Abstract
Calcium homeostasis modulator protein-1 (CALHM1) and its Caenorhabditis elegans (ce) homolog, CLHM-1, belong to a new family of physiologically important ion channels that are regulated by voltage and extracellular Ca2+ (Ca2+o) but lack a canonical voltage-sensing domain. Consequently, the intrinsic voltage-dependent gating mechanisms for CALHM channels are unknown. Here, we performed voltage-clamp experiments on ceCLHM-1 chimeric, deletion, insertion, and point mutants to assess the role of the NH2 terminus (NT) in CALHM channel gating. Analyses of chimeric channels in which the ceCLHM-1 and human (h)CALHM1 NH2 termini were interchanged showed that the hCALHM1 NT destabilized channel-closed states, whereas the ceCLHM-1 NT had a stabilizing effect. In the absence of Ca2+o, deletion of up to eight amino acids from the ceCLHM-1 NT caused a hyperpolarizing shift in the conductance-voltage relationship with little effect on voltage-dependent slope. However, deletion of nine or more amino acids decreased voltage dependence and induced a residual conductance at hyperpolarized voltages. Insertion of amino acids into the NH2-terminal helix also decreased voltage dependence but did not prevent channel closure. Mutation of ceCLHM-1 valine 9 and glutamine 13 altered half-maximal activation and voltage dependence, respectively, in 0 Ca2+. In 2 mM Ca2+o, ceCLHM-1 NH2-terminal deletion and point mutant channels closed completely at hyperpolarized voltages with apparent affinity for Ca2+o indistinguishable from wild-type ceCLHM-1, although the ceCLHM-1 valine 9 mutant exhibited an altered conductance-voltage relationship and kinetics. We conclude that the NT plays critical roles modulating voltage dependence and stabilizing the closed states of CALHM channels.
Keywords: channel gating, voltage sensing, Ca2+ regulation, calcium homeostasis modulator channel
in a search for genes linked to late onset Alzheimer’s disease (AD), calcium homeostasis modulator 1 (CALHM1) was originally identified as a gene of unknown function (8). Human CALHM1 (hCALHM1) and its Caenorhabditis elegans homolog (ceCLHM-1) have now been shown to be plasma membrane channels with gating regulated by voltage and extracellular Ca2+ concentration ([Ca2+]o; 20, 22, 43). In the presence of Ca2+o, membrane depolarization induces slowly activating currents that deactivate upon membrane hyperpolarization. Removal of divalent cations causes a left shift in the conductance-voltage (G–V) relationship, enabling CALHM channels to open at hyperpolarized voltages (20, 43). CALHM1 is expressed in type II taste bud cells and the brain, where it plays important roles in taste perception and cortical neuron excitability, respectively (20, 44). Voltage-dependent gating of CALHM1 is required for the perception of sweet, bitter, and umami taste compounds (44). Low [Ca2+]o-induced gating of CALHM1 alters action potential firing in cultured mouse cortical neurons (20). Although CALHM1 integrates electrical and extracellular Ca2+ signals for its physiological roles, its channel-gating mechanism is not yet understood. The molecular determinants of the CALHM activation gate have not been established, nor is it known how these channels sense voltage or [Ca2+]o. Here, we sought to develop an understanding of the structural basis for the voltage-dependent gating of CALHM channels.
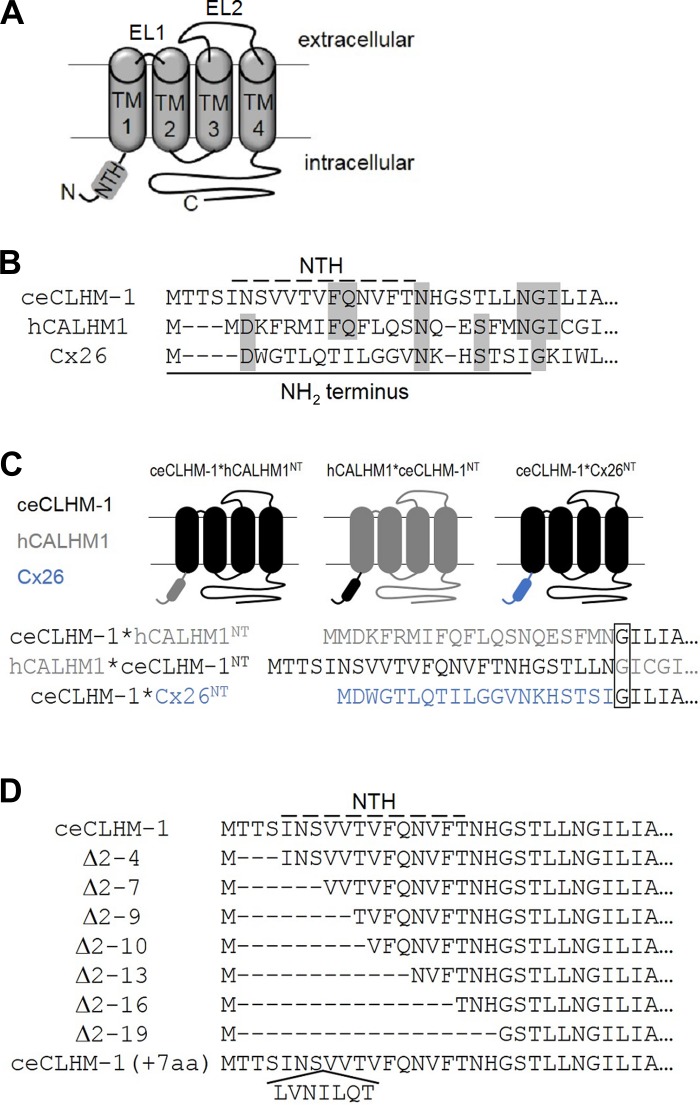
CALHM monomers have four transmembrane domains with a cytoplasmic amino (NH2) terminus (NT) that contains a short helix (NTH). Monomers oligomerize to form hexameric ion channels that are permeable to monovalent and divalent cations, monovalent anions, and ATP with poor selectivity due to a wide pore diameter of ~14 Å (20, 40, 43, 44). CALHM channels lack significant sequence similarity with other known channels and possess a unique pharmacological profile, suggesting that they belong to a distinct, evolutionarily conserved class of ion channels (8, 20, 22, 40, 43). Although hCALHM1 and ceCLHM-1 share only 16% sequence identity, they exhibit similar biophysical properties when expressed in Xenopus oocytes and functional conservation when expressed in C. elegans (20, 43). This suggests that structure rather than simply the amino acid sequence is critical for ion permeation and channel-gating properties. Both connexins, which can form either intercellular gap junctional channels or unopposed hemichannels, and pannexins exhibit structural and regulatory similarities with CALHM channels (40). Like CALHMs, connexin monomers have four transmembrane helixes with cytoplasmic amino and carboxyl termini and an NT that contains a short NTH. Connexin monomers assemble into a hexameric nonselective ion channel with a wide permeation pore (3, 11, 23, 46, 53). Both families of ion channels are regulated by voltage and [Ca2+]o (2, 12, 26, 29, 36, 41, 45, 48). Although hCALHM1 cannot form intercellular channels (40), we considered that structural insights into connexin hemichannel gating could shed light on CALHM gating mechanisms.
Connexins display two distinct gating mechanisms (45). The NTH contains a transjunctional voltage (Vj) sensor and forms a “plug” gate (23, 27, 30, 33, 41, 47), whereas the interface of the first transmembrane domain (TM1) and first extracellular loop (EL1) constitutes a “loop” gate that responds to membrane potential (Vm) and the concentration of extracellular divalent cations (9, 13, 16, 19, 31, 37, 42, 45, 48, 49). The plug and loop gates act in concert to close the gap junction conduction pore, which is lined by residues of the NTH and TM1 (14, 23, 54). Whereas gap junction channels sense both Vj and Vm, Vj is equivalent to Vm in connexin hemichannels (2, 26). CALHM proteins have an NT and NTH similar in length to connexins but lack a large EL1. Therefore we explored the role of the NT in voltage-dependent gating of CALHM channels.
To define the role of the NT in CALHM channel gating, we performed two-electrode voltage-clamp recordings of chimeric CALHM channels as well as ceCLHM-1 deletion, insertion, and point mutants expressed in Xenopus oocytes. Our results show that the NT regulates voltage dependence and the stability of CALHM channel-closed states in the absence of Ca2+o, establishing an important role for the NT in the voltage-dependent gating of CALHM channels. NT point mutations and deletions also affected the kinetics and half-maximal activation in the presence of Ca2+o but did not alter apparent affinity for [Ca2+]o or prevent channel closure. Thus the CALHM channel NT modulates voltage-dependent gating but is unlikely to act as the voltage sensor, [Ca2+]o sensor, or sole channel gate.
MATERIALS AND METHODS
Ethical approval.
The University of Pennsylvania Institutional Animal Care and Use Committee approved all procedures involving the use of Xenopus laevis for this study. Adult female X. laevis were obtained from Xenopus One (Dexter, MI) and maintained in university facilities. X. laevis were anesthetized by immersion in 0.15% ethyl 3-aminobenzoate methanesulfonate salt (Sigma-Aldrich) for 15 min before part of an ovary was removed. Oocytes were defolliculated with type 2 collagenase (Worthington Biochemical) in OR2 solution (85 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, and 5 mM HEPES, pH 7.6).
Molecular biology.
Human CALHM1 and C. elegans clhm-1 cDNAs were in the pBF oocyte expression vector. Deletion, insertion, and chimeric mutants were made using a PCR-based approach; the desired NH2-terminal sequence was introduced into the forward primer along with clhm-1 sequence to create and amplify mutant cDNAs. The seven amino acids added to the NT in the insertion mutant are predicted to lengthen the NTH on the basis of α-helical predictions made with JPred3 (http://www.compbio.dundee.ac.uk/jpred/). Restriction enzymes BamHI and SalI were used to insert all mutant cDNAs into pBF with the exception of ceCLHM-1*hCALHM1NTH, which was created by cutting hCALHM1 with PstI and SalI and inserting a ceCLHM-1 PCR product. All constructs were verified by sequencing. More detailed descriptions of plasmids and the primers used to make them are available upon request. All ceCLHM-1 point mutations were created using the QuikChange II site-directed mutagenesis kit (Agilent Technologies). RNA was synthesized from linearized plasmids with SP6 RNA polymerase (mMessage mMachine kit; Ambion).
Electrophysiology of Xenopus oocytes.
Stage IV–VI Xenopus oocytes were injected with 2.5–10 ng of cRNA along with 15 ng of Xenopus connexin-38 antisense oligonucleotide to inhibit endogenous connexin 38 (Cx38) currents. Oocytes were incubated for 2 days at 16°C in ND96 medium (96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, 2.5 mM Na-pyruvate, and 10 mM penicillin-streptomycin, pH 7.6). At least 30 min before recording, oocytes were injected with 46 nl of a 20 mM BAPTA-10 mM Ca2+ solution to prevent contamination from endogenous Ca2+-activated Cl− currents.
Standard bath solutions contained (in mM) 100 Na+, 100 Cl−, 2 K+, and 10 HEPES with various concentrations of Ca2+ (0.01, 0.1, 0.5, 2, or 5 mM). A divalent cation-free solution was created by adding 0.5 mM EGTA and 0.5 mM EDTA to the standard bath solution lacking divalent cations. All recording solutions were pH 7.2, adjusted with methanesulfonic acid. Recordings were acquired at room temperature with an OC-725C amplifier (Warner Instruments) at 1 kHz with a 16-bit analog-to-digital converter (Instrutech ITC-16) with TW100F-6 glass electrodes (World Precision Instruments) filled with 3 M KCl.
To study G–V relations, the voltage was stepped from the holding potential to test pulses in 10-mV increments ranging from −120 to +50 mV (divalent-free solution) or from −80 to +60 mV (Ca2+o-containing solutions) for 5 s. After each test pulse, the voltage was stepped to −80 mV for 5 s and then back to the holding potential for 45 s. Following recording in divalent cation-free solution, currents were recorded in the presence of 1 mM Gd3+ to establish the magnitude of nonspecific current from endogenous channels or exposure to 0-Ca2+ solution. To determine the appropriate holding potential in divalent cation-free experiments, preliminary studies were undertaken in which ceCLHM-1 currents were recorded with holding potentials at either −15 or −40 mV. The holding potential affected voltage-dependent gating (data not shown). Nevertheless, holding at −40 mV in the absence of divalent cations proved technically difficult and generated a constant inward current that likely altered intracellular ion concentrations. Thus, to reduce leak, a holding potential of −15 mV, which is near the resting potential in divalent cation-free solution, was used when recording in 0-Ca2+o solution. In the presence of Ca2+o, a −40 mV holding potential was used to keep channels in the closed state between test pulses.
To investigate activation kinetics in divalent-free solution, the voltage was stepped from the holding potential of −15 mV to −120 mV for 2 s and then to test pulses in 5-mV increments ranging from +15 to +35 mV for 5 s. After each test pulse, the voltage was stepped to −80 mV for 0.5 s and then back to the holding potential of −15 mV for 15 s. Deactivation kinetics were examined by stepping from the −15 mV holding potential to +30 mV for 3 s and then to test pulses in 10-mV increments ranging from −120 to −70 mV for 3 s in divalent-free solution. Results were unchanged when the reverse order of the voltage steps was performed.
Data analysis.
All recordings were analyzed with Igor Pro. To determine G–V relationships in the presence and absence of Ca2+o, currents were measured 3 ms after stepping from the test pulse to −80 mV to avoid contamination from the membrane capacitive current. For each oocyte, the half-maximal activation voltage (V0.5), voltage-dependent slope (Z), and maximal conductance (Gmax) were determined by fitting 0-Ca2+o data with a Boltzmann function without significant constraints. These were reasonable estimates because the measured data were within ~80% of the fitting range and the Boltzmann fits were not dependent on the last one or two data points. Means ± SE are presented in Figs. 4, E and F, and 7, A and C, and Tables 1–3. The currents in Ca2+o-containing solutions were normalized to Gmax (determined from the 0-Ca2+o data from the same oocyte) with the assumption that Ca2+o does not affect Gmax because Ca2+o does not alter single-channel conductance (20). All normalized data were fitted with Boltzmann functions to determine the V0.5 and Z presented in Figs. 1B; 3, B and D; 4, B–D and G; 6B; 7, B and D; 8, B–E; 9A; and 10C. ΔG0, the free energy of channel close-to-open transition at zero voltage, was calculated as ΔG0 = 0.24Z·F·V0.5, where F is the Faraday constant. For mutants for which Gmax could not be determined from the 0-Ca2+o data, conductances at −80 mV were normalized to the conductance evoked by a 5-s +50-mV pulse for 0-Ca2+o solution or the 5-s +60-mV voltage pulse in 2 mM Ca2+o solution.
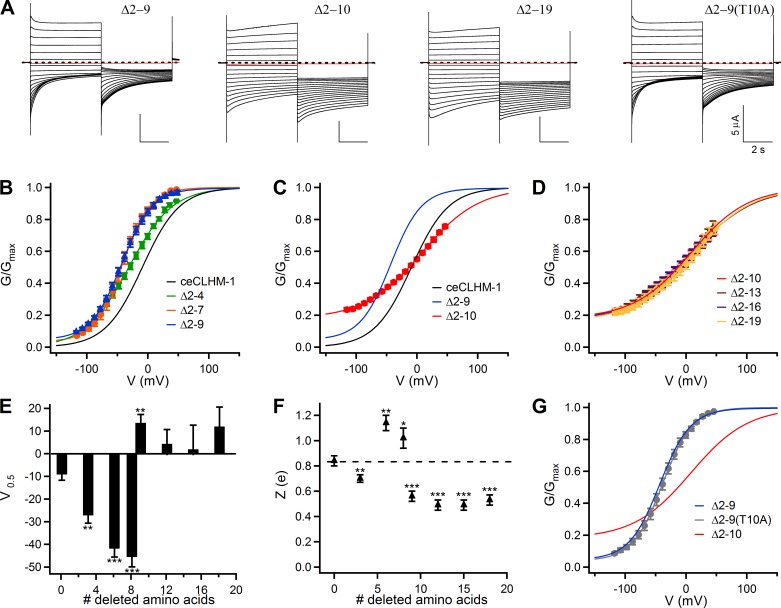
Fig. 4.
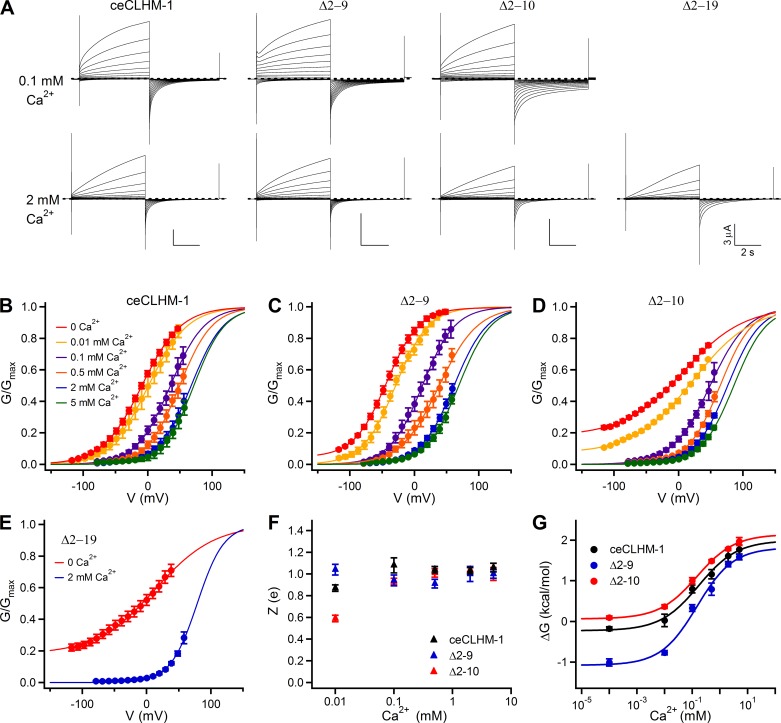
Deletion of NH2-terminal amino acids alters the voltage-dependent gating of ceCLHM-1. A: families of currents observed in oocytes expressing Δ2–9, Δ2–10, Δ2-19, and Δ2–9(T10A) channels in divalent cation-free solution; protocol as in Fig. 1A. Dashed lines indicate zero current, and red traces show current at −80 mV following the addition of Gd3+. B: G–V relationship for Δ2-4 (green squares; V0.5 = −31.4 ± 0.6 mV, Z = 0.71 ± 0.01 e, n = 7), Δ2-7 (orange circles; V0.5 = −41.9 ± 0.5 mV, Z = 1.05 ± 0.03 e, n = 5), and Δ2–9 (blue triangles; V0.5 = −43.1 ± 0.2 mV, Z = 1.00 ± 0.01 e, n = 9) channels compared with ceCLHM-1 (black line). C: G–V relationship for the Δ2–10 channel (red circles; V0.5 = 7.6 ± 0.4 mV, Z = 0.54 ± 0.01 e, n = 11) compared with ceCLHM-1 (black line) and the Δ2–9 channel (blue line); the Δ2–10 channel cannot close completely at hyperpolarized voltages in the absence of divalent cations. D: G–V relationships for the Δ2-13 (brown circles; V0.5 = 6.6 ± 0.6 mV, Z = 0.49 ± 0.01 e, n = 5), Δ2-16 (purple circles; V0.5 = 7.2 ± 0.5 mV, Z = 0.49 ± 0.01 e, n = 6), and Δ2-19 (yellow circles; V0.5 = 12.4 ± 0.8 mV, Z = 0.55 ± 0.01 e, n = 7) channels compared with the Δ2–10 channel (red line) in the absence of divalent cations. E: average half-maximal activation voltage V0.5 (mV). F: voltage-dependent slope Z (e). For E and F, error bars show SE of average data presented in Table 1; *P < 0.05, **P < 0.01, ***P < 0.001 vs. ceCLHM-1. In F, the dashed line shows Z for ceCLHM-1. G: mutation of Thr10 to Ala in the Δ2–9 background gave rise to a G–V relationship with a V0.5 = −37.8 ± 0.4 mV and Z = 1.05 ± 0.02 e (n = 6) in the absence of divalent cations, similar to the Δ2–9 channel (blue line), but not the Δ2–10 channel (red line). All G–V relationships were determined in divalent-free conditions and fitted with Boltzmann functions as described in Fig. 1. In B–D and G, error bars show SE of normalized data.
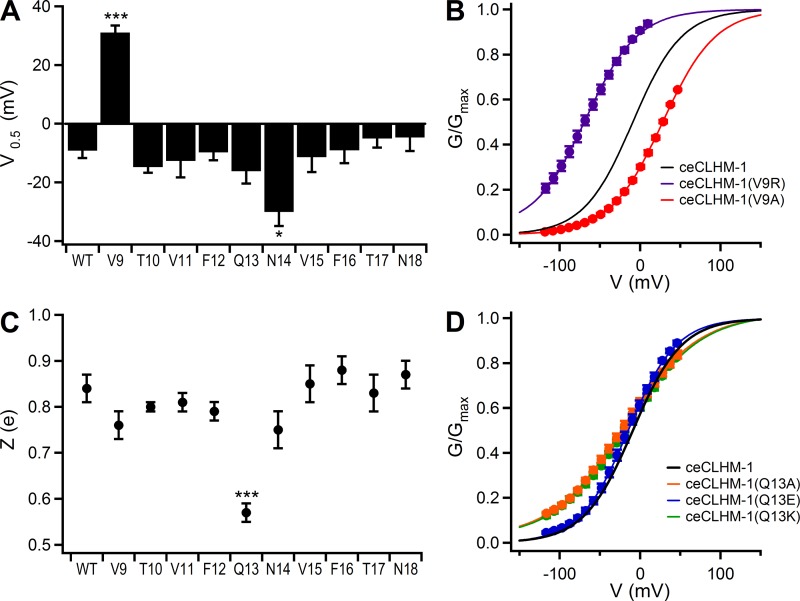
Fig. 7.
Mutation of ceCLHM-1 Val9, Gln13, and Asn14 alters voltage-dependent gating. A: average half-maximal activation voltage for alanine substitution mutants in divalent cation-free solution. WT, wild type. B: G–V relationship for ceCLHM-1(V9A) (red circles; V0.5 = 28.1 ± 0.2 mV, Z = 0.77 ± 0.01 e, n = 5) and ceCLHM-1(V9R) (purple circles; V0.5 = −64.8 ± 0.5 mV, Z = 0.76 ± 0.01 e, n = 6) compared with ceCLHM-1 (black line). C: average voltage-dependent slope Z (e) for alanine substitution mutants in divalent cation-free solution. D: G–V relationship for ceCLHM-1(Q13A) (orange circles; V0.5 = −17.1 ± 0.2 mV, Z = 0.58 ± 0.01 e, n = 7), ceCLHM-1(Q13K) (green circles; V0.5 = −16.6 ± 0.4 mV, Z = 0.54 ± 0.01 e, n = 5), and ceCLHM-1(Q13E) (blue circles; V0.5 = −20.9 ± 0.3 mV, Z = 0.85 ± 0.01 e, n = 5) compared with ceCLHM-1 (black line). For A and C, half-maximal activation voltage and voltage-dependent slope were determined by fitting G–V relationships with Boltzmann functions; error bars show SE of average data presented in Table 2. *P < 0.05; ***P < 0.001. For B and D, normalized G–V relationships were determined as in Fig. 1; error bars show SE of normalized data.
Table 1.
Average V0.5 and Z in the absence of divalent cations
| V0.5, mV | Z, e | |
|---|---|---|
| hCALHM1 | −61.6 ± 6.9‡ | 0.59 ± 0.04‡ |
| ceCLHM-1 | −9.2 ± 2.5 | 0.84 ± 0.03 |
| Δ2–4 | −27.2 ± 3.4† | 0.7 ± 0.03† |
| Δ2–7 | −41.9 ± 3.7‡ | 1.14 ± 0.06† |
| Δ2–9 | −45.6 ± 4.3‡ | 1.02 ± 0.08* |
| Δ2–9(T10A) | −36.8 ± 3.5‡ | 1.06 ± 0.03† |
| Δ2–9(T10G) | −41.4 ± 5.5‡ | 0.92 ± 0.05 |
| Δ2–10 | 13.6 ± 3.7† | 0.56 ± 0.04‡ |
| Δ2–13 | 4.4 ± 6.3 | 0.49 ± 0.04‡ |
| Δ2–16 | 2.0 ± 10.6 | 0.49 ± 0.04‡ |
| Δ2–19 | 12.0 ± 9.6 | 0.53 ± 0.04‡ |
| ceCLHM-1(+7aa) | −26.4 ± 4.3† | 0.62 ± 0.02‡ |
| ceCLHM-1*hCALHM1NT | −84.7 ± 3.5‡ | 0.54 ± 0.02‡ |
| hCALHM1*ceCLHM-1NT | −24 ± 3.9§ | 0.63 ± 0.03 |
| ceCLHM-1*Cx26NT | 43.5 ± 2.5‡ | 1.65 ± 0.12‡ |
Values are means ± SE.
P < 0.05, †P < 0.01, ‡P < 0.001 vs. ceCLHM-1, §P < 0.001 vs. hCALHM1.
Table 3.
Average V0.5, Z, and ΔG0 for ceCLHM-1, Δ2-9, Δ2-10, and ceCLHM- 1(V9R) in [Ca2+]o solutions
| 0.01 mM Ca2+ | 0.1 mM Ca2+ | 0.5 mM Ca2+ | 2 mM Ca2+ | 5 mM Ca2+ | |
|---|---|---|---|---|---|
| ceCLHM-1 | |||||
| V0.5, mV | 0.9 ± 8.4 | 31.9 ± 3.3 | 48.1 ± 4.3 | 67.6 ± 5.4 | 72.3 ± 3.9 |
| Z, e | 0.87 ± 0.03 | 1.08 ± 0.07 | 1.04 ± 0.03 | 1.03 ± 0.03 | 1.06 ± 0.04 |
| ΔG0, kcal/mol | 0.03 ± 0.15 | 0.80 ± 0.10 | 1.16 ± 0.11 | 1.61 ± 0.13 | 1.77 ± 0.11 |
| Δ2–9 | |||||
| V0.5, mV | −32.4 ± 2.9* | 13.1 ± 5.2* | 37.5 ± 6.5 | 61.2 ± 2.6 | 68.7 ± 3.7 |
| Z, e | 1.04 ± 0.05* | 0.94 ± 0.05 | 0.91 ± 0.04* | 1.00 ± 0.07 | 1.00 ± 0.04 |
| ΔG0, kcal/mol | −0.77 ± 0.1* | 0.26 ± 0.10† | 0.79 ± 0.15 | 1.41 ± 0.06 | 1.58 ± 0.07 |
| Δ2–10 | |||||
| V0.5, mV | 26.6 ± 2.3* | 47.0 ± 4.2* | 64.2 ± 1.8* | 74.8 ± 1.0 | 85.5 ± 2.2* |
| Z, e | 0.59 ± 0.03‡ | 0.93 ± 0.03 | 1.01 ± 0.04 | 1.04 ± 0.02 | 1.00 ± 0.06 |
| ΔG0, kcal/mol | 0.36 ± 0.05 | 1.00 ± 0.08 | 1.49 ± 0.04 | 1.79 ± 0.02 | 1.97 ± 0.10 |
| ceCLHM-1(V9R) | |||||
| V0.5, mV | −38.3 ± 9.8† | 3.3 ± 2.1‡ | 23.6 ± 3.6† | 43.5 ± 5.8† | 49.9 ± 2.7† |
| Z, e | 0.8 ± 0.10 | 0.88 ± 0.07* | 0.92 ± 0.07 | 1.02 ± 0.04 | 1.01 ± 0.03 |
| ΔG0, kcal/mol | −0.7 ± 0.08* | 0.06 ± 0.03† | 0.49 ± 0.05† | 1.02 ± 0.08† | 1.16 ± 0.05† |
Values are means ± SE.
P < 0.05, †P < 0.01, ‡P < 0.001 vs. ceCLHM-1 at the same [Ca2+].
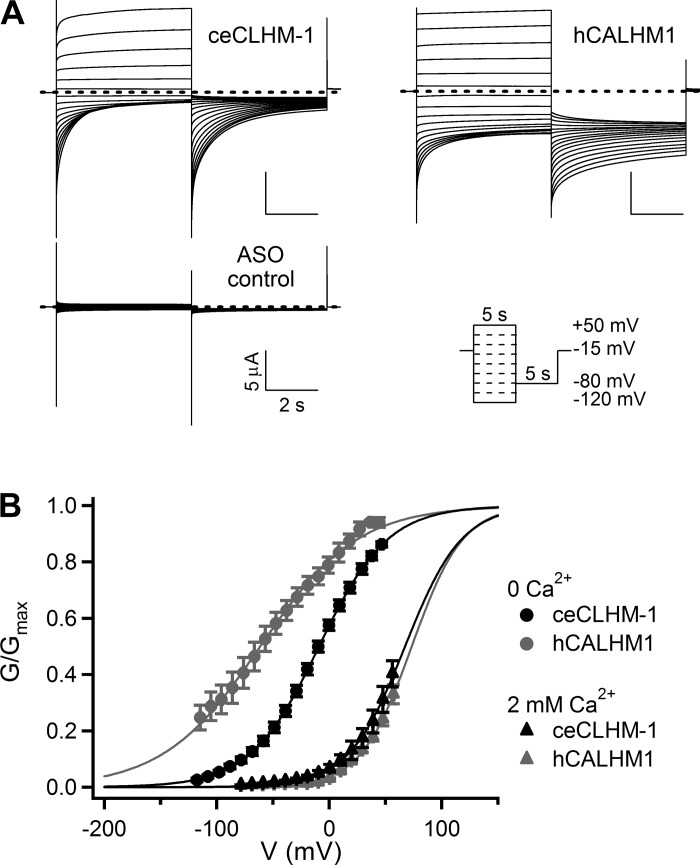
Fig. 1.
Intrinsic voltage-dependent gating is exhibited by ceCLHM-1 and hCALHM1. A: currents observed in oocytes expressing ceCLHM-1, hCALHM1, or a Xenopus Cx38 antisense oligonucleotide alone (ASO control) in response to 5-s voltage pulses from −120 to +50 mV from a holding potential of −15 mV in divalent cation-free solution. Cx38 ASO and BAPTA were injected into all oocytes to inhibit endogenous currents; dashed lines indicate zero current. B: currents at −80 mV were measured after a series of voltage pulses as in A to determine conductance-voltage (G–V) relations. For each oocyte, Gmax was determined by fitting 0-Ca2+o data with a Boltzmann function, and conductances were then normalized to Gmax. Conductances measured in the presence of 2 mM Ca2+o were normalized to Gmax determined by fitting 0-Ca2+o data from the same oocyte, on the basis of the observation that Ca2+o does not affect Gmax (20). All normalized data were fitted with Boltzmann functions (lines) to produce apparent G–V relationships. hCALHM1 in 0 Ca2+o (gray circles), V0.5 = −61.4 ± 0.9 mV, Z = 0.58 ± 0.01 e, n = 7; ceCLHM-1 in 0 Ca2+o (●), V0.5 = −9.1 ± 0.1 mV, Z = 0.83 ± 0.01 e, n = 9; hCALHM1 in 2 mM Ca2+o (gray triangles), V0.5 = 72.3 ± 0.5 mV, Z = 1.14 ± 0.02 e, n = 5; ceCLHM-1 in 2 mM Ca2+o (▲), V0.5 = 67.4 ± 0.8 mV, Z = 1.00 ± 0.02 e, n = 5. Apparent G–V relationships for ceCLHM-1 and hCALHM1 in the presence of 2 mM Ca2+o are not significantly different. Error bars show SE of normalized data.
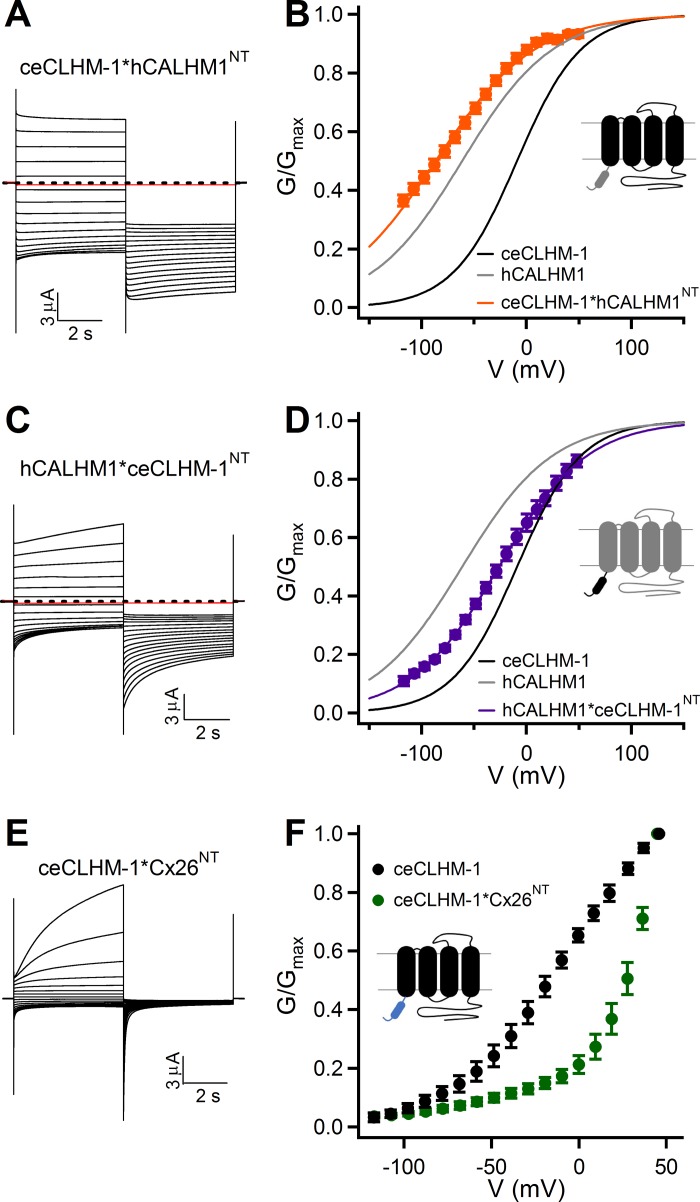
Fig. 3.
NT regulates the stability of CALHM channel-closed states in the absence of divalent cations. A: currents observed in oocytes expressing ceCLHM-1*hCALHM1NT in divalent cation-free solution. Dashed line shows zero current, and the red trace shows current at −80 mV following the addition of Gd3+. B: G–V relationship for ceCLHM-1*hCALHM1NT (orange circles) in the absence of Ca2+o (V0.5 = −86.7 ± 0.9 mV, Z = 0.53 ± 0.01 e, n = 8) compared with that of ceCLHM-1 (black line) and hCALHM1 (gray line). C: currents observed in oocytes expressing hCALHM1*ceCLHM-1NT in divalent cation-free solution. D: G–V relationship for hCALHM1*ceCLHM-1NT (purple circles) in the absence of Ca2+o (V0.5 = −26.3 ± 0.2 mV, Z = 0.60 ± 0.01 e, n = 6) compared with that of ceCLHM-1 (black line) and hCALHM1 (gray line). E: currents observed in oocytes expressing ceCLHM-1*Cx26NT in 0-Ca2+o solution. F: G–V relationship for ceCLHM-1*Cx26NT (green circles) in the absence of Ca2+o compared with that of ceCLHM-1 (black circles). In F, G–V relationships were determined by measuring conductances at −80 mV evoked by a series of voltage prepulses, normalized to the conductance at −80 mV evoked by a +50-mV prepulse. For all recordings, pulse protocol is as in Fig. 1A. G–V relationships were determined as in Fig. 1. Error bars show SE of normalized data. Chimera schematics are described in Fig. 2D.
Fig. 6.
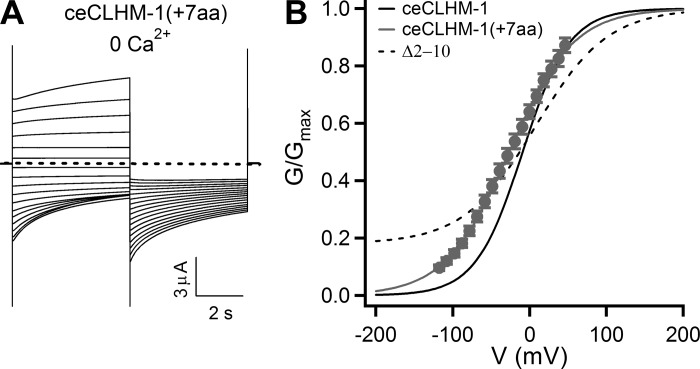
Insertion of amino acids into the ceCLHM-1 NT affects intrinsic gating properties but does not prevent channel closure. A: currents observed in an oocyte expressing ceCLHM-1(+7aa) in the absence of divalent cations; pulse protocol as in Fig. 1A. Dashed line shows zero current. B: G–V relationship for ceCLHM-1(+7aa) in the absence of divalent cations (gray circles; V0.5 = −26.5 ± 0.5 mV, Z = 0.61 ± 0.01 e, n = 7) compared with ceCLHM-1 (solid black line) and the Δ2–10 channel (dashed line). Error bars show SE of normalized data.
Fig. 8.
Ca2+o sensing is not disrupted in ceCLHM-1 NH2-terminal deletion mutants. A: from left to right, families of currents observed in oocytes expressing ceCLHM-1, Δ2–9, Δ2–10, and Δ2-19 channels in response to 5-s voltage pulses from −80 to +60 mV in 0.1 mM Ca2+o (top) and 2 mM Ca2+o (bottom) solutions. For ceCLHM-1, Δ2–9, and Δ2–10 channels, recordings in 0.1 and 2 mM Ca2+o solutions were performed on the same oocyte; activation kinetics become slower while deactivation kinetics become faster with increasing [Ca2+]o. The Δ2–9, Δ2–10, and Δ2-19 mutants, as well as ceCLHM-1, closed completely in the presence of Ca2+o. Holding potential was −40 mV; dashed lines indicate zero current. B–E: apparent G–V relationships for ceCLHM-1 (B), Δ2–9 (C), Δ2–10 (D), and Δ2-19 (E) channels in various [Ca2+]o solutions. Currents at −80 mV were measured after a series of voltage pulses as described in A to determine G–V relationships in the presence of 0.01 (yellow), 0.1 (purple), 0.5 (orange), 2 (blue), and 5 mM Ca2+o (green) and 0 Ca2+o (red). G–V relationships were determined as described in Fig. 1B. Error bars show SE of normalized data; n = 4–11. F: average voltage-dependent slope Z (e) for ceCLHM-1 (▲), Δ2–9 (blue triangles), and Δ2–10 (red triangles) channels across all [Ca2+]o tested. G: free energy of channel opening ΔG0 was fitted with a Hill equation to determine the IC50 for [Ca2+]o: ceCLHM-1, IC50 = 170 ± 89 μM, Hill coefficient = 0.62 ± 0.18; Δ2–9, IC50 = 165 ± 75 μM, Hill coefficient = 0.66 ± 0.18; Δ2–10, IC50 = 147 ± 23 μM, Hill coefficient = 0.64 ± 0.06.
Fig. 9.
Chimeric CALHM channels exhibit Ca2+o regulation. A: apparent G–V relations for ceCLHM-1*hCALHM1NT (gray triangles; V0.5 = 79.8 ± 2.2 mV, Z = 1.02 ± 0.06 e) and hCALHM1*ceCLHM-1NT (▲; V0.5 = 75.2 ± 1.1 mV, Z = 1.03 ± 0.03 e) in 2 mM Ca2+o compared with ceCLHM-1*hCALHM1NT (gray circles) and hCALHM1*ceCLHM-1NT (●) 0-Ca2+o data, previously presented in Fig. 3, B and D. Currents at −80 mV were measured after a series of voltage pulses and analyzed as in Fig. 1B. Note that the ceCLHM-1*hCALHM1NT and hCALHM1*ceCLHM-1NT chimeras closed at hyperpolarized voltages in the presence of 2 mM Ca2+o just as ceCLHM-1 and hCALHM1. Error bars show SE of normalized data; n = 6–8. B: families of currents observed in oocytes expressing ceCLHM-1*Cx26NT in response to 5-s voltage pulses from −80 to +60 mV in 2 mM Ca2+o. C: G–V relationship for ceCLHM-1*Cx26NT (gray diamonds) and ceCLHM-1 (◆) in the presence of 2 mM Ca2+o. In C, Gmax was not determined for each oocyte. G–V relationships were determined by measuring conductances at −80 mV evoked by a series of voltage prepulses, normalized to the conductance at −80 mV evoked by a +60-mV voltage prepulse. From −20 to +50 mV, P < 0.05 comparing ceCLHM-1*Cx26NT with ceCLHM-1. Error bars show SE of normalized data.
Fig. 10.
Mutation of ceCLHM-1 Val9 alters channel gating in the presence of Ca2+o. A: currents observed in oocytes expressing ceCLHM-1(V9R) in response to 5-s voltage pulses from −80 to +60 mV in 2 mM Ca2+o solution. B: representative current trace for ceCLHM-1 (black) and ceCLHM-1(V9R) (gray) in response to a 5-s voltage pulse at +50 mV followed by a 5-s voltage pulse at −80 mV in 2 mM Ca2+o; holding potential was −40 mV. Time course of activation at +50 mV was determined by fitting data as in B with a single exponential. ceCLHM-1(V9R) τ = 1.59 ± 0.10 s (n = 5) compared with τ ~7.03 ± 0.96 s for ceCLHM-1 (n = 5). Tail currents recorded upon stepping to −80 mV were best fitted with a double exponential, with faster deactivation time constants for ceCLHM-1(V9R) (τ1 = 0.024 ± 0.001 s, τ2 = 0.24 ± 0.04 s; n = 5) compared with ceCLHM-1 (τ1 = 0.036 ± 0.008 s, τ2 = 0.44 ± 0.02 s; n = 5). C: apparent G–V relations for ceCLHM-1 (◆; V0.5 = 67.4 ± 0.8 mV, Z = 1.00 ± 0.02 e, n = 5) and ceCLHM-1(V9R) (gray diamonds; V0.5 = 44.1 ± 0.4 mV, Z = 1.02 ± 0.02 e, n = 5) in the presence of 2 mM Ca2+o are different (P < 0.01; data presented as in Fig. 8). D: free energy of channel-opening ΔG0 was fitted with a Hill equation to determine the IC50 for [Ca2+]o; ceCLHM-1(V9R), IC50 = 208 ± 111 μM, Hill coefficient = 0.44 ± 0.10, compared with ceCLHM-1, as previously described in Fig. 8G. Error bars show SE of normalized data.
To analyze activation and deactivation kinetics, currents 3 ms after stepping to the test pulse to the end of the pulse were fit with an exponential function. ceCLHM-1 and the Δ2–9 amino acid deletion channel kinetic data were fit with a double exponential using the following equation: I(t) = I0 + A1·exp [−(t − t0)/τ1] + A2·exp [−(t − t0)/τ2], where τ1 is the time constant of the fast process, τ2 is the time constant of the slow process, I0 is the steady-state current, t0 is the time at the start of the fit, and A is the current amplitude at time t0. The Δ2–10 amino acid deletion channel currents were fit with a single exponential, and the resulting τ was compared with ceCLHM-1 τ2.
Sequence analysis.
A multiple sequence alignment (Fig. 2) was created from the C. elegans CLHM-1 NP_495403 and human CALHM1 NP_001001412 sequences with MegAlign (Lasergene); the Cx26 sequence NP_003995.2 was aligned manually. The ceCLHM-1 NH2-terminal α-helix predictions were made with JPred3 (http://www.compbio.dundee.ac.uk/jpred/).
Fig. 2.
Sequences of wild-type and NH2-terminal mutant proteins. A: predicted topology of CALHM proteins. NH2-terminal helix (NTH), transmembrane domains (TM) and extracellular loops (EL) are indicated. N, NH2 terminus; C, COOH terminus. B: sequence alignment of the NT of ceCLHM-1, hCALHM1, and Cx26; solid line indicates the ceCLHM-1 NT, dashed line indicates the NTH as predicted with JPred3, and gray shading shows identical residues. C: ceCLHM-1*hCALHM1NT, hCALHM1*ceCLHM-1NT, and ceCLHM-1*Cx26NT chimeras used in this study. ceCLHM-1 (black), hCALHM1 (gray), and Cx26 (blue) sequences as indicated; box shows the Gly at the beginning of TM1. D: sequences of deletion and insertion mutants; dashed line shows predicted NTH of ceCLHM-1.
RESULTS
Intrinsic voltage-dependent gating is exhibited by hCALHM1 and ceCLHM-1.
The two CALHM family members known to form functional ion channels, hCALHM1 and ceCLHM-1, are regulated by voltage and [Ca2+]o. Because the two modes of regulation are allosterically coupled, we examined intrinsic voltage-dependent gating by recording channel currents in a divalent cation-free solution. Recombinant hCALHM1 and ceCLHM-1 were expressed in Xenopus oocytes, and whole cell currents were recorded (Fig. 1A) using established protocols (20, 43). G–V relations were determined by measuring tail currents at −80 mV following a series of voltage prepulses and fitting the G–V curves with Boltzmann functions to determine voltage-dependent gating parameters. hCALHM1 had a half-maximal activation voltage (V0.5) of −61.4 mV and voltage-dependent slope (Z) of 0.58 e, where e is the number of elementary charges. ceCLHM-1 opened at significantly more depolarized voltages (V0.5 = −9.1 mV) and exhibited greater voltage dependence than hCALHM1 (Z = 0.83 e; Fig. 1B). These results demonstrate that in the absence of extracellular divalent cations, CALHM channels possess an intrinsic voltage sensor coupled to an activation gate.
Ca2+o stabilizes channel closed-states of both hCALHM1 and ceCLHM-1 (20, 43). Addition of 2 mM Ca2+o caused a +134-mV depolarizing shift in the G–V relationship for hCALHM1 and a +77-mV shift for ceCLHM-1 (Fig. 1B). Although quantitatively different, these results demonstrate that both channels are regulated by voltage and [Ca2+]o, with the activation gate opening in response to depolarization and Ca2+ unbinding. Because ceCLHM-1 can close completely at hyperpolarized voltages in the absence of divalent cations (Fig. 1B) and oocytes expressing ceCLHM-1 are more stable than those expressing hCALHM1, we focused subsequent experiments on ceCLHM-1.
NH2 terminus is important for voltage-dependent gating of CALHM channels.
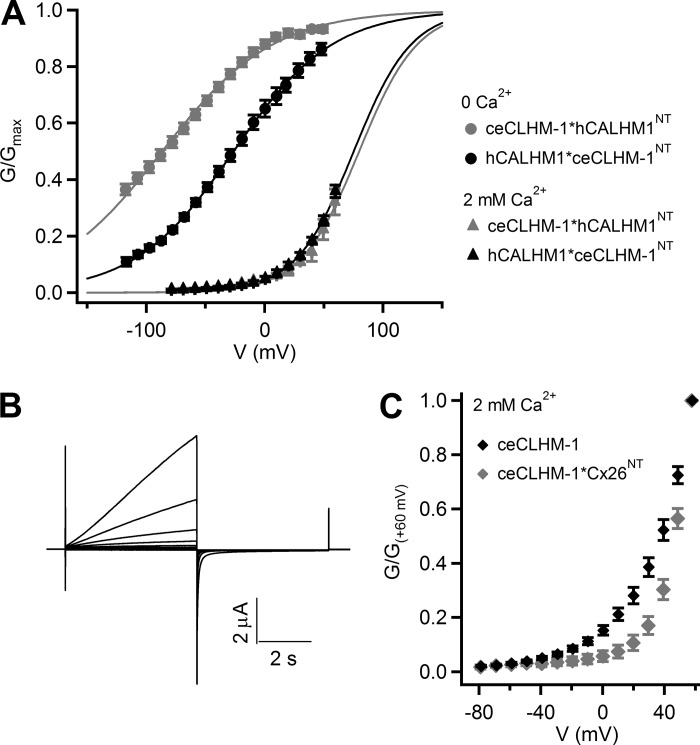
On the basis of structural and functional similarities with connexin hemichannels, we hypothesized that the NT, which is important for connexin gating, may also play roles in CALHM channel gating. To assess a possible role of the NT in channel gating, we exploited the different voltage dependencies of hCALHM1 and ceCLHM-1 in the absence of Ca2+o (Fig. 1B) by analyzing voltage-dependent gating of chimeric CALHM channels (Fig. 2). We created a chimeric channel in which the ceCLHM-1 NT was replaced with that of hCALHM1 (ceCLHM-1*hCALHM1NT) and another in which the hCALHM1 NT was replaced with that of ceCLHM-1 (hCALHM1*ceCLHM-1NT; Fig. 2C). The ceCLHM-1*hCALHM1NT chimera was functional, exhibiting a −77.6-mV shift in the G–V relationship compared with ceCLHM-1 (Fig. 3, A and B). Thus replacement of the ceCLHM-1 NT with the hCALHM1 NT shifted channel inactivation to more hyperpolarized voltages, suggesting that channel-closed states are destabilized in the ceCLHM-1*hCALHM1NTchannel. Given very slow time-dependent deactivation at −80 mV (Fig. 3A), we confirmed the voltage dependence of ceCLHM-1*hCALHM1NT gating by dividing the I-V relations by the driving force to determine the G–V relationship (V0.5 = −87.8 mV; Z = 0.63 e). Replacement of the hCALHM1 NT with that of ceCLHM-1 resulted in a +35.1-mV shift in the G–V relationship compared with hCALHM1, suggesting that the channel-closed states are stabilized in the hCALHM1*ceCLHM-1NT channel (Fig. 3, C and D). These results suggest that the NT is involved in the intrinsic voltage-dependent gating mechanism of CALHM channels, with the hCALHM1 NT destabilizing, and the ceCLHM-1 NT stabilizing, chimeric channel-closed states at hyperpolarized voltages.
To further explore the importance of the NT in voltage-dependent gating, we created a chimeric channel in which the ceCLHM-1 NT was replaced with that of Cx26 (ceCLHM-1*Cx26NT; Fig. 2C). Remarkably, this chimera was functional, with slowed activation and accelerated deactivation kinetics compared with ceCLHM-1 (Fig. 3E). The ceCLHM-1*Cx26NT chimera exhibited a depolarizing shift in the G–V relationship (Fig. 3F), indicating that the Cx26 NT stabilized ceCLHM-1 channel-closed states. Interestingly, the Cx26 NT and hCALHM1 NT had opposite effects on ceCLHM-1 gating despite the NH2 termini differing by only one amino acid in length (Figs. 2B and 3, B and F). These results suggest that although CALHM channels are remarkably tolerant of NT replacement, the NT plays a role in CALHM channel gating.
Length of the NH2 terminus affects voltage-dependent gating of ceCLHM-1.
A crystal structure of Cx26 in an open state revealed that the NTH folds into the channel pore, where it may act as a plug gate (23). Although CALHM and connexin NH2 termini lack sequence conservation, their lengths are similar (Fig. 2B) and a chimeric channel in which the ceCLHM-1 NT was replaced by that of Cx26 (ceCLHM-1*Cx26NT) was functional (Fig. 3, E and F). We predicted that if the NT forms a plug gate in CALHM channels, then ceCLHM-1 NH2-terminal deletions would result in channels that cannot close. Accordingly, we analyzed a series of ceCLHM-1 NH2-terminal deletion mutants (Fig. 2D). We first deleted three amino acids (Δ2-4) to create a ceCLHM-1 channel with an NT of the same length as the human channel (Fig. 2, B and D). The G–V relationship of the Δ2-4 channel exhibited a −22.3-mV hyperpolarizing shift and decrease in the voltage-dependent slope compared with ceCLHM-1 (Fig. 4B). Nevertheless, the first three residues of the NT are not required for channel function or for intrinsic voltage dependence, nor do they account for the quantitative differences in intrinsic voltage dependence observed between hCALHM1 and ceCLHM-1 (Fig. 1B).
To assess the role of the NTH, which was not disrupted in the ceCLHM-1 Δ2-4 channel, we analyzed a series of NTH deletion mutants (Fig. 2D). All NTH deletion mutant channels were functional and exhibited voltage-dependent gating (Fig. 4 and Table 1). This contrasts with connexin hemichannels, which require a fully intact NT for channel function (17). Compared with ceCLHM-1 (Fig. 1B), the Δ2-7 and Δ2–9 channels had altered gating, exhibiting greater than −30-mV hyperpolarizing shifts in G–V relations and increased voltage-dependent slopes compared with ceCLHM-1 (Fig. 4, B, E, and F, and Table 1). Thus residues Thr2-Val9 modulate ceCLHM-1 gating, but they are not required for voltage sensing or for closing the channel gate. Channels with 9 (Δ2–10), 12 (Δ2-13), 15 (Δ2-16), or 18 (Δ2-19) amino acids deleted (Fig. 2D) were also functional but exhibited a substantial (~23%) residual conductance at −120 mV (Fig. 4, A, C, and D). The magnitude of this residual conductance was determined by fitting the G–V data with a Boltzmann function, which showed a conductance plateau at hyperpolarized voltages. The residual conductance did not appear to be from endogenous channels, because oocytes expressing wild-type ceCLHM-1 channels recorded on the same day did not exhibit it. Furthermore, addition of the ceCLHM-1 inhibitor Gd3+ eliminated the residual conductance (Fig. 4A), indicating that it was not simply a nonspecific leak due to recording in 0-Ca2+ solution. The inability of the Δ2–10, Δ2-13, Δ2-16, and Δ2-19 channels to close completely at hyperpolarized voltages in the absence of Ca2+o suggests that these channels either lack part of the intrinsic voltage-dependent gate or that the coupling between the voltage sensor and the gate is altered. However, because voltage dependence was not eliminated in the NTH deletion mutants, a voltage sensor was still present even with the entire NTH deleted.
Although the Δ2–9 and Δ2–10 channels differ by only one amino acid in NT length, their G–V relationships and voltage dependencies were markedly different (Fig. 4, C, E, and F). Deletion of up to 18 amino acids, which removes the entire ceCLHM-1 NTH (Fig. 2D), had no additional effect, as the V0.5 and Z for the Δ2–10 and Δ2-19 channels were not different (Fig. 4, D–F, and Table 1). Thus removal of residues 2–10 of the NT had the greatest impact on voltage-dependent gating of ceCLHM-1. To understand why deletion of the 10th amino acid generated dramatically different gating properties between the Δ2–9 and Δ2–10 channels, we asked whether the presence of a Thr at residue 10 was required. A channel that lacked eight NT residues as in the Δ2–9 construct but had the Thr at position 10 replaced with Ala [Δ2–9(T10A)] exhibited a G–V relationship that was not different from that of the Δ2–9 channel (Fig. 4G). In addition, mutation of Thr10 to glycine in the Δ2–9 background did not prevent channel closure at hyperpolarized voltages or disrupt voltage dependence (Table 1). These results indicate that the presence of a Thr at position 10 is not required and that a critical determinant of the distinct voltage-dependent gating properties of the Δ2–9 and Δ2–10 channels is the length of the NT.
NH2-terminal deletions alter both activation and deactivation kinetics.
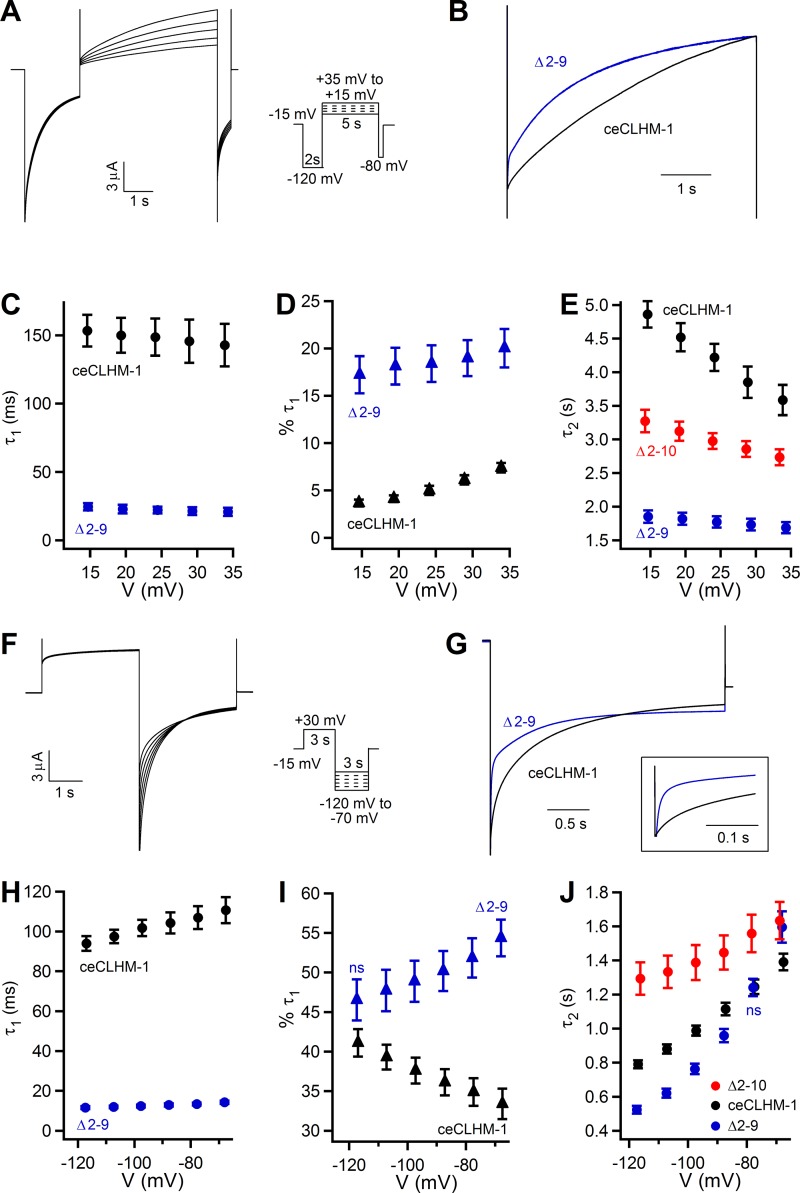
To more specifically define how ceCLHM-1 gating is affected by the NT deletions, we compared the activation and deactivation kinetics of wild-type, Δ2–9, and Δ2–10 ceCLHM-1 channels. To examine activation kinetics, the voltage was stepped to −120 mV to drive channels into the closed state and then to a series of activating test pulses (Fig. 5A). The time courses of activation for ceCLHM-1 and the Δ2–9 channel were best fitted by double exponentials, whereas Δ2–10 currents could only be fitted with a single exponential, with the time constant more comparable to the slower process (τ2) for ceCLHM-1. This suggests that either a channel gating transition is absent in the Δ2–10 channel or that the time constant of the faster activation process (τ1) was too fast to be measured in our experiments. Only a small percentage of the total current was defined by τ1, which was sixfold faster in the Δ2–9 channel compared with ceCLHM-1 (Fig. 5, B–D). Voltage dependence was determined from a linear fit analysis of the natural logarithm of the time constants presented in Fig. 5 across the voltages tested. ceCLHM-1 τ2 was voltage dependent, becoming smaller at more depolarized potentials (Fig. 5E). Compared with the wild type, the rate of activation of the Δ2–9 channel was ≥2-fold faster with a 70% decrease in voltage dependence (Fig. 5E), which accounts for the left shift observed in the Δ2–9 G–V relationship (Fig. 4B). The Δ2–10 channel activation time constant was also reduced and exhibited a 42% decrease in voltage dependence compared with the wild type (Fig. 5E), consistent with the difference in the voltage-dependent slopes observed between the steady-state G–V relationships (Fig. 4C).
Fig. 5.
Disruption of the ceCLHM-1 NT affects both activation and deactivation kinetics. A: ceCLHM-1 currents observed in response to a 2-s voltage pulse at −120 mV to close channels, followed by a series of 5-s activating test pulses from +15 to +35 mV and then a step to −80 mV for 0.5 s. Time course of activation was determined by fitting data as in A with a double exponential for ceCLHM-1 and Δ2–9 and a single exponential for Δ2–10. B: representative current traces for ceCLHM-1 (black) and Δ2–9 (blue) at +20 mV following a −120-mV prepulse. C: time constant of the faster activation process (τ1). D: percent of current described by τ1. E: time constant of the slower activation process (τ2) for ceCLHM-1 (black; n = 6), Δ2–9 (blue; n = 6), and Δ2–10 (red; n = 6) channels. In E, τ2 at +15 mV was significantly different from τ2 at +35 mV for ceCLHM-1 (P < 0.01) and Δ2–10 (P < 0.05) channels. F: currents observed in oocytes expressing ceCLHM-1 in response to a 3-s voltage pulse at +30 mV followed by stepping to different deactivating potentials from −120 to −70 mV. Time course of deactivation was determined by fitting data as in F with a double exponential for ceCLHM-1 and Δ2–9 and a single exponential for the Δ2–10 channel. G: representative current traces for ceCLHM-1 (black) and Δ2–9 (blue) channels at −80 mV following a +30-mV prepulse. H: τ1 at −120 mV was significantly different from that at −70 mV for both ceCLHM-1 (black; n = 7) and Δ2–9 (blue; n = 8) channels (P < 0.05). I: percentage of the tail current described by τ1 was voltage dependent (P < 0.05), increasing at hyperpolarized voltages for ceCLHM-1 and decreasing at hyperpolarized voltages for the Δ2–9 mutant. J: time constant of the slower deactivation process (τ2) exhibits significant voltage dependence from −120 to −70 mV for ceCLHM-1 (black; P < 0.001), Δ2–9 (blue; P < 0.001), and Δ2–10 (red; P < 0.05) channels. Activation and deactivation pulse protocols were performed in divalent cation-free solution; holding potential was −15 mV. In C–E and H–J, all Δ2–9 and Δ2–10 values are significantly different from ceCLHM-1 (P < 0.05) except the two marked not significant (ns). Error bars show SE.
Deactivation kinetics were determined by measuring tail currents produced after stepping to hyperpolarized potentials following a +30-mV depolarizing pulse (Fig. 5F). For both ceCLHM-1 and the Δ2–9 channel, τ1 and the percentage of current defined by this time constant were voltage dependent (Fig. 5, G–I). Strikingly, Δ2–9 currents decayed faster with τ1 ~8-fold smaller than ceCLHM-1, suggesting a lower energy barrier for the open-to-close transition in the Δ2–9 channel (Fig. 5H). For ceCLHM-1, Δ2–9, and Δ2–10 channels, τ2 was smaller at more hyperpolarized voltages and showed significant voltage dependence (Fig. 5J). Although τ2 was similar for ceCLHM-1 and the mutant channels, the Δ2–9 channel displayed a twofold increase in voltage dependence, and the Δ2–10 channel exhibited a twofold decrease in voltage dependence compared with the wild-type channel (Fig. 5J). This is consistent with the respective increase and decrease in voltage dependence observed in the steady-state G–V relations of the Δ2–9 and Δ2–10 channels compared with ceCLHM-1. In summary, these results indicate that disruption of the NT affects both the time course and voltage dependence of ceCLHM-1 activation and deactivation in 0-Ca2+o.
NH2 terminus is unlikely to form a plug gate in ceCLHM-1.
In Cx26, interactions between the NTH and TM1 allow for ion permeation, whereas in the closed state the NTH plugs the pore (23). The presence of a residual conductance in the absence of divalent cations for ceCLHM-1 channels with nine or more NH2-terminal amino acids deleted is consistent with the idea that the NT could contribute to a plug gate. To test this, we inserted seven amino acids into the NTH of ceCLHM-1 [ceCLHM-1(+7aa)], predicting that if the ceCLHM-1 NT forms a plug gate, then extending the length of the NTH should prevent channels from closing as it is unlikely that the NTH extension could be physically accommodated within the pore. ceCLHM-1(+7aa) was functional, with altered gating compared with the wild-type channel (Fig. 6, A and B). Notably, the ceCLHM-1(+7aa) conductance did not plateau at hyperpolarized voltages as observed for the Δ2–10 channel (Fig. 6B), suggesting that the channel was able to close. Whereas the NT is unlikely to form a plug gate in ceCLHM-1, insertion of NH2-terminal amino acids affected intrinsic voltage-dependent gating, providing further evidence that there is an optimal NT length.
Identification of NT residues that regulate voltage-dependent gating.
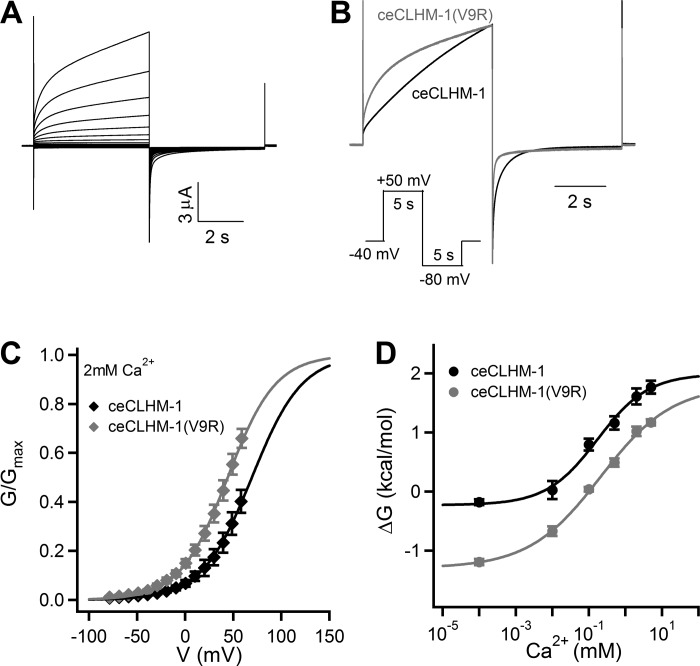
To identify specific residues in the NTH that contribute to modulation of voltage-dependent gating, we made a series of 10 alanine [Ala (A)] substitution mutants starting with ceCLHM-1 Val9, which is mutated to the initiation methionine in the Δ2–9 channel, and analyzed G–V relations. Compared with the wild type, ceCLHM-1(V9A) exhibited a +40.3-mV depolarizing shift in the G–V relationship, indicating that the Ala substitution at this residue stabilized closed-channel states (Fig. 7, A and B, and Table 2). Conversely, mutation of ceCLHM-1 Val9 to arginine [Arg (R)], the residue found at the corresponding position in hCALHM1, caused a −51.6-mV hyperpolarizing shift in the G–V relationship, indicating that it destabilized channel-closed states (Fig. 7B and Table 2). Our results showing that mutation of Val9 shifted the G–V relationship and that the absence of an amino acid at this position in the Δ2–10 channel caused drastic changes in channel gating indicate that this residue is important for voltage-dependent gating of ceCLHM-1. Mutation of ceCLHM-1 asparagine 14 to Ala also shifted the G–V relationship (Fig. 7A). However, other mutations at this site resulted in nonfunctional channels. Alanine substitution at Gln13 decreased the voltage-dependent slope (Fig. 7, C and D, and Table 2). Because this residue is conserved among CALHM family members, we also introduced a positively charged lysine [Lys (K)] and negatively charged glutamate [Glu (E)] at this site to determine the effects of charge substitutions. ceCLHM-1(Q13K) exhibited gating properties indistinguishable from ceCLHM-1(Q13A). However, substitution with Glu, which differs from Gln in that its side chain contains an oxygen instead of an amino group, resulted in voltage-dependent gating that was not different from ceCLHM-1 (Fig. 7D and Table 2). Notably, charge substitutions at Gln13 did not change the gating polarity, suggesting that although this residue plays a role in voltage dependence, it is not the voltage sensor. In summary, NT point mutations affect the stability of channel-closed states and voltage dependence of ceCLHM-1, indicating that the NT sequence plays an important role in channel gating.
Table 2.
Average V0.5 and Z for ceCLHM-1 point mutants in the absence of divalent cations
| V0.5, mV | Z, e | |
|---|---|---|
| ceCLHM-1 | −9.2 ± 2.5 | 0.84 ± 0.03 |
| ceCLHM-1(V9A) | 31.1 ± 2.4† | 0.76 ± 0.03 |
| ceCLHM-1(V9R) | −60.8 ± 1.9† | 0.85 ± 0.02 |
| ceCLHM-1(T10A) | −14.8 ± 1.9 | 0.8 ± 0.01 |
| ceCLHM-1(V11A) | −12.7 ± 5.6 | 0.81 ± 0.02 |
| ceCLHM-1(F12A) | −9.8 ± 2.7 | 0.79 ± 0.02 |
| ceCLHM-1(Q13A) | −16.2 ± 4.2 | 0.57 ± 0.02† |
| ceCLHM-1(Q13K) | −13.4 ± 2.4 | 0.55 ± 0.02† |
| ceCLHM-1(Q13E) | −15.5 ± 3.0 | 0.85 ± 0.02 |
| ceCLHM-1(N14A) | −30.1 ± 4.8* | 0.75 ± 0.04 |
| ceCLHM-1(V15A) | −11.4 ± 5.1 | 0.85 ± 0.04 |
| ceCLHM-1(F16A) | −9.1 ± 4.4 | 0.88 ± 0.03 |
| ceCLHM-1(T17A) | −5.1 ± 3.1 | 0.83 ± 0.04 |
| ceCLHM-1(N18A) | −4.7 ± 6.4 | 0.87 ± 0.03 |
Values are means ± SE.
P < 0.05, †P < 0.001 vs. ceCLHM-1.
NH2-terminal deletion channels retain Ca2+o regulation.
Ca2+o regulates CALHM channel gating by stabilizing channels in closed states at hyperpolarized voltages (20, 43). As our results suggest that the NT plays a role in intrinsic voltage-dependent gating, we determined the effect of the NH2-terminal deletions on ceCLHM-1 gating in the presence of Ca2+o. Surprisingly, the Δ2–10 channel that could not close at hyperpolarized voltages in divalent cation-free solution closed completely at hyperpolarized voltages in the presence of as little as 0.1 mM Ca2+o (Fig. 8, A and D). The Δ2-19 channel, with the entire NTH deleted, also closed completely in the presence of Ca2+o (Fig. 8, A and E). As observed for ceCLHM-1, Ca2+o slowed activation and accelerated deactivation kinetics of the Δ2–9, Δ2–10, and Δ2-19 channels (Fig. 8A). These results demonstrate that similar to wild-type ceCLHM-1, gating of the Δ2–9, Δ2–10, and Δ2-19 channels is regulated by both voltage and [Ca2+]o. Importantly, the Δ2-19 channel closed completely at hyperpolarized voltages in the presence of 2 mM Ca2+o, indicating that the NTH is not required to form an intact Ca2+o- and voltage-regulated channel gate.
Ca2+o caused depolarizing shifts in the apparent G–V relationships (Fig. 8, B–E, and Table 3). Five millimolar Ca2+o right shifted the ceCLHM-1 G–V relationship by +81 mV (Fig. 8B and Table 3). For the Δ2–9 and Δ2–10 channels, 5 mM Ca2+o caused +112- and +78-mV shifts in the apparent G–V relationships, respectively (Fig. 8, C and D, and Table 3). In the presence of 5 mM Ca2+o, the voltage-dependent slope was not different for the Δ2–9 and Δ2–10 mutants compared with ceCLHM-1 (Fig. 8F), suggesting that voltage dependence in the presence of Ca2+o is unaltered by NH2-terminal deletions. To determine whether NH2-terminal deletions affected the apparent affinity for [Ca2+]o, we calculated the effect of [Ca2+]o on the free energy difference between the closed and open states at zero voltage (ΔG0). For ceCLHM-1 and both deletion mutants, increasing [Ca2+]o increased ΔG0 (Table 3). ΔG0 values determined at various [Ca2+]o were fitted with a Hill equation to determine the IC50. The [Ca2+]o IC50 calculated for the Δ2–9 channel (165 ± 75 µM) and the Δ2–10 channel (147 ± 23 µM) was not different from that of ceCLHM-1 (170 ± 89 µM; Fig. 8G). These data indicate that NT truncations of ceCLHM-1 leave intact a voltage- and Ca2+o-dependent gate and suggest that the [Ca2+]o sensor is unaffected in these mutants.
NT sequence affects kinetics and G-V relationships in the presence of Ca2+o.
To assess the impact of NT sequence changes on CALHM channel gating in the presence of Ca2+o, we analyzed ceCLHM-1*hCALHM1NT, hCALHM1*ceCLHM-1NT, and ceCLHM-1*Cx26NT chimeric channels (Fig. 9). In 2 mM Ca2+o the apparent G–V relationships for ceCLHM-1*hCALHM1NT and hCALHM1*ceCLHM-1NT were indistinguishable from each other and the wild-type channels. However, the ceCLHM-1*Cx26NT chimera, with slowed activation kinetics and accelerated deactivation kinetics, exhibited a depolarizing shift in the apparent G–V relationship compared with ceCLHM-1 (Figs. 8A and 9, B and C). This suggests that the NT affects the stability of ceCLHM-1 channel-closed states in both the absence and presence of Ca2+o.
Because mutation of ceCLHM-1 Val9 to Arg destabilized channel-closed states in the absence of Ca2+o, we also investigated the effect of this mutation in Ca2+-containing solutions (Fig. 10A). The time course of activation in 2 mM Ca2+o at +50 mV, best fitted with a single exponential, was 4.4-fold faster for ceCLHM-1(V9R) compared with ceCLHM-1. Tail currents recorded upon stepping to −80 mV following a +50 mV depolarizing pulse were best fitted with a double exponential, with τ1 1.5-fold and τ2 1.8-fold faster for ceCLHM-1(V9R) vs. the wild-type channel. The V9R mutant channel also closed more rapidly because 81% of the current was defined by the faster deactivation process (τ1) compared with 49% for ceCLHM-1 (Fig. 10B). Five millimolar Ca2+o caused a +111-mV depolarizing shift in the apparent G–V relationship for ceCLHM-1(V9R) (Table 3). Compared with the wild-type channel, ceCLHM-1(V9R) opened at significantly more hyperpolarized voltages across all [Ca2+]o (Fig. 10B and Table 3). To determine whether the V9R mutation affected apparent affinity for [Ca2+]o, we fitted ΔG0 values determined at various [Ca2+]o with a Hill equation to determine the IC50. The [Ca2+]o IC50 for ceCLHM-1(V9R) (208 ± 111 µM) was not different from that of ceCLHM-1 (170 ± 89 µM) suggesting that the [Ca2+]o sensor is intact in the V9R mutant channel. These results demonstrate that a single point mutation at ceCLHM-1 Val9 affects the V0.5 of the steady-state G–V relations as well as the activation and deactivation kinetics in the presence of Ca2+o. Thus the NT plays a role in modulating voltage-dependent gating in both the presence and absence of Ca2+o.
DISCUSSION
The physiological roles of CALHM channels and their involvement in pathophysiological processes are only now starting to be defined. CALHM1 is an essential component of the voltage-gated ATP release channel in type II taste bud cells required for perception of sweet, bitter, and umami compounds (21, 44). In mouse cortical neurons, CALHM1 plays a role in enhanced excitability in response to decreases in [Ca2+]o that can exist in synaptic clefts during high excitatory activity (20, 35). Human genetic studies suggest that a P86L polymorphism in CALHM1 influences the age of AD onset and loss of Calhm1 in mice causes increased steady-state levels of toxic Aβ, impairs memory flexibility, and disrupts long-term potentiation (18, 50, 51). In C. elegans, CLHM-1 is required for coordinated locomotion; however, overexpression in neurons is toxic, inducing necrotic-like neurodegeneration that is partially Ca2+ dependent (43). Given the emerging insight into the physiological significance of CALHM channels, there is a need to understand how their gating is regulated.
Expression of the P86L-CALHM1 and R154H-CALHM1 variants in mammalian cells resulted in reduced Ca2+ uptake and increased Aβ secretion compared with CALHM1, but neither mutant channel had altered voltage-dependent gating or ionic permeability properties (52). A molecular understanding of how CALHM channels function is currently lacking; therefore we performed a structure-function investigation to define the molecular determinants that regulate voltage-dependent gating. To this end, we considered structural determinants of the gating of structurally and functionally similar connexin channels (40), wherein the NT plays a critical role. We found that in chimeric CALHM channels the hCALHM1 NT destabilized channel-closed states of ceCLHM-1 and, conversely, the ceCLHM-1 NT stabilized channel-closed states at hyperpolarized voltages. Whereas deletion of nine or more NH2-terminal amino acids from ceCLHM-1 decreased voltage dependence and led to residual conductance in the absence of divalent cations, considerable extension of the NT altered voltage-dependent gating but did not prevent channel closure at hyperpolarized voltages. All NH2-terminal mutant channels were able to close completely at hyperpolarized voltages in the presence of Ca2+o, and the Δ2–9 and Δ2–10 deletion channels exhibited voltage dependencies and apparent [Ca2+]o affinities indistinguishable from wild-type ceCLHM-1. Mutation of a single residue, ceCLHM-1 Val9, shifted the apparent G–V relationship in both the presence and absence of Ca2+o, but it did not affect apparent [Ca2+]o affinity. Together, the analyses of mutant channels with deletions, insertions, and point mutations as well as chimeric channels show that the NT plays an important role in CALHM channel gating but that it is unlikely to act as the voltage sensor, [Ca2+]o sensor, or sole channel gate.
Replacement of the ceCLHM-1 NT with the hCALHM1 NT or Cx26 NT, which are of comparable lengths, resulted in functional channels with destabilized and stabilized channel-closed states, respectively, in the absence of Ca2+o. These opposite effects on gating could result from differences in the orientations of the Cx26 and hCALHM1 NTHs. Although a glycine at residue 12 acts as a hinge in the Cx26 NT, the hCALHM1 NT is not predicted to form a kinked structure. Alternatively, specific nonconserved residues in the hCALHM1 and Cx26 NH2 termini could affect the stability of channel-closed states. Notably, the V9R point mutation in ceCLHM-1 caused a hyperpolarizing shift in the G–V relationship in the absence and presence of physiological [Ca2+]o suggesting that specific residues may be important gating determinants in the chimeric channels. The Val9-corresponding residue in Cx32 (Gly5) is important for gating and ion selectivity. Negative charge substitutions at Gly5 reverse the gating polarity of Cx32 and increase cation selectivity, whereas positive charge substitutions at this residue decrease conductance and result in nonselective channels (28, 33). A crystal structure of Cx26 indicates that Gly5 forms a hydrogen bond with Asp2 in the neighboring protomer, stabilizing the funnel organization of the NH2 termini in the channel pore (23). Because the plug gate model may not be relevant for CALHM channels, Val9 likely plays a different role in ceCLHM-1. We note that Val9 and Gln13, the position at which mutations altered voltage-dependent slope, fall on the same face of the NTH. Identification of regions of the channel that interact with this face of the NTH may provide deeper insights into the role of the NT in CALHM channel gating.
Although the NT plays a role in stabilizing closed states of CALHM channels, the location of the CALHM channel gate(s) remains unknown. Connexin hemichannels utilize two gating mechanisms that have distinct voltage sensors and gates; the “fast” Vj gate and a “slow” loop gate (2, 26, 45). Residues in TM1 and EL1 contribute to loop gate closure, which occurs at hyperpolarized voltages with the closed state stabilized by extracellular divalent cations (13, 16, 19, 31, 32, 37, 42, 45, 48, 49). CALHM channels have a short EL1 (<10 amino acids) compared with connexin hemichannels (~30 amino acids), although they possess a large second extracellular loop (EL2). Notably, mutation of a conserved Asp at the TM3-EL2 interface alters ion selectivity and conductance of CALHM channels (20, 43). In connexins, Vj and loop gating were defined by analyses of single-channel gating transitions (41, 45) and by charge substitutions that generated hemichannels with the gates exhibiting reversed polarities (15, 25, 27, 33, 47). If there are two gates in CALHM channels, then both must respond similarly to membrane voltage, and we have yet to identify a residue that can be mutated to reverse the polarity of CALHM channel gating.
Although we undertook these studies with the Cx26 structure in mind, it is possible that not all connexin family members share the same fast gating mechanism. On the basis of the Cx26 structure, deletion of up to 12 amino acids of the Cx37 NT would be expected to result in a constitutively open channel, but it instead results in a nonconducting channel (17). A different gating mechanism termed the “particle receptor” model involving interactions between the COOH terminus and cytoplasmic loop has been proposed to contribute to the fast gating mechanism of Cx40 and Cx43 hemichannels (1, 6, 24, 34, 39), although truncation of the COOH terminus does not disrupt Vj gating in Cx32 (15). Recent analysis of a Cx43*NT37 chimeric channel suggested that changes in the conformation of the COOH terminus affect gating by another domain, such as the NT (10). In pannexins, the COOH terminus also plays a role in gating and has been proposed to form a plug gate through nonspecific interactions with the channel pore (4, 7) such that caspase cleavage of the COOH terminus in response to apoptotic signals results in a constitutively open channel (5, 38). It is possible that voltage-dependent gating in CALHM channels could result from interactions between the NT, the cytoplasmic loop, and/or the COOH terminus.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grants R01-DC-012538 (J. K. Foskett and Z. Ma) and R03-DC-014328 (J. E. Tanis) and an American Heart Postdoctoral Fellowship 12POST11940054 (J. E. Tanis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.E.T., Z.M., and J.K.F. conceived and designed research; J.E.T. performed experiments; J.E.T. and Z.M. analyzed data; J.E.T., Z.M., and J.K.F. interpreted results of experiments; J.E.T. prepared figures; J.E.T. drafted manuscript; J.E.T., Z.M., and J.K.F. edited and revised manuscript; J.E.T., Z.M., and J.K.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Wint Thu Saung for assistance with oocyte procurement and Xenopus maintenance and Drs. Roberto Dominguez, Horia Vais, and D. D.-O. Mak for helpful discussions.
REFERENCES
- 1.Anumonwo JM, Taffet SM, Gu H, Chanson M, Moreno AP, Delmar M. The carboxyl terminal domain regulates the unitary conductance and voltage dependence of connexin40 gap junction channels. Circ Res 88: 666–673, 2001. doi: 10.1161/hh0701.088833. [DOI] [PubMed] [Google Scholar]
- 2.Bargiello TA, Tang Q, Oh S, Kwon T. Voltage-dependent conformational changes in connexin channels. Biochim Biophys Acta 1818: 1807–1822, 2012. doi: 10.1016/j.bbamem.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beblo DA, Veenstra RD. Monovalent cation permeation through the connexin40 gap junction channel. Cs, Rb, K, Na, Li, TEA, TMA, TBA, and effects of anions Br, Cl, F, acetate, aspartate, glutamate, and NO3. J Gen Physiol 109: 509–522, 1997. doi: 10.1085/jgp.109.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bond SR, Naus CC. The pannexins: past and present. Front Physiol 5: 58, 2014. doi: 10.3389/fphys.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467: 863–867, 2010. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA 100: 11388–11393, 2003. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dourado M, Wong E, Hackos DH. Pannexin-1 is blocked by its C-terminus through a delocalized non-specific interaction surface. PLoS One 9: e99596, 2014. doi: 10.1371/journal.pone.0099596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreses-Werringloer U, Lambert JC, Vingtdeux V, Zhao H, Vais H, Siebert A, Jain A, Koppel J, Rovelet-Lecrux A, Hannequin D, Pasquier F, Galimberti D, Scarpini E, Mann D, Lendon C, Campion D, Amouyel P, Davies P, Foskett JK, Campagne F, Marambaud P. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell 133: 1149–1161, 2008. doi: 10.1016/j.cell.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebihara L, Liu X, Pal JD. Effect of external magnesium and calcium on human connexin46 hemichannels. Biophys J 84: 277–286, 2003. doi: 10.1016/S0006-3495(03)74848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ek Vitorín JF, Pontifex TK, Burt JM. Determinants of Cx43 channel gating and permeation: the amino terminus. Biophys J 110: 127–140, 2016. doi: 10.1016/j.bpj.2015.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong XQ, Nicholson BJ. Size selectivity between gap junction channels composed of different connexins. Cell Commun Adhes 8: 187–192, 2001. doi: 10.3109/15419060109080721. [DOI] [PubMed] [Google Scholar]
- 12.González D, Gómez-Hernández JM, Barrio LC. Molecular basis of voltage dependence of connexin channels: an integrative appraisal. Prog Biophys Mol Biol 94: 66–106, 2007. doi: 10.1016/j.pbiomolbio.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Kronengold J, Trexler EB, Bukauskas FF, Bargiello TA, Verselis VK. Single-channel SCAM identifies pore-lining residues in the first extracellular loop and first transmembrane domains of Cx46 hemichannels. J Gen Physiol 122: 389–405, 2003. doi: 10.1085/jgp.200308861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kronengold J, Trexler EB, Bukauskas FF, Bargiello TA, Verselis VK. Pore-lining residues identified by single channel SCAM studies in Cx46 hemichannels. Cell Commun Adhes 10: 193–199, 2003. doi: 10.1080/cac.10.4-6.193.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon T, Dowd TL, Bargiello TA. The carboxyl terminal residues 220–283 are not required for voltage gating of a chimeric connexin32 hemichannel. Biophys J 105: 1376–1382, 2013. doi: 10.1016/j.bpj.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon T, Tang Q, Bargiello TA. Voltage-dependent gating of the Cx32*43E1 hemichannel: conformational changes at the channel entrances. J Gen Physiol 141: 243–259, 2013. doi: 10.1085/jgp.201210839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyle JW, Minogue PJ, Thomas BC, Domowicz DA, Berthoud VM, Hanck DA, Beyer EC. An intact connexin N-terminus is required for function but not gap junction formation. J Cell Sci 121: 2744–2750, 2008. doi: 10.1242/jcs.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert JC, Sleegers K, González-Pérez A, Ingelsson M, Beecham GW, Hiltunen M, Combarros O, Bullido MJ, Brouwers N, Bettens K, Berr C, Pasquier F, Richard F, Dekosky ST, Hannequin D, Haines JL, Tognoni G, Fiévet N, Dartigues JF, Tzourio C, Engelborghs S, Arosio B, Coto E, De Deyn P, Del Zompo M, Mateo I, Boada M, Antunez C, Lopez-Arrieta J, Epelbaum J, Schjeide BM, Frank-Garcia A, Giedraitis V, Helisalmi S, Porcellini E, Pilotto A, Forti P, Ferri R, Delepine M, Zelenika D, Lathrop M, Scarpini E, Siciliano G, Solfrizzi V, Sorbi S, Spalletta G, Ravaglia G, Valdivieso F, Vepsäläinen S, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Hanon O, Piccardi P, Annoni G, Mann D, Marambaud P, Seripa D, Galimberti D, Tanzi RE, Bertram L, Lendon C, Lannfelt L, Licastro F, Campion D, Pericak-Vance MA, Soininen H, Van Broeckhoven C, Alpérovitch A, Ruiz A, Kamboh MI, Amouyel P. The CALHM1 P86L polymorphism is a genetic modifier of age at onset in Alzheimer’s disease: a meta-analysis study. J Alzheimers Dis 22: 247–255, 2010. doi: 10.3233/JAD-2010-100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez W, Liu Y, Harris AL, Contreras JE. Divalent regulation and intersubunit interactions of human connexin26 (Cx26) hemichannels. Channels (Austin) 8: 1–4, 2014. doi: 10.4161/chan.26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Z, Siebert AP, Cheung KH, Lee RJ, Johnson B, Cohen AS, Vingtdeux V, Marambaud P, Foskett JK. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc Natl Acad Sci USA 109: E1963–E1971, 2012. doi: 10.1073/pnas.1204023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Z, Saung WT, Foskett JK. Action potentials and ion conductances in wild-type and CALHM1-knockout type II taste cells. J Neurophysiol 117: 1865–1876, 2017. doi: 10.1152/jn.00835.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Z, Tanis JE, Taruno A, Foskett JK. Calcium homeostasis modulator (CALHM) ion channels. Pflugers Arch 468: 395–403, 2016. doi: 10.1007/s00424-015-1757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458: 597–602, 2009. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 24.Moreno AP, Chanson M, Elenes S, Anumonwo J, Scerri I, Gu H, Taffet SM, Delmar M. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ Res 90: 450–457, 2002. doi: 10.1161/hh0402.105667. [DOI] [PubMed] [Google Scholar]
- 25.Oh S, Abrams CK, Verselis VK, Bargiello TA. Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J Gen Physiol 116: 13–31, 2000. doi: 10.1085/jgp.116.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh S, Bargiello TA. Voltage regulation of connexin channel conductance. Yonsei Med J 56: 1–15, 2015. doi: 10.3349/ymj.2015.56.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys J 87: 912–928, 2004. doi: 10.1529/biophysj.103.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh S, Verselis VK, Bargiello TA. Charges dispersed over the permeation pathway determine the charge selectivity and conductance of a Cx32 chimeric hemichannel. J Physiol 586: 2445–2461, 2008. doi: 10.1113/jphysiol.2008.150805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 115: 1077–1089, 1991. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peracchia C, Peracchia LL. Inversion of both gating polarity and CO2 sensitivity of voltage gating with D3N mutation of Cx50. Am J Physiol Cell Physiol 288: C1381–C1389, 2005. doi: 10.1152/ajpcell.00348.2004. [DOI] [PubMed] [Google Scholar]
- 31.Pfahnl A, Dahl G. Localization of a voltage gate in connexin46 gap junction hemichannels. Biophys J 75: 2323–2331, 1998. doi: 10.1016/S0006-3495(98)77676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinto BI, García IE, Pupo A, Retamal MA, Martínez AD, Latorre R, González C. Charged residues at the first transmembrane region contribute to the voltage dependence of the slow gate of connexins. J Biol Chem 291: 15740–15752, 2016. doi: 10.1074/jbc.M115.709402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J 79: 2403–2415, 2000. doi: 10.1016/S0006-3495(00)76485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Revilla A, Castro C, Barrio LC. Molecular dissection of transjunctional voltage dependence in the connexin-32 and connexin-43 junctions. Biophys J 77: 1374–1383, 1999. doi: 10.1016/S0006-3495(99)76986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rusakov DA, Fine A. Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron 37: 287–297, 2003. doi: 10.1016/S0896-6273(03)00025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez HA, Mese G, Srinivas M, White TW, Verselis VK. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J Gen Physiol 136: 47–62, 2010. doi: 10.1085/jgp.201010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez HA, Villone K, Srinivas M, Verselis VK. The D50N mutation and syndromic deafness: altered Cx26 hemichannel properties caused by effects on the pore and intersubunit interactions. J Gen Physiol 142: 3–22, 2013. doi: 10.1085/jgp.201310962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandilos JK, Chiu YH, Chekeni FB, Armstrong AJ, Walk SF, Ravichandran KS, Bayliss DA. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J Biol Chem 287: 11303–11311, 2012. doi: 10.1074/jbc.M111.323378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seki A, Duffy HS, Coombs W, Spray DC, Taffet SM, Delmar M. Modifications in the biophysical properties of connexin43 channels by a peptide of the cytoplasmic loop region. Circ Res 95: e22–e28, 2004. doi: 10.1161/01.RES.0000140737.62245.c5. [DOI] [PubMed] [Google Scholar]
- 40.Siebert AP, Ma Z, Grevet JD, Demuro A, Parker I, Foskett JK. Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins. J Biol Chem 288: 6140–6153, 2013. doi: 10.1074/jbc.M112.409789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J 88: 1725–1739, 2005. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang Q, Dowd TL, Verselis VK, Bargiello TA. Conformational changes in a pore-forming region underlie voltage-dependent “loop gating” of an unapposed connexin hemichannel. J Gen Physiol 133: 555–570, 2009. doi: 10.1085/jgp.200910207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanis JE, Ma Z, Krajacic P, He L, Foskett JK, Lamitina T. CLHM-1 is a functionally conserved and conditionally toxic Ca2+-permeable ion channel in Caenorhabditis elegans. J Neurosci 33: 12275–12286, 2013. doi: 10.1523/JNEUROSCI.5919-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495: 223–226, 2013. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trexler EB, Bennett MV, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci USA 93: 5836–5841, 1996. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science 283: 1176–1180, 1999. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- 47.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature 368: 348–351, 1994. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 48.Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol 132: 315–327, 2008. doi: 10.1085/jgp.200810029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verselis VK, Trelles MP, Rubinos C, Bargiello TA, Srinivas M. Loop gating of connexin hemichannels involves movement of pore-lining residues in the first extracellular loop domain. J Biol Chem 284: 4484–4493, 2009. doi: 10.1074/jbc.M807430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vingtdeux V, Chandakkar P, Zhao H, Blanc L, Ruiz S, Marambaud P. CALHM1 ion channel elicits amyloid-β clearance by insulin-degrading enzyme in cell lines and in vivo in the mouse brain. J Cell Sci 128: 2330–2338, 2015. doi: 10.1242/jcs.167270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vingtdeux V, Chang EH, Frattini SA, Zhao H, Chandakkar P, Adrien L, Strohl JJ, Gibson EL, Ohmoto M, Matsumoto I, Huerta PT, Marambaud P. CALHM1 deficiency impairs cerebral neuron activity and memory flexibility in mice. Sci Rep 6: 24250, 2016. doi: 10.1038/srep24250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vingtdeux V, Tanis JE, Chandakkar P, Zhao H, Dreses-Werringloer U, Campagne F, Foskett JK, Marambaud P. Effect of the CALHM1 G330D and R154H human variants on the control of cytosolic Ca2+ and Aβ levels. PLoS One 9: e112484, 2014. doi: 10.1371/journal.pone.0112484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang HZ, Veenstra RD. Monovalent ion selectivity sequences of the rat connexin43 gap junction channel. J Gen Physiol 109: 491–507, 1997. doi: 10.1085/jgp.109.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou XW, Pfahnl A, Werner R, Hudder A, Llanes A, Luebke A, Dahl G. Identification of a pore lining segment in gap junction hemichannels. Biophys J 72: 1946–1953, 1997. doi: 10.1016/S0006-3495(97)78840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]