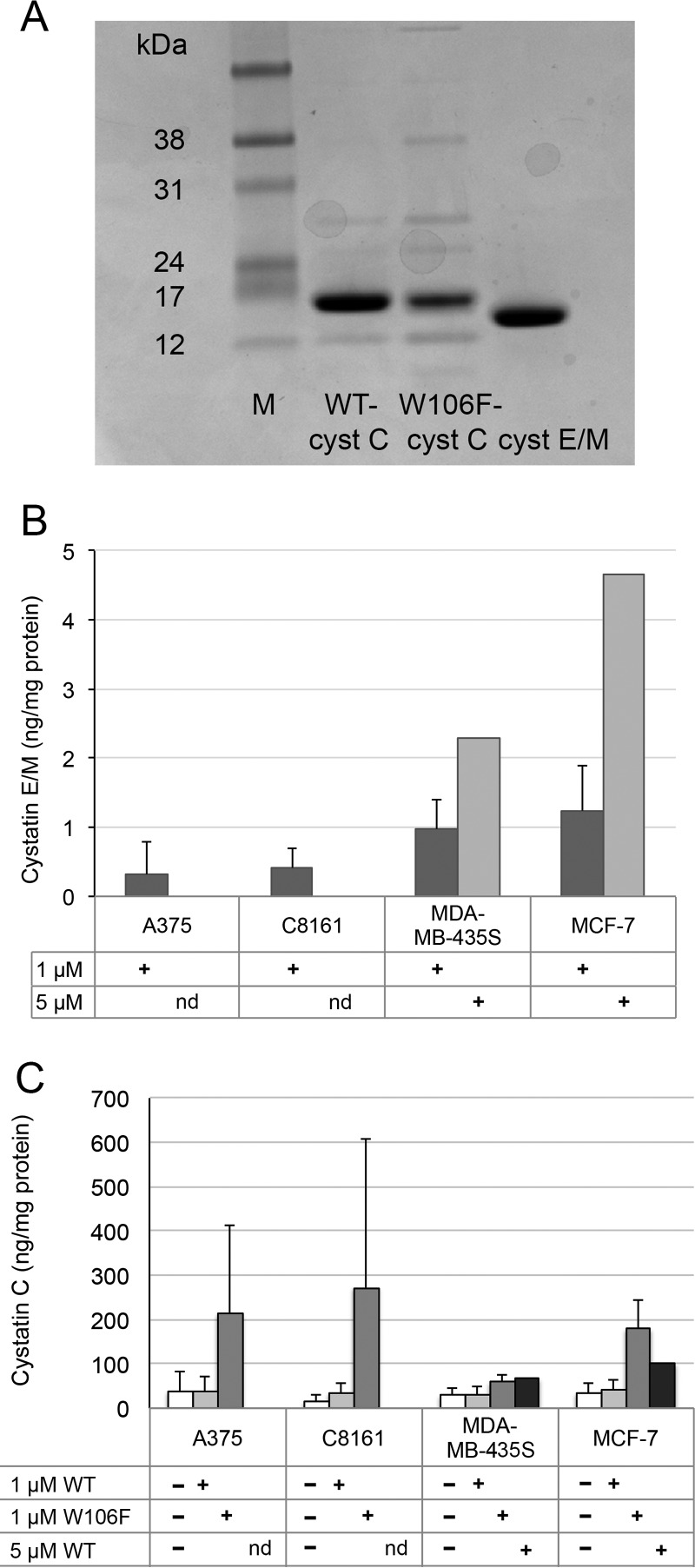
Cystatin E/M shows the tightest binding of legumain among the known cystatins (Ki 0.0016 nm; Ref. 8). Despite a 100-fold lower affinity for legumain (Ki 0.2 nm; Ref. 24), the more available cystatin C is also an efficient legumain inhibitor in vitro. In addition to comparing these two cystatins, we included W106F-cystatin C in the experiment, as this cystatin variant in other cell lines has been shown to be more efficiently internalized than wild-type cystatin C (22). W106F-cystatin C is somewhat impeded to inhibit cysteine cathepsins, but the legumain-inhibiting capacity is unmodified. The purity of the recombinant proteins used was >90% as analyzed by SDS-PAGE (Fig. 3A).
Figure 3.
Cellular uptake of cystatins. A, the quality of recombinant proteins used for cell experiments was analyzed by SDS-PAGE. M, size marker. B and C, cystatin (cyst) E/M (B) and cystatin C (C) were analyzed in cell homogenates by ELISA, and values were correlated to total protein content. Cells were incubated with 1 or 5 μm cystatin E/M, wild-type cystatin C, or W106F-cystatin C for 6 h. In control cells incubated without cystatin E/M addition, intracellular cystatin E/M levels were below the detection limit. Bars represent mean values of duplicate wells from 1–7 experiments, with error bars indicating the S.D. ELISA measurements were performed in duplicate wells. nd, not determined.
The melanoma cell lines and MCF-7 were incubated for 6 h with the addition of either 1 μm wild-type cystatin C, W106F-cystatin C, or cystatin E/M. The cells were then lysed, and cystatin C and E/M were analyzed by ELISA. Control cells were incubated in standard medium without any cystatin addition.
Cystatin E/M levels in the control cells were below the detection limit, as stated earlier. In lysates from cells incubated with cystatin E/M, low levels could be measured. The highest degree of uptake was shown in MCF-7 cells (1.3 ng/mg protein), and the lowest was in C8161 cells (0.45 ng/mg protein) (Fig. 3B). The cystatin C content of the control cells was by contrast relatively high, at a level ∼100-fold higher than the internalized cystatin E/M (see above).
Increased levels of both wild-type and W106F-cystatin C were seen in all cell lines after the addition of the variants to culture medium, with the highest values for W106F-cystatin C. The MDA-MB-435S cells showed the lowest uptake. In these cells the level of cystatin C increased to 123% that of the control for wild-type cystatin C and to 204% for the W106F variant. The C8161 cell line showed the most efficient uptake of cystatin C compared with the control cells, with levels at 207% for wild-type and 525% for W106F-cystatin C (Fig. 3C).
As the uptake of cystatin E/M was the highest in MCF-7 and MDA-MB-435S, these cells were used for a dose-response experiment to further compare the uptake of cystatin E/M and cystatin C. The cells were incubated without or with either 1 or 5 μm cystatin E/M or wild-type cystatin C for 6 h. A more pronounced uptake was seen in cells of both cell lines after incubation with the higher concentration of the different cystatins, but the magnitude of cystatin uptake was much higher for cystatin C at 1 μm than for cystatin E/M at 5 μm (Fig. 3, B and C).
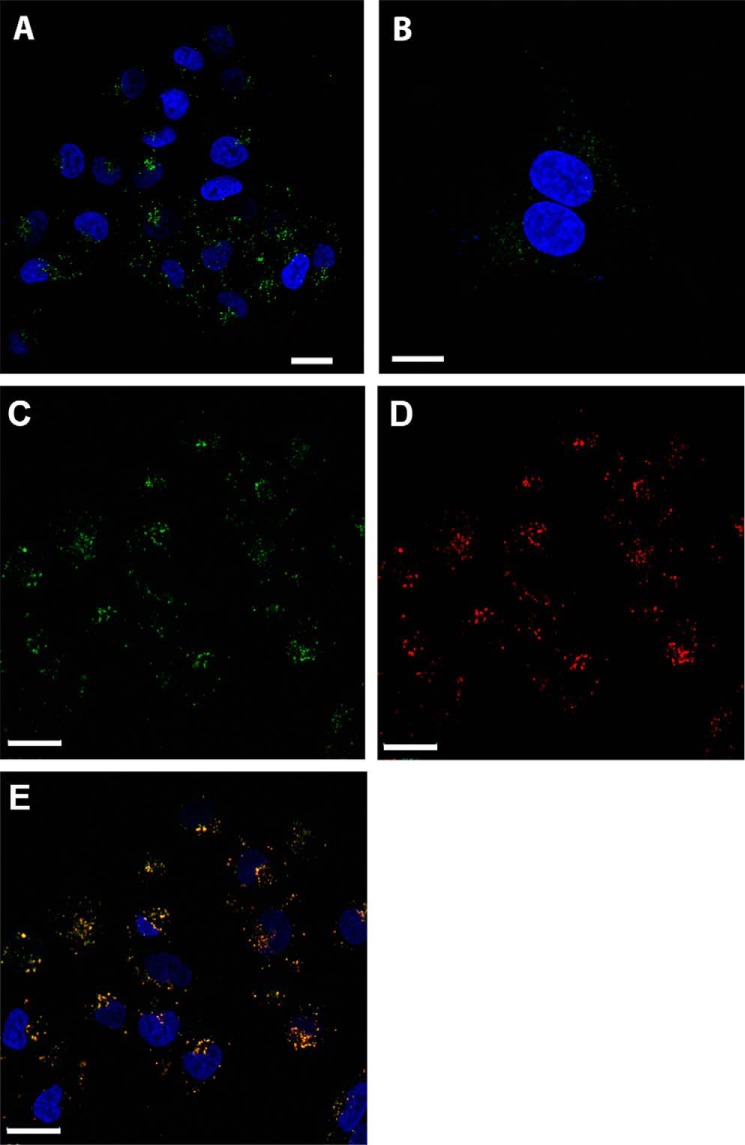
A375 and MDA-MB-435S cells were incubated with Alexa Fluor-488 conjugated cystatin E/M and visualized by confocal microscopy. Fluorescence was detected in cytoplasmic vesicles resembling endolysosomal compartments in both cell lines, in a pattern that recalls cystatin C uptake in other cell lines (Fig. 4, A, B, and C). To further define the localization of the internalized cystatin E/M, A375 cells were simultaneously incubated with Alexa Fluor-568-labeled cystatin C (Fig. 4D), which after internalization has been shown to end up in vesicles containing cysteine cathepsins and legumain in MCF-7 breast cancer cells (22). The results demonstrate that cystatin E/M and cystatin C perfectly co-locate in the same vesicles after uptake (Fig. 4E).
Figure 4.
Subcellular localization of internalized cystatins in melanoma cells. A and B, when analyzed by confocal laser-scanning microscopy, internalized cystatin E/M (green) was seen in perinuclear vesicles all over the cytoplasm in A375 (A) and MDA-MB-435S cells (B). C–E, cystatin E/M (green, C) and cystatin C (red, D) was internalized by A375 cells and co-located (yellow, E) in endolysosomal compartments. Nuclei were stained with DAPI (blue). The Scale bar indicates 20 μm in A, C, D, and E and 10 μm in B.
Thus, melanoma cells internalize both cystatin C and cystatin E/M into endolysosomal compartments. The internalization can be augmented by modification of the cystatin molecule and/or by increasing the dose of added cystatin.
Cysteine protease activity in melanoma cell lines
For detection of basal enzyme activities, cells were incubated in standard medium for 24 h and subsequently lysed. Activity buffer and a fluorescent substrate for legumain (Z-Ala-Ala-Asn-NMec) or cathepsin B (Z-Arg-Arg-NMec) was then added to a portion of the lysate. Enzyme activity was monitored by the cleavage of the fluorescent substrates and measured as the increase of fluorescence per min correlated to total protein content of the cell homogenate. Both legumain (Fig. 5A) and cathepsin B (data not shown) activity could be measured in all four cell lines.
Figure 5.
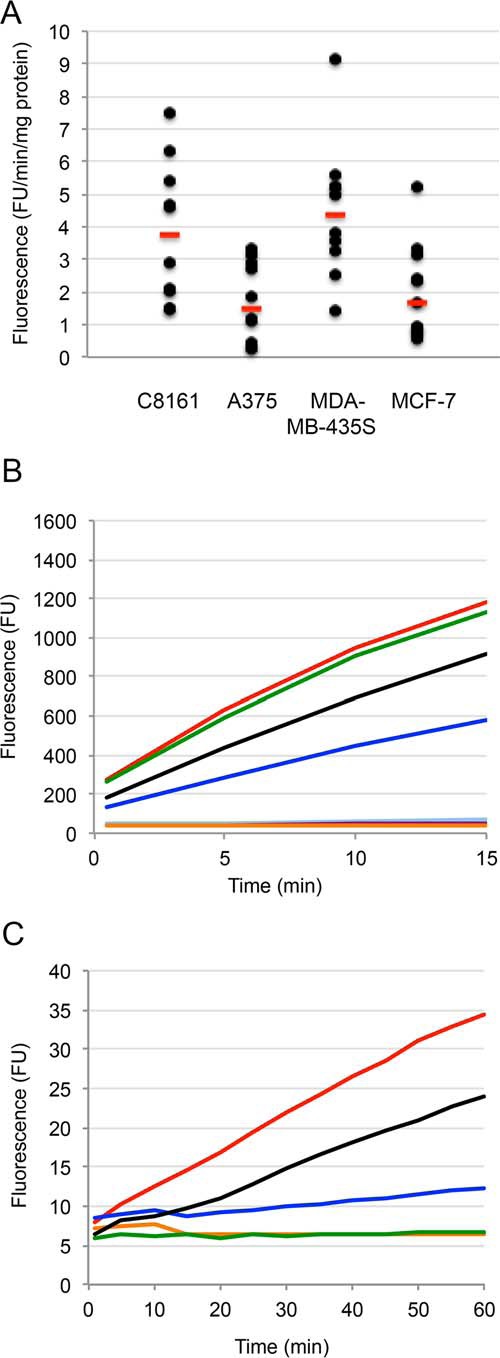
Legumain activity measured by the substrate Z-Ala-Ala-Asn-NMec. A, basal Z-Ala-Ala-Asn-NMec-degrading activity in melanoma cell lines was analyzed and expressed as increase of fluorescence units (FU)/min/mg of total protein. Each black dot represents the mean value of duplicate wells in one experiment. Red bars represent median values. B, inhibition of pig kidney legumain activity by cystatins C and E/M. Fluorescent product was continuously measured every 30 s for 15 min. Orange curve, addition of 75 nm cystatin E/M at time 0; purple, 7.5 nm cystatin E/M; black, 0.75 nm cystatin E/M; light blue, 75 nm cystatin C; dark blue, 7.5 nm cystatin C; green, 0.75 nm cystatin C; red, no inhibitor added. C, inhibition of Z-Ala-Ala-Asn-NMec-degrading activity in lysates of MDA-MB-435S melanoma cells, after the addition of cystatin E/M to the lysate. An increase of fluorescence was measured every minute for 60 min. Orange, addition of 1 μm cystatin E/M at time 0; green, 0.1 μm cystatin E/M; blue, 0.01 μm cystatin E/M; black, 1 μm E-64; red, no inhibitor added.
To address how specific the Z-Ala-Ala-Asn-NMec substrate used is to assay legumain in such cell lysates, containing a wide repertoire of proteases of different classes, two control experiment were performed under the same assay conditions. When purified pig legumain (6) was added to the assay at ∼1 nm concentration, the recombinant Escherichia coli produced cystatin E/M showed ∼25% inhibition when added to a concentration of 0.75 nm and complete inhibition at 7.5 and 75 nm (Fig. 5B). Recombinant wild-type cystatin C showed 0, 50, and 100% inhibition of legumain activity when added to 0.75, 7.5, and 75 nm final concentrations, respectively (Fig. 5B). This result verified that the cystatin preparations used indeed can fully inhibit legumain and illustrates that the Ki for legumain inhibition by cystatin C of 0.2 nm results in less efficient inhibition than seen for cystatin E/M under the assay conditions with quite dilute enzyme, as expected (6, 8, 10). In the second control experiment, varying amounts of recombinant E. coli-produced cystatin E/M was added to a melanoma cell lysate under the same assay conditions. Cystatin E/M added to final concentrations of 1 or 0.1 μm resulted in complete inhibition of the enzyme(s) degrading the Z-Ala-Ala-Asn-NMec substrate used, whereas 0.01 μm cystatin E/M showed ∼75% inhibition (Fig. 5C). This strongly indicated that the enzyme responsible for substrate hydrolysis was legumain. As cystatin E/M is an inhibitor of both cysteine cathepsins and legumain (8, 9), we used the cysteine cathepsin inhibitor E-64, which shows no reactivity against legumain (6), for an additional control. When E-64 was added to final concentrations of 0.01 or 0.1 μm, no signs of inhibition were seen (not shown). At 1 μm, a small effect was seen in the rate of Z-Ala-Ala-Asn-NMec hydrolysis the first 10 min, but the enzyme rate was just marginally reduced compared with when no inhibitor was added to the lysate after that (Fig. 5C, black curve). Thus, a small degrading effect of the Z-Ala-Ala-Asn-NMec substrate by a cysteine protease other than legumain cannot be ruled out completely, but we conclude that the vast majority of enzyme showing activity in the assay must be legumain.
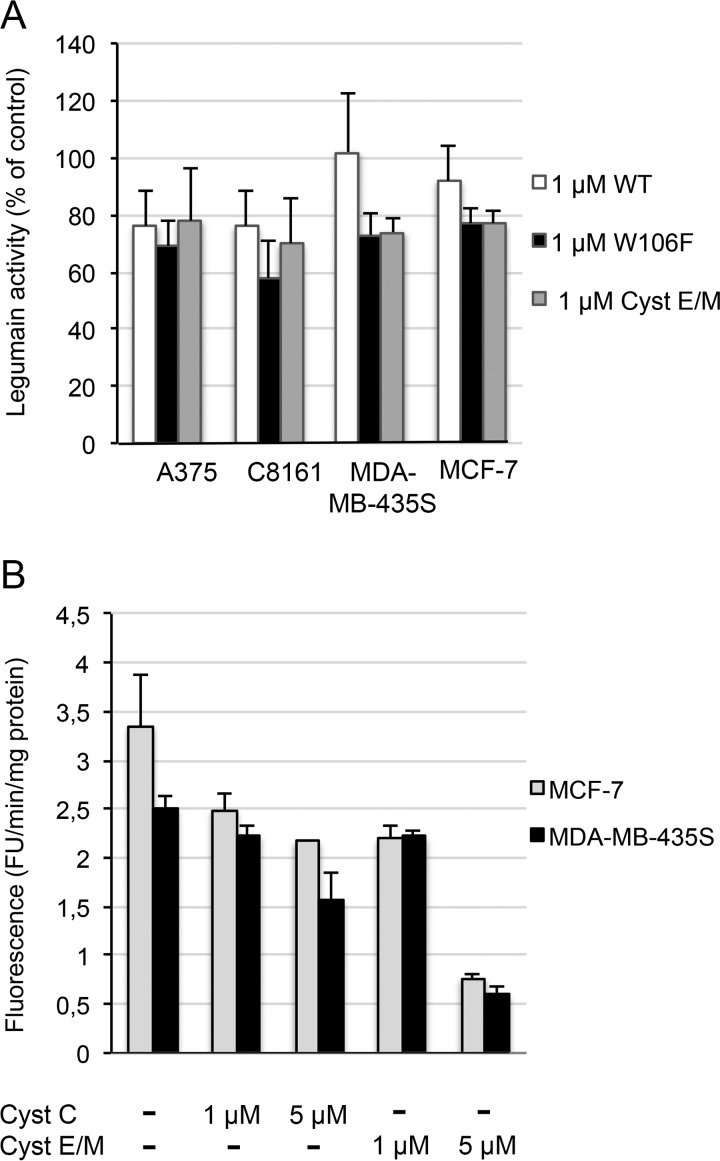
The effects of cystatin uptake on legumain activity was measured in cell lysates after incubation with either cystatin E/M, wild-type cystatin, or W106F-cystatin C and compared with lysates of control cells incubated in medium without cystatin addition (set to 100%). The activity of legumain in lysates of melanoma A375, C8161, and MDA-MB-435S cells and the reference MCF-7 cells was clearly inhibited when they were incubated with either of the three cystatin variants (Fig. 6A). In lysates of cells incubated with W106F-cystatin C, which was more efficiently internalized according to the ELISA results, the legumain activity was also more efficiently inhibited. The incubation with cystatin E/M led to at least the same degree of inhibition of legumain activity as incubation with cystatin C. This is remarkable because the intracellular content of wild-type cystatin C after uptake was ∼100 times higher than that of cystatin E/M in ng of cystatin/mg of protein according to the ELISA results.
Figure 6.
Legumain activity in melanoma cells after cystatin uptake. The three melanoma cell lines and the reference cells MCF-7 were seeded in 6-well plates and incubated for 6 h after the addition of different cystatin variants. Legumain activity was measured using Z-Ala-Ala-Asn-NMec as the substrate. A, legumain activity was compared in lysates of four cell lines incubated with 1 μm concentrations of either wild-type cystatin C (WT), W106F-cystatin C, or cystatin E/M. In each experiment the activity was related to that in control cells incubated in standard medium, set to 100%. Bars represent mean values of legumain activity in duplicate wells in 3–5 experiments, with error bars indicating the S.D. B, a dose-response effect of cystatin uptake was demonstrated by measuring legumain activity in lysates of MDA-MB-435S and MCF-7 cells incubated with either 1 or 5 μm cystatin C or cystatin E/M. Bars represent the mean value of duplicate wells in one experiment. Activity measurements were analyzed in duplicate.
In another experiment we increased the cystatin C or E/M concentration of the medium to 5 μm. This led to even more efficient inhibition of the intracellular legumain activity in both MDA-MB-435S and MCF-7 cells, reflecting the dose-dependent uptake shown by ELISA. The remaining legumain activity in homogenates of cells incubated with 5 μm cystatin E/M was only 20% that of the activity in the control cells (Fig. 6B). Thus, internalization of cystatin C and E/M could be an important way to regulate the intracellular legumain activity.
Uptake effects on migration and invasion in Matrigel
A375 melanoma cells were incubated in inserts containing Matrigel or in control inserts in cell culture medium with the addition of 5 μm cystatin E/M or W106F-cystatin C. After 72 h the cells that had migrated through the membrane in the inserts were stained and lysed before the absorbance was measured. Cell ability to migrate through the transwell membrane was significantly decreased by both cystatin E/M (p = 0.04) and W106F-cystatin C (p = 0.02) addition compared with control cells without cystatin addition (Fig. 7A). A tendency to decreased invasion of the Matrigel was also seen, with p values of 0.09 and 0.13, respectively (Fig. 7B).
Figure 7.
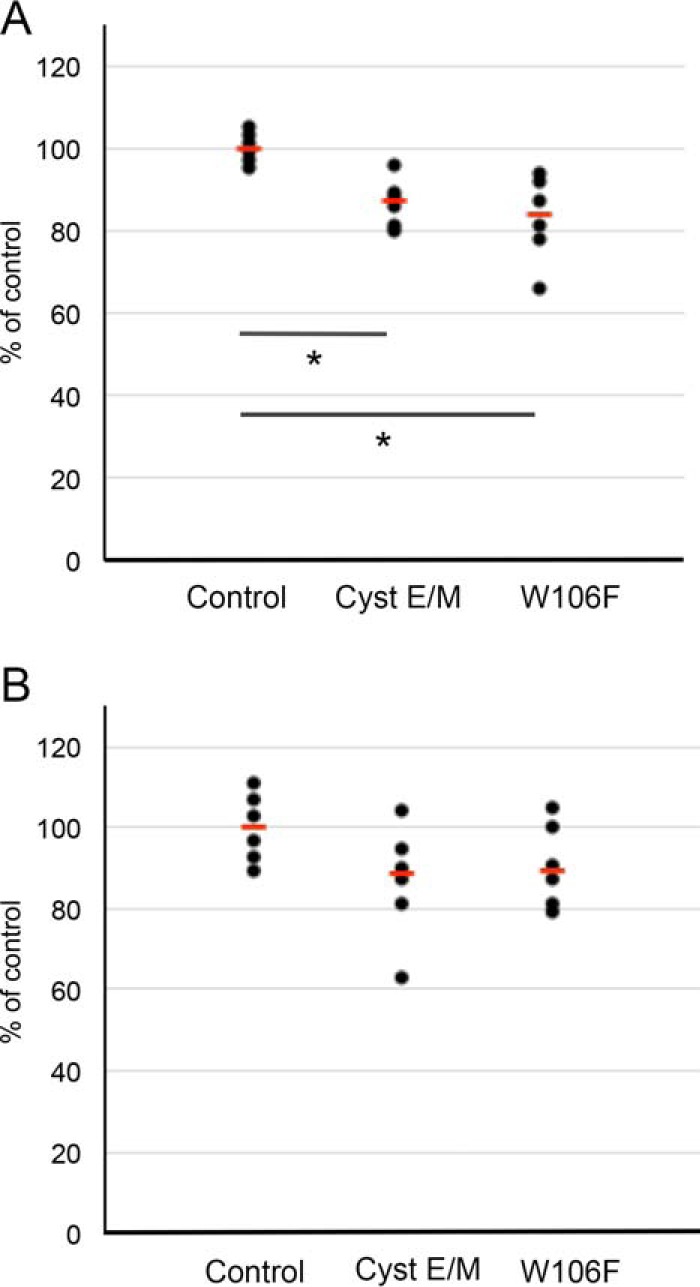
Effects by cystatin uptake on migration and invasion of melanoma cells. A375 cells were starved and then incubated for 72 h with and without either 5 μm cystatin (Cyst) E/M or 5 μm W106F-cystatin C in transwell filters with or without Matrigel coating. A and B, the mean value of the migration (A) and invasion (B) of the control cells incubated without cystatin addition was set to 100% in each experiment. For comparison, 1 μm concentrations of the known inhibitor of cell invasion/migration, E-64, resulted in a median reduction of migration and invasion to 70 and 85%, respectively, when MCF-7 cells were analyzed in the same assay (22). p values in A: *, <0.05. p values in B: not significant (0.09 and 0.13, respectively). Black dots represent results from single wells. Red bars represent median values.
Cystatins in malignant melanoma
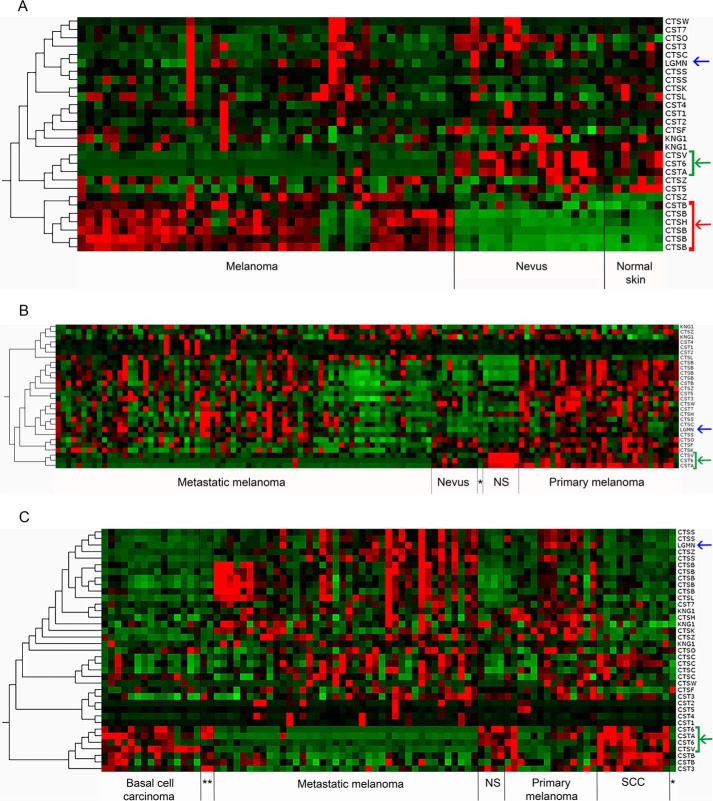
Many reports suggest that an impaired balance between proteases and inhibitors is at hand in cancer and that some cystatins may have tumor-suppressing properties (25–27). Given our results on melanoma cell lines, clearly demonstrating that the external addition of cystatins E/M and C can shift the protease/inhibitor balance within tumor cells especially with respect to down-regulation of legumain activity, we sought evidence for a mis-balance in tumor tissue. To address this we analyzed the gene expression of the human cystatins and their potential in vivo target cysteine proteases, the 11 cysteine cathepsins and legumain, in a microarray dataset containing 45 primary melanoma, 18 benign skin nevi, and 7 normal skin samples (28). Clustering made by Qlucore Omics revealed a gene expression signature with down-regulated expression of the cystatins E/M (CST6) and A (CSTA). Expression of cathepsin V (CTSV) was also down-regulated in the melanoma samples, whereas expression of cathepsin B (CTSB) and H (CTSH) was up-regulated compared with nevi and normal skin. No special gene expression signature was seen for legumain (LGMN) (Fig. 8A). Another dataset contained gene expression data from normal skin and nevi and melanoma tissue, the latter divided into two groups of either primary or metastatic melanoma. Renewed clustering again revealed a signature with down-regulated expression of cystatin E/M, cystatin A, and cathepsin V but now being a signature to distinguish metastatic melanoma from primary melanoma, nevi, and normal skin. In this case, however, the clustering did not reveal a corresponding pattern of up-regulated expression of any cathepsin or legumain (Fig. 8B). A third dataset contained samples from other skin cancers (basal cell carcinoma and squamous cell carcinoma), melanoma (primary and metastatic), and normal skin. Interestingly, clustering analysis using this dataset revealed a signature of down-regulated expression of cystatin E/M, cystatin A, and cathepsin V in melanoma tissue; either the tumor was categorized as primary or metastatic compared with the other skin cancers or normal skin. No clear expression signature concerning up-regulation of the cysteine proteases was seen (Fig. 8C).
Figure 8.
Gene expression levels for cystatins, cysteine cathepsins, and legumain in tissue samples. The heat maps show relative up-regulation of mRNA expression in red and down-regulation in green. CTS, cathepsin; CST, cystatin; LGMN, legumain; KNG, kininogen. Expression array datasets were downloaded from www.ebi.ac.uk/arrayexpress3 and analyzed using Qlucore Omics Exploration software. A, a dataset (accession number GSE3189) including 45 samples from melanoma tissue, 18 nevi, and 7 normal skin showed that the levels of mRNA for cystatin E/M (CST6), cystatin A (CSTA), and cathepsin V (CTSV) are down-regulated in melanoma, whereas cathepsin B (CTSB) and cathepsin H (CTSH) are up-regulated. B, a dataset (GSE46517) including tissue samples from normal epithelial melanocytes (*, n = 1), normal skin (NS, n = 7), nevi (n = 9), primary, and metastatic melanoma (n = 31 and 73, respectively) showed that the levels of mRNA for cystatin E/M (CST6), cystatin A (CSTA), and cathepsin V (CTSV) are down-regulated in metastatic melanoma. C, a third dataset (GSE7553), including tissue samples from normal human epidermal melanocytes (*, n = 1), normal skin (NS, n = 4), basal cell carcinoma (n = 15), squamous cell carcinoma (SCC, n = 11), melanoma in situ (**, n = 2), primary and metastatic melanoma (n = 14 and 40, respectively) again showed that the levels of mRNA for cystatin E/M (CST6), cystatin A (CSTA) and cathepsin V (CTSV) are clearly down-regulated in metastatic melanoma. Green arrows and brackets: cystatin E/M, cystatin A, and cathepsin V. Red arrows and brackets: cathepsin B and cathepsin H. Blue arrows: relative expression level of legumain (LGMN) mRNA.
Discussion
It is commonly suggested that the balance between some proteases and their inhibitors is disturbed in malignancies such as cancer of the breast, ovaries, and skin. The cysteine proteases (11 cysteine cathepsins and legumain) are often increased in activity or expression, and they can be recovered in unusual subcellular compartments (1–4, 6–7). The presence of their inhibitors, the cystatins, is often decreased. Of the 12 cystatins, cystatin E/M has been suggested to be down-regulated at the gene level in several cancers (25). In our gene expression analysis we could corroborate that cystatin E/M was down-regulated in most of the melanoma samples compared with normal skin and nevi, whereas there were no obvious signs of up-regulation of expression of legumain or any of the other potential target enzymes for the cystatins. Clustering analysis focusing on all genes of the cysteine protease/inhibitor system in three independent data sets in all cases revealed a strong gene signature with decreased expression of cystatin E/M, cystatin A, and cathepsin V coupled to melanoma and especially metastatic melanoma. This gene signature should be interesting to explore further for possible value as a biomarker for melanoma diagnosis. Another aspect, because we also demonstrate in the this study that the intracellular activity of legumain in melanoma cells can be regulated by uptake of externally added cystatins C and E/M, is that it seems possible that the normal balance of the cysteine protease/inhibitor system could be restored by therapeutic addition of cystatin E/M.
We analyzed the expression of all type 2 cystatins in three melanoma cell lines and one breast cancer cell line and found that the cystatin C expression is by far the dominant of the cystatins. A low level of mRNA for cystatin F was found in MDA-MB-435S cells. Additionally, a low level of mRNA for cystatin SN was found in these cells and also in MCF-7 cells. Cystatin F is normally only expressed in white blood cells, and cystatin SN is normally only expressed in salivary tissues. Interestingly, recent reports indicate that expression of cystatin SN in non-small cell lung cancer was correlated to recurrence, metastasis, and survival and that expression of cystatin SN could be used as a biomarker for esophageal, gastric, colorectal, and pancreatic cancer (18, 29–32).
Melanoma cells were incubated with either wild-type cystatin C, W106F-cystatin C, or cystatin E/M, and it was seen that the uptake of the cystatin C variants was much more effective than that of cystatin E/M. Even so, the effects on legumain activity in cell lysates were similar, possibly reflecting the lower Ki value for cystatin E/M inhibition of legumain. This indicates that even if cystatin C is the most abundant of the type 2 cystatins, other cystatins with low expression levels might be of importance in some cases and have specialized functions. Furthermore, we saw that the engineered cystatin variant, W106F-cystatin C, was more efficiently internalized than wild-type cystatin C and also resulted in more efficient inhibition of legumain activity. The cysteine cathepsin-inhibiting capacity of this variant is somewhat decreased in comparison with wild-type cystatin C, as one of the amino acids in the cathepsin-binding surface is exchanged, but the legumain-inhibiting capacity is unaffected (22). This indicates that it may be possible to customize a “super cystatin” that is directly targeted to a special process.
The uptake enables a way for the interaction between the secreted inhibitor and the possible target enzyme, legumain, in lysosome-like compartments. We have here studied the effects of cystatin uptake on enzyme regulation in homogenates of whole cells, but the effect is likely even more pronounced in the compartments where the enzymes are naturally found. The cells used for this study showed no signs of legumain-staining in unexpected compartments or on the cell surface (data not shown).
Our results clearly show internalization of cystatin E/M in contrast to another study (33) in which no indication of uptake was seen. In this study naïve cells were incubated in conditioned medium from cells producing FLAG-tagged cystatin E/M. A possible explanation to this discrepancy could be that the FLAG-tag addition affects the uptake properties of cystatin E/M.
It has been suggested that melanoma cells transfected to overproduce cystatin E/M have suppressed invasiveness in Matrigel, likely via inhibition of legumain (19). Here we show that uptake of cystatin E/M will occur when the culturing medium contains the inhibitor and that the legumain activity is decreased in lysates of these cells as a consequence. We also show that cells incubated in cystatin E/M-containing medium for 72 h exhibit decreased invasiveness in Matrigel and decreased migration through non-coated membranes. Although the evidence is circumstantial, this favors the hypothesis that inhibition of intracellular legumain by the internalized cystatin E/M is responsible for the decrease, either directly or via another enzyme.
In conclusion, cystatin E/M appears to be a good candidate to efficiently down-regulate the increased legumain activity possibly being important for the malignant phenotype of melanoma cells. This might be an important way to influence tumor cell properties, which should be further pursued.
Experimental procedures
Cells and reagents
The human breast adenocarcinoma cell line MCF-7 and the three human melanoma cell lines MDA-MB-435S, A375, and C8161 were used. C8161 was kindly provided by Dr. Bo Jansson (Department of Oncology, Lund University, Sweden), and the other cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA).
Cell lines MCF-7 and A375 were cultured in Dulbecco's modified Eagle's medium with 4500 mg/liter of glucose, GlutaMAX-I and pyruvate, C8161 in RPMI 1640 medium, and MDA-MB-435S in Leibowitz's L-15 medium. All media were supplemented with 100 IU/ml penicillin/streptomycin and 10% fetal bovine serum (FBS). Cells were detached using 0.05% trypsin-EDTA. Trypsin was inactivated by the addition of complete culturing medium to the detached cells. The cells were transferred to a tube, and after centrifugation the cell pellet was washed once with PBS before lysis in 0.2% Triton X-100 (Sigma) in either PBS, pH 7.4, or 50 mm sodium citrate, pH 5.0. A protease inhibitor “mixture” was added to all cell homogenates and samples of conditioned medium to a final concentration of 5 mm benzamidine hydrochloride, 15 mm NaN3, and 10 mm EDTA. Cell media, antibiotics, trypsin, and PBS was purchased from Life Technologies.
qRT-PCR
The expression of mRNA for the type 2 cystatins C, D, E/M, F, S, SA, and SN as well as the proteases cathepsin B and legumain was measured in the three melanoma cell lines.
TRIzol reagent was used for isolation of total RNA according to the manufacturer's recommendations (Invitrogen). The RNA was further purified by RNeasy Mini kit (Qiagen AB, Solna, Sweden) before cDNA synthesis from 25 ng of total RNA according to the manufacturer's guidelines. The quality and the concentration of the isolated RNA were determined by electrophoresis in a non-denaturing agarose gel and by measurement of the A260/A280 ratio (NanoDrop 2000, Thermo Fisher Scientific Inc. Rockford, IL).
Analysis of 18S rRNA was used as an endogenous control, and relative expression was calculated using the comparative Ct method. Values were presented multiplied by a factor of 106. Real-time PCR experiments were performed on a StepOnePlus real-time PCR device (Applied Biosystems, Foster City, CA). All reagents for qRT-PCR was purchased from Life Technologies.
Proteins
Wild-type human cystatin C and the variant W106F-cystatin C were expressed in E. coli and purified by ion-exchange chromatography as earlier described (22, 34–35).
To produce cystatin E/M in E. coli, the expression vector pHD389, based on the cystatin C expression vector pHD313 but with a short polylinker replacing the cystatin C cDNA insert, was used (36). A cystatin E/M-encoding segment was amplified by PCR from the human cDNA clone HAQBM60 (10) using primers MA552 (5′-TATAT-GGATCC-CGGCCGCAGGAGCG CAT-3′ (up-stream, with flanking BamHI site underlined)) and MA553 (5′-TACAT-GAATTC-TCACATCTGCACACAGTTGTG-3′ (down-stream, with flanking EcoRI site underlined)), purified, digested, and ligated into BamHI/EcoRI-restricted pHD389. The construct encodes the bacterial OmpA signal peptide sequence to direct the recombinant protein to the periplasm followed by Ala-Pro encoded by the BamHI site used for cloning and then the 121-amino acid residues of mature human cystatin E/M (amino acids 29–149 of pre-cystatin E/M; Ref. 10). Clones with the correct insert sequence were identified by DNA sequencing and transformed into E. coli MC1061. Bacteria containing the plasmid were used for production of cystatin E/M as previously described for cystatin C (34). In short, bacteria were cultured overnight in TB medium containing 100 μg/ml ampicillin. Expression of cystatin E/M was induced by increasing the temperature from 30 to 42 °C for 3 h before centrifugation. The pelleted bacteria were resuspended in 20% sucrose, centrifuged again, and resuspended in H2O. After another centrifugation step to remove cell debris, the supernatant consisted of the periplasmic extract.
Cystatin E/M was purified from the periplasmic extract by incubation with CM-papain coupled to CNBr-activated Sepharose® 4B (GE Healthcare) overnight. After washing, the inhibitor was eluted with a buffer containing 50 mm Na3PO4 and 500 mm NaCl, pH 11.5. The pH of the eluate was immediately adjusted with 1 m Tris-HCl, pH 6.5. A final purification step was achieved by size-exclusion chromatography (SephadexTM 75; GE Healthcare) in 50 mm ammonium bicarbonate buffer, pH 8.0.
The concentration of purified E. coli-produced cystatin E/M was determined according to A280 measurements and a Coomassie protein assay (Thermo Fisher Scientific, Inc.). The purity of the protein was >90% according to SDS-PAGE (NuPAGE, 4–12% Bis-Tris; Life Technologies). The expected N-terminal sequence APRPQE was verified by Edman degradation, and size as expected was confirmed by MALDI-TOF MS (22).
The enzyme-inhibiting capacity of the purified E. coli-expressed human cystatin E/M was measured by titration of papain activity as described for recombinant baculovirus/insect cell-produced cystatin E/M (10) using the substrate Bz-Arg-pNA (Bachem, Bubendorf, Switzerland), titration of legumain activity using the substrate Bz-Asn-pNA (Bachem) as described (6, 8), and in assays with fluorescent substrates suitable for the enzymes as earlier described (22) and detailed under “Intracellular protease activity” below. Papain for these enzyme assays was purchased from Sigma, and legumain was purified from fresh pig kidneys using the method of Chen et al. (6).
Quantification of cystatins
Cystatins C, D, E/M, and F were quantified by ELISA both in conditioned culture media and in cell lysates for determination of the basal levels of the different cystatins.
A total of 500,000 cells were seeded in 24-well culture plates (Thermo Fischer Scientific.) and allowed to settle for 24 h. At harvest the medium was removed, and the cells were washed with PBS twice and finally incubated with 300 μl of trypsin at 37 °C for 10 min. Detached cells were pelleted, and cell pellets were lysed in 250 μl of lysis buffer, pH 7.4, and incubated overnight at 4 °C.
Cystatin C was quantified by a double-sandwich ELISA-specific for human cystatin C (37). Briefly, wells in a 96-well microtiter plate were coated with a polyclonal rabbit anti-(human cystatin C) antibody (antiserum 8206) for capture of the antigen. A secondary biotinylated monoclonal mouse anti-(human cystatin C) antibody was then added followed by horseradish peroxidase (HRP)-conjugated streptavidin and substrate for detection. For quantification of cystatins D (38), E/M (10), and F (39), similar ELISA methods were used but used one fraction of a monospecific polyclonal antiserum against the recombinant cystatin for capture and a second biotinylated fraction of IgG from the same antiserum for detection (40). Highly purified preparations of recombinantly produced cystatin D, E/M, and F was used as standards in these assays to construct appropriate calibration curves. The levels of the cystatins in the lysates were correlated to total protein content measured by Coomassie protein assay.
To analyze presence of cystatins S, SA, and SN, immunoblotting was performed preceded by affinity precipitation, necessitated by the low secretion and intracellular content of these cystatins. Ten μl of CM-papain-Sepharose was added to a pool of medium or lysate followed by 120 h of incubation on a shaker at 4 °C. After centrifugation, the supernatant was discarded, and the remaining gel pellet was resuspended in reducing NuPAGE LDS sample buffer (Life Technologies). Sample proteins were separated in a NuPAGE 4–12% Bis-Tris gel (Life Technologies) before electroblotting to a poly(vinylidene difluoride) membrane (Immobilon-P, Millipore, Bedford, MA). Blotted proteins were immunodetected using polyclonal rabbit antisera raised against human cystatin S or SN purified from human urine (12). As a secondary antibody a HRP conjugated goat anti-(rabbit IgG) fraction (DAKO, Copenhagen, Denmark) was used. The blotted proteins were visualized by chemiluminescence (ECL Plus reagent, Amersham Biosciences). Cystatin S or cystatin SN purified from human saliva was co-electrophoresed and blotted as controls.
Analysis of internalized proteins
A total of 500,000–1,000,000 cells were seeded in 6-well culture plates (Fischer) and allowed to settle for 24 h. The cells were then washed with PBS twice and incubated in 500 μl of fresh medium without or with the addition of 1–5 μm cystatin E/M, wild-type cystatin C, or W106F-cystatin C for 6 h. At harvest the cells were incubated with 300 μl of trypsin at 37 °C for 10 min. Cell pellets were lysed in 250 μl of lysis buffer, pH 5.0, and incubated at room temperature for 1 h. Finally, the cystatin E/M or cystatin C levels in the lysates were quantified by ELISA (described above) and correlated to the total protein content measured by Coomassie protein assay.
Intracellular protease activity
First, an experiment was performed to show that the substrate Z-Ala-Ala-Asn-NMec, meant to be quite specific to assay legumain activity (5, 6), could be cleaved by enzymes in a crude melanoma cell homogenate and that this enzyme activity could be inhibited by the addition of cystatin E/M to the reaction. Cystatin E/M was added to portions of a cell lysate generated as described in the previous paragraph to final concentrations of 1 μm, 0.1 μm, or 0.01 μm. As cystatin E/M is an inhibitor of both cysteine cathepsins and legumain, we used E-64 (final concentrations 1 μm, 0.1 μm, and 0.01 μm) to determine if cysteine cathepsins also contributed to the cleaving of the substrate. The buffer used was 0.1 m Na2HPO4 buffer, pH 5.8, with 0.1% CHAPS, 1 mm EDTA, and 4 mm DTT (6). The fluorescence was monitored at 355 nm excitation/460 nm emission in a Fluoroscan Ascent plate reader every min for 1 h at 37 °C.
To determine basic enzyme levels in the cell lines studied and to assess intracellular legumain inhibition, the experimental setup was basically the same as for the analysis of internalized cystatins described above. Legumain and cathepsin B activity in cell lysates was monitored by the cleavage of the fluorescent substrates Z-Ala-Ala-Asn-NMec (10 μm) and Z-Arg-Arg-NMec (10 μm), respectively. Cathepsin B activity was measured in 0.1 m phosphate buffer, pH 6.0, containing 1 mm EDTA and 4 mm DTT, and legumain activity was measured as mentioned before. The fluorescence was monitored at 355 nm excitation/460 nm emission in a Fluoroscan Ascent plate reader for up to 5 h at 37 °C. Enzyme activity calculations were based on the initial rate when the assay showed a linear response. To calculate the rate of the enzyme activity in relation to cell protein content, 2 μl of each cell lysate was transferred to a new tube for the Coomassie protein assay.
Migration studies
To study the migration in vitro, 50,000 A375 cells were starved for 4 h in basal medium with the addition of 0.1% bovine serum albumin (BSA, Sigma) instead of 10% FBS. The cells were then seeded in this starving medium in 6.5-mm cell culture inserts with an 8 μm PET (polyethylene terephthalate) membrane in a 24-well plate (BD Biosciences) without or with the addition of either 5 μm cystatin E/M or W106F-cystatin C. BSA was replaced by 10% FBS in medium added to the lower wells as chemoattractant. After 72 h of incubation in 37 °C, the invasive cells on the lower surface of the membrane were stained with 0.25% crystal violet and rinsed with water. The stained cells were dissolved in 10% acetic acid, and the absorbance of the solution was measured at 560 nm. The results from the control cells were set to 100% in each experiment, and the results from the cells to which cystatins had been added were expressed as the percentage of the results for the control cells. Statistical evaluations were conducted using SPSS release 22.0.0.1 (IBM Corp., Armonk, NY) focusing on nonparametric test for independent samples (Mann-Whitney U test).
Confocal laser-scanning microscopy
100,000 A375 or MDA-MB-435S cells/well were seeded on coverslips in a 6-well cell culture plate (Thermo Fischer Scientific) and incubated for 6 h without or with 5 μm Alexa Fluor 488-labeled cystatin E/M or 5 μm Alexa Fluor 568-labeled cystatin C. After washing, the cells were fixed with 4% paraformaldehyde for 20 min in the cold. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole; Molecular Probes Europe BV, Leiden, The Netherlands). Coverslips were mounted on microscope slides with Vectashield mounting medium (Vector Laboratories, Cambridge, UK).
To label the proteins, pure preparations of cystatin C and E/M were incubated with either coupling-activated Alexa Fluor 568 or Alexa Fluor 468 according to the manufacturer's advice (Molecular Probes), and the labeled proteins were purified from unbound fluorophore by PD-10 column chromatography.
A Zeiss LSM 510 meta microscope was used to detect fluorescence. The settings were optimized for each fluorophore, and images were separately grabbed and then merged with the overlay function.
Microarray analysis
To investigate the gene expression in melanoma with respect to cystatins, cysteine cathepsins and legumain several publicly available microarrays were analyzed. The microarrays were selected from the ArrayExpress website (www.ebi.ac.uk/arrayexpress).3 We searched for datasets containing both normal samples (normal skin, nevi) and metastatic melanoma. The data were then downloaded into Qlucore Omics Explorer v3.0 (Qlucore AB, Lund, Sweden). All known human cathepsins, cystatins, and legumain were selected as our genes of interest, clustering analysis was performed, and heat maps were produced. In all cases the tissue samples were clustered hierarchically with respect to the expression of our selected variables.
Author contributions
H. W., U. E., and M. A. conceived and coordinated the study and drafted the paper. H. W. designed, performed, and analyzed the experiments shown in Figs. 3–6. J. A. designed and performed the experiments shown in Figs. 1, 2, and 7. F. A. performed the analyses shown in Fig. 8 under supervision of H. W., U. E., and M. A. All authors reviewed the results and approved the final version of the manuscript.
This work was supported by the Faculty of Medicine and BioCARE at Lund University and by a national 'ALF' program grant (to M. A.). The authors declare that they have no conflicts of interest with the contents of this article.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- qRT
- quantitative real-time
- CM
- carboxymethylated
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- Z
- carboxybenzyl
- NMec
- 7-amino-4-methylcoumarin
- Bz
- benzoyl
- pNA
- para-nitro-aniline.
References
- 1. Coulibaly S., Schwihla H., Abrahamson M., Albini A., Cerni C., Clark J. L., Ng K. M., Katunuma N., Schlappack O., Glössl J., and Mach L. (1999) Modulation of invasive properties of murine squamous carcinoma cells by heterologous expression of cathepsin B and cystatin C. Int. J. Cancer 83, 526–531 [DOI] [PubMed] [Google Scholar]
- 2. Vasiljeva O., Papazoglou A., Krüger A., Brodoefel H., Korovin M., Deussing J., Augustin N., Nielsen B. S., Almholt K., Bogyo M., Peters C., and Reinheckel T. (2006) Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 66, 5242–5250 [DOI] [PubMed] [Google Scholar]
- 3. Vasiljeva O., and Turk B. (2008) Dual contrasting roles of cysteine cathepsins in cancer progression: apoptosis versus tumour invasion. Biochimie 90, 380–386 [DOI] [PubMed] [Google Scholar]
- 4. Koblinski J. E., Ahram M., and Sloane B. F. (2000) Unraveling the role of proteases in cancer. Clin. Chim. Acta 291, 113–135 [DOI] [PubMed] [Google Scholar]
- 5. Rawlings N. D., Waller M., Barrett A. J., and Bateman A. (2014) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 42, D503–D509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J. M., Dando P. M., Stevens R. A., Fortunato M., and Barrett A. J. (1998) Cloning and expression of mouse legumain, a lysosomal endopeptidase. Biochem. J. 335, 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu C., Sun C., Huang H., Janda K., and Edgington T. (2003) Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 63, 2957–2964 [PubMed] [Google Scholar]
- 8. Alvarez-Fernandez M., Barrett A. J., Gerhartz B., Dando P. M., Ni J., and Abrahamson M. (1999) Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J. Biol. Chem. 274, 19195–19203 [DOI] [PubMed] [Google Scholar]
- 9. Cheng T., Hitomi K., van Vlijmen-Willems I. M., de Jongh G. J., Yamamoto K., Nishi K., Watts C., Reinheckel T., Schalkwijk J., and Zeeuwen P. L. (2006) Cystatin M/E is a high affinity inhibitor of cathepsin V and cathepsin L by a reactive site that is distinct from the legumain-binding site: a novel clue for the role of cystatin M/E in epidermal cornification. J. Biol. Chem. 281, 15893–15899 [DOI] [PubMed] [Google Scholar]
- 10. Ni J., Abrahamson M., Zhang M., Fernandez M. A., Grubb A., Su J., Yu G. L., Li Y., Parmelee D., Xing L., Coleman T. A., Gentz S., Thotakura R., Nguyen N., Hesselberg M., and Gentz R. (1997) Cystatin E is a novel human cysteine proteinase inhibitor with structural resemblance to family 2 cystatins. J. Biol. Chem. 272, 10853–10858 [DOI] [PubMed] [Google Scholar]
- 11. Sotiropoulou G., Anisowicz A., and Sager R. (1997) Identification, cloning, and characterization of cystatin M, a novel cysteine proteinase inhibitor, down-regulated in breast cancer. J. Biol. Chem. 272, 903–910 [DOI] [PubMed] [Google Scholar]
- 12. Abrahamson M., Barrett A. J., Salvesen G., and Grubb A. (1986) Isolation of six cysteine proteinase inhibitors from human urine: their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J. Biol. Chem. 261, 11282–11289 [PubMed] [Google Scholar]
- 13. Zeeuwen P. L., Van Vlijmen-Willems I. M., Jansen B. J., Sotiropoulou G., Curfs J. H., Meis J. F., Janssen J. J., Van Ruissen F., and Schalkwijk J. (2001) Cystatin M/E expression is restricted to differentiated epidermal keratinocytes and sweat glands: a new skin-specific proteinase inhibitor that is a target for cross-linking by transglutaminase. J. Invest. Dermatol. 116, 693–701 [DOI] [PubMed] [Google Scholar]
- 14. Pulukuri S. M., Gorantla B., Knost J. A., and Rao J. S. (2009) Frequent loss of cystatin E/M expression implicated in the progression of prostate cancer. Oncogene 28, 2829–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qiu J., Ai L., Ramachandran C., Yao B., Gopalakrishnan S., Fields C. R., Delmas A. L., Dyer L. M., Melnick S. J., Yachnis A. T., Schwartz P. H., Fine H. A., Brown K. D., and Robertson K. D. (2008) Invasion suppressor cystatin E/M (CST6): high-level cell type-specific expression in normal brain and epigenetic silencing in gliomas. Lab. Invest. 88, 910–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schagdarsurengin U., Pfeifer G. P., and Dammann R. (2007) Frequent epigenetic inactivation of cystatin M in breast carcinoma. Oncogene 26, 3089–3094 [DOI] [PubMed] [Google Scholar]
- 17. Ai L., Kim W. J., Kim T. Y., Fields C. R., Massoll N. A., Robertson K. D., and Brown K. D. (2006) Epigenetic silencing of the tumor suppressor cystatin M occurs during breast cancer progression. Cancer Res. 66, 7899–7909 [DOI] [PubMed] [Google Scholar]
- 18. Chen X., Cao X., Dong W., Xia M., Luo S., Fan Q., and Xie J. (2010) Cystatin M expression is reduced in gastric carcinoma and is associated with promoter hypermethylation. Biochem. Biophys. Res. Commun. 391, 1070–1074 [DOI] [PubMed] [Google Scholar]
- 19. Briggs J. J., Haugen M. H., Johansen H. T., Riker A. I., Abrahamson M., Fodstad Ø., Maelandsmo G. M., and Solberg R. (2010) Cystatin E/M suppresses legumain activity and invasion of human melanoma. BMC Cancer 10, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith R., Johansen H. T., Nilsen H., Haugen M. H., Pettersen S. J., Mælandsmo G. M., Abrahamson M., and Solberg R. (2012) Intra- and extracellular regulation of activity and processing of legumain by cystatin E/M. Biochimie 94, 2590–2599 [DOI] [PubMed] [Google Scholar]
- 21. Ekström U., Wallin H., Lorenzo J., Holmqvist B., Abrahamson M., and Avilés F. X. (2008) Internalization of cystatin C in human cell lines. FEBS J. 275, 4571–4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wallin H., Abrahamson M., and Ekström U. (2013) Cystatin C properties crucial for uptake and inhibition of intracellular target enzymes. J. Biol. Chem. 288, 17019–17029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wallin H., Bjarnadottir M., Vogel L. K., Wassélius J., Ekström U., and Abrahamson M. (2010) Cystatins: extra- and intracellular cysteine protease inhibitors: High-level secretion and uptake of cystatin C in human neuroblastoma cells. Biochimie 92, 1625–1634 [DOI] [PubMed] [Google Scholar]
- 24. Abrahamson M., Alvarez-Fernandez M., and Nathanson C.-M. (2003) Cystatins. Biochem. Soc. Symp. 70, 179–199 [DOI] [PubMed] [Google Scholar]
- 25. Zhang J., Shridhar R., Dai Q., Song J., Barlow S. C., Yin L., Sloane B. F., Miller F. R., Meschonat C., Li B. D., Abreo F., and Keppler D. (2004) Cystatin M: a novel candidate tumor suppressor gene for breast cancer. Cancer Res. 64, 6957–6964 [DOI] [PubMed] [Google Scholar]
- 26. Alvarez-Díaz S., Valle N., García J. M., Peña C., Freije J. M., Quesada V., Astudillo A., Bonilla F., López-Otín C., and Muñoz A. (2009) Cystatin D is a candidate tumor suppressor gene induced by vitamin D in human colon cancer cells. J. Clin. Invest. 119, 2343–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sokol J. P., and Schiemann W. P. (2004) Cystatin C antagonizes transforming growth factor β signaling in normal and cancer cells. Mol. Cancer Res. 2, 183–195 [PubMed] [Google Scholar]
- 28. Talantov D., Mazumder A., Yu J. X., Briggs T., Jiang Y., Backus J., Atkins D., and Wang Y. (2005) Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin. Cancer Res. 11, 7234–7242 [DOI] [PubMed] [Google Scholar]
- 29. Yoneda K., Iida H., Endo H., Hosono K., Akiyama T., Takahashi H., Inamori M., Abe Y., Yoneda M., Fujita K., Kato S., Nozaki Y., Ichikawa Y., Uozaki H., Fukayama M., et al. (2009) Identification of cystatin SN as a novel tumor marker for colorectal cancer. Int. J. Oncol. 35, 33–40 [PubMed] [Google Scholar]
- 30. Choi E. H., Kim J. T., Kim J. H., Kim S. Y., Song E. Y., Kim J. W., Kim S. Y., Yeom Y. I., Kim I. H., and Lee H. G. (2009) Up-regulation of the cysteine protease inhibitor, cystatin SN, contributes to cell proliferation and cathepsin inhibition in gastric cancer. Clin. Chim. Acta 406, 45–51 [DOI] [PubMed] [Google Scholar]
- 31. Cao X., Li Y., Luo R. Z., Zhang L., Zhang S. L., Zeng J., Han Y. J., and Wen Z. S. (2015) Expression of cystatin SN significantly correlates with recurrence, metastasis, and survival duration in surgically resected non-small cell lung cancer patients. Sci. Rep. 5, 8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang J., Liu H. L., Liu Z. H., Tan S. W., and Wu B. (2015) Identification of cystatin SN as a novel biomarker for pancreatic cancer. Tumour Biol. 36, 3903–3010 [DOI] [PubMed] [Google Scholar]
- 33. D'Costa Z. C., Higgins C., Ong C. W., Irwin G. W., Boyle D., McArt D. G., McCloskey K., Buckley N. E., Crawford N. T., Thiagarajan L., Murray J. T., Kennedy R. D., Mulligan K. A., Harkin D. P., Waugh D. J., et al. (2014) TBX2 represses CST6 resulting in uncontrolled legumain activity to sustain breast cancer proliferation: a novel cancer-selective target pathway with therapeutic opportunities. Oncotarget 5, 1609–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abrahamson M., Dalbøge H., Olafsson I., Carlsen S., and Grubb A. (1988) Efficient production of native, biologically active human cystatin C by Escherichia coli. FEBS Lett. 236, 14–18 [DOI] [PubMed] [Google Scholar]
- 35. Björk I., Brieditis I., Raub-Segall E., Pol E., Håkansson K., and Abrahamson M. (1996) The importance of the second hairpin loop of cystatin C for proteinase binding: characterization of the interaction of Trp-106 variants of the inhibitor with cysteine proteinases. Biochemistry 35, 10720–10726 [DOI] [PubMed] [Google Scholar]
- 36. Freije J. P., Balbín M., Abrahamson M., Velasco G., Dalbøge H., Grubb A., and López-Otín C. (1993) Human cystatin D: cDNA cloning, characterization of the Escherichia coli expressed inhibitor, and identification of the native protein in saliva. J. Biol. Chem. 268, 15737–15744 [PubMed] [Google Scholar]
- 37. Olafsson I., Löfberg H., Abrahamson M., and Grubb A. (1988) Production, characterization and use of monoclonal antibodies against the major extracellular human cysteine proteinase inhibitors cystatin C and kininogen. Scand. j. Clin. Lab. Invest. 48, 573–582 [DOI] [PubMed] [Google Scholar]
- 38. Balbín M., Hall A., Grubb A., Mason R. W., López-Otín C., and Abrahamson M. (1994) Structural and functional characterization of two allelic variants of human cystatin D sharing a characteristic inhibition spectrum against mammalian cysteine proteinases. J. Biol. Chem. 269, 23156–23162 [PubMed] [Google Scholar]
- 39. Ni J., Fernandez M. A., Danielsson L., Chillakuru R. A., Zhang J., Grubb A., Su J., Gentz R., and Abrahamson M. (1998) Cystatin F is a glycosylated human low molecular weight cysteine proteinase inhibitor. J. Biol. Chem. 273, 24797–24804 [DOI] [PubMed] [Google Scholar]
- 40. Werle B., Sauckel K., Nathanson C. M., Bjarnadottir M., Spiess E., Ebert W., and Abrahamson M. (2003) Cystatins C, E/M, and F in human pleural fluids of patients with neoplastic and inflammatory lung disorders. Biol. Chem. 384, 281–287 [DOI] [PubMed] [Google Scholar]