Abstract
Bispecific antibodies show great promise as intrinsic combination therapies, but often suffer from poor physiochemical properties, many times related to poor heterodimerization. De Nardis et al. identify specific electrostatic interactions that facilitate efficient heterodimerization, resulting in bispecific antibodies with physiochemical properties very similar to those of naturally occurring antibodies. This provides a new platform for the treatment of an array of diseases from cancer and autoimmune diseases to infectious diseases.
Introduction
Bispecific antibodies (bsAbs)3 represent a fast-growing field of research, with seemingly endless opportunities. Whereas naturally occurring antibodies bind bivalently to one antigen by means of their two Fab arms, bsAbs are engineered to contain the arms of two different antibodies, allowing them to bind simultaneously to two distinct antigens (Fig. 1). Since the 1980s, many different bsAbs have been generated against a variety of targets and more than 20 bsAbs are currently in clinical development, whereas many more are in the pipeline for clinical testing (1, 2). The therapeutic application of bsAbs is very broad, ranging from immunotherapy against cancer and immune disorders to applications against infectious diseases. However, applying bsAbs requires maintaining the natural antibody structure and the ability for large-scale production of high-quality material, which has proven challenging with these engineered sequences. A study by De Nardis et al. (3) now demonstrates one solution to this problem, facilitated by pairing oppositely charged amino acids across the heterodimer interface.
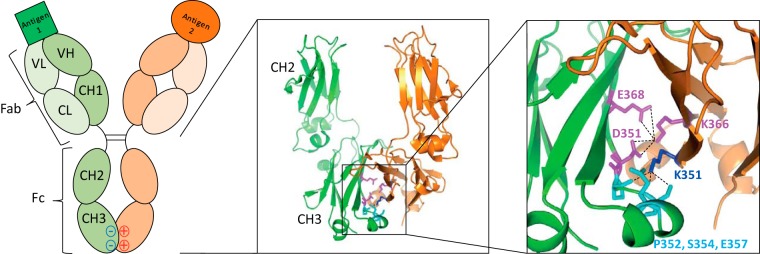
Figure 1.
Engineering of a DEKK bispecific antibody. A cartoon of a heterodimeric antibody with the different domains of the heavy and light chain labeled is shown on the left. The middle panel shows the protein structure of the DEKK CH3 domains (PDB entry 5NSC), while the right panel reveals the molecular interactions of the DEKK substitutions (Asp-351, Glu-368, Lys-351, Lys-366) in which the individual substitutions are highlighted; interacting residues are indicated in similar colors. Fab, antigen-binding fragment; Fc, crystallizable fragment; VL, light-chain variable region; CL, light-chain constant region; VH, heavy-chain variable region; CH1–3, heavy-chain constant regions 1–3.
Engineered bsAbs have been designed to act by a variety of mechanisms. For example, bsAb MM-111 acts on solid tumors by targeting two different antigens, human epidermal growth factor receptors (HER) 2 and 3, simultaneously (4). bsAb DVD-Ig FVM09∼MR72 protects against ebolavirus by using one arm to bind to the virus and enter the endosome and using the other arm to block viral entry in the host cell (5). An alternative use of bsAbs that has proven to be successful in cancer therapy involves recruiting specific immune cells with one arm and binding to target cells with the other arm. Blinatumomab, which is approved for acute lymphocytic leukemia (ALL) therapy, recognizes the CD19 receptor on leukemic B cells with one arm while recruiting killer T cells that mediate lysis of the tumor cells with the other arm (6).
There are many methods used to produce bsAbs, including quadroma technology (fusing two hybridoma cell lines), chemical cross-linking, and genetic engineering. However, these modifications often result in lower production yields, lower stability, reduced antibody half-life in vivo, and higher immunogenicity compared with regular antibodies. Therefore, bsAbs that mimic intact IgG antibodies are favored, and extensive efforts have been aimed at optimizing their expression and production. Such bsAbs obviously also have the advantage of retaining antibody functions mediated through their Fc domain.
The assembly of natural IgG antibodies is mediated by homodimerization of the third constant domain (CH3) within the heavy chain (Fig. 1). In engineered bsAbs, homodimer-favoring interactions must be replaced with interactions that favor heterodimerization, and high quality bsAbs are then selected based in part on a favorable ratio of the heterodimeric form over unwanted homodimeric species. Previous work has used rational design or directed evolution to create symmetric–asymmetric steric complementarity (using “knobs-into-holes” technology), charge-to-charge swaps, charge-to-steric complementarity swaps plus introduction of long-range electrostatic interactions, and isotype strand swaps (7). The first charge-to-charge swap (called DD-KK), based on computational modeling, demonstrated the potential of such a strategy, yielding greater than 90% heterodimers and retaining in vivo half-life (8). Further improvements on these existing scaffolds will increase the potential and feasibility for therapeutic applications.
De Nardis et al. (3) now use a combined computational modeling and iterative experimental validation strategy to identify a novel charge-to-charge paired construct, termed “DEKK,” by introducing altered charge polarity to support electrostatic interactions that favor heterodimer formation, while disfavoring homodimerization by charge repulsion. De Nardis et al. (3) first scanned a large series of mutants to identify positively and negatively charged substitutions in the CH3 domain that impaired the formation of homodimers, followed by the introduction of oppositely charged amino acids into the complementary CH3 domain. The L351D/T366K pair in particular resulted in the efficient formation of heterodimers and barely any homodimers. Further computational analysis was used to introduce additional electrostatic interactions at the core of the heterodimeric CH3 interface, yielding the L351D,L368E/L351K,T366K variant or DEKK, which showed 100% heterodimer formation. Furthermore, the level of aggregation, unfolding, and in vivo half-life was similar to that of natural antibodies. Structural analysis of the DEKK bsAb revealed unexpected interactions. As predicted by the computational modeling, residues Asp-351 and Glu-368 interacted with residue Lys-366 in the opposite CH3 domain. However, as a result of local shifts in the IgG backbone forming stabilizing salt-bridge interactions, residue Lys-351 did not interact with Asp-351 as predicted but formed interactions with residues Pro-352, Ser-354, and Glu-357 (Fig. 1). These unexpected backbone rearrangements and novel interactions may account for the favorable stability of the molecule, making it a very attractive platform for the development of novel bsAbs. De Nardis et al. (3) further demonstrated the utility of the DEKK design to construct a highly stable bsAb targeting HER2 and HER3 and to produce it using known protocols and, under GMP conditions, providing proof-of-concept that the DEKK design can yield bsAbs that can be used in the clinic.
The discovery of the DEKK substitutions provides a new platform from which bsAbs can be developed more easily. Furthermore, the DEKK platform can also be exploited for the generation of tri- and tetraspecific antibodies, by adding additional Fab regions on top of the Fab regions of bispecific antibodies or on the opposite site of the CH3 domain, as well as heterodimerization of other proteins and cytokines that have been engineered to include CH3 domains. However, despite the similarity in terms of stability, the melting temperature of the DEKK bispecific antibody was lower compared with other bsAbs and natural antibodies, raising the question of whether optimizing thermal stability is a requirement. The DEKK design is somewhat similar to the previously published DDKK design that involves opposite charges at slightly different positions (8). It would be appropriate to evaluate the two designs in a side-by-side comparison and assess whether they could be combined to produce bsAbs even more efficiently. For bsAbs to be successful, they will need to be amenable to straightforward, high-yield antibody production processes that fit GMP manufacturing conditions but also have physiochemical properties similar to that of naturally occurring antibodies. Design improvements to overcome these critical hurdles, such as the one described by De Nardis et al. (3), are important for the success of bsAbs as future therapeutics.
The authors declare that they have no conflicts of interest with the contents of this article.
- bsAbs
- bispecific antibodies
- DEKK
- L351D, L368E/L351K, T366K.
References
- 1. Fan G., Wang Z., Hao M., and Li J. (2015) Bispecific antibodies and their applications. J. Hematol. Oncol. 8, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu H., Saxena A., Sidhu S. S., and Wu D. (2017) Fc engineering for developing therapeutic bispecific antibodies and novel scaffolds. Front. Immunol. 8, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Nardis C., Hendriks L. J. A., Poirier E., Arvinte T., Gros P., Bakker A. B. H., and de Kruif J. (2017) A new approach for generating bispecific antibodies based on a common light-chain format and the stable architecture of human immunoglobulin G1. J. Biol. Chem. 292, 14706–14717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McDonagh C. F., Huhalov A., Harms B. D., Adams S., Paragas V., Oyama S., Zhang B., Luus L., Overland R., Nguyen S., Gu J., Kohli N., Wallace M., Feldhaus M. J., Kudla A. J., Schoeberl B., and Nielsen U. B. (2012) Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol. Cancer Ther. 11, 582–593 [DOI] [PubMed] [Google Scholar]
- 5. Wec A. Z., Nyakatura E. K., Herbert A. S., Howell K. A., Holtsberg F. W., Bakken R. R., Mittler E., Christin J. R., Shulenin S., Jangra R. K., Bharrhan S., Kuehne A. I., Bornholdt Z. A., Flyak A. I., Saphire E. O., Crowe J. E. Jr., Aman M. J., Dye J. M., Lai J. R., and Chandran K. (2016) A “Trojan horse” bispecific-antibody strategy for broad protection against ebolaviruses. Science 354, 350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kantarjian H., Stein A., Gökbuget N., Fielding A. K., Schuh A. C., Ribera J. M., Wei A., Dombret H., Foà R., Bassan R., Arslan Ö., Sanz M. A., Bergeron J., Demirkan F., Lech-Maranda E., Rambaldi A., Thomas X., Horst H. A., Brüggemann M., Klapper W., Wood B. L., Fleishman A., Nagorsen D., Holland C., Zimmerman Z., and Topp M. S. (2017) Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ha J. H., Kim J. E., and Kim Y. S. (2016) Immunoglobulin Fc heterodimer platform technology: From design to applications in therapeutic antibodies and proteins. Front. Immunol. 7, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gunasekaran K., Pentony M., Shen M., Garrett L., Forte C., Woodward A., Ng S. B., Born T., Retter M., Manchulenko K., Sweet H., Foltz I. N., Wittekind M., and Yan W. (2010) Enhancing antibody Fc heterodimer formation through electrostatic steering effects. J. Biol. Chem. 285, 19637–19646 [DOI] [PMC free article] [PubMed] [Google Scholar]